Lepidium sativum Sprouts Grown under Elevated CO2 Hyperaccumulate Glucosinolates and Antioxidants and Exhibit Enhanced Biological and Reduced Antinutritional Properties
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Experimental Conditions
2.2. Evaluation of the Contents of Pigments
2.3. Amino Acid Determination
2.4. Evaluation of Phenol Content
2.5. Glucosinolate Extraction and Determination
2.6. Evaluation of Myrosinase Enzyme Activity
2.7. Inhibition of Micellar Solubility of Cholesterol
2.8. Lipase and Amylase Inhibition Assays
2.9. Antioxidant Activity
2.10. Lipoxygenase (LOX) Assay
2.11. Evaluation of Cyclooxygenase 2
2.12. Antibacterial Activities
2.13. Anticancer Activities
2.14. Statistical Analyses
3. Results and Discussion
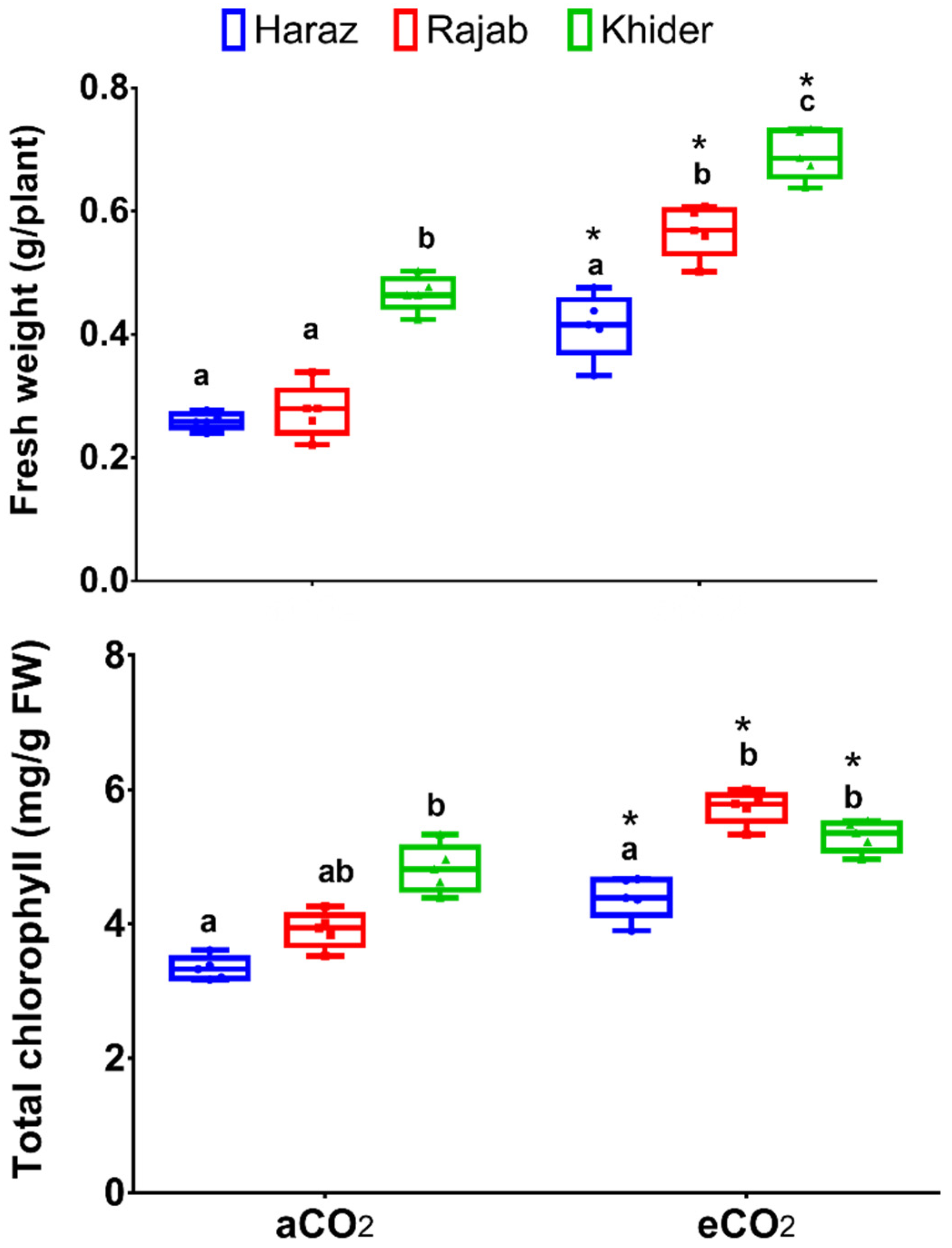
3.1. Elevated CO2 Improves the Biomass Production of L. sativum Sprouts
3.2. L. sativum Sprouts Produced under eCO2 Showed Reduced Content of Antinutrients
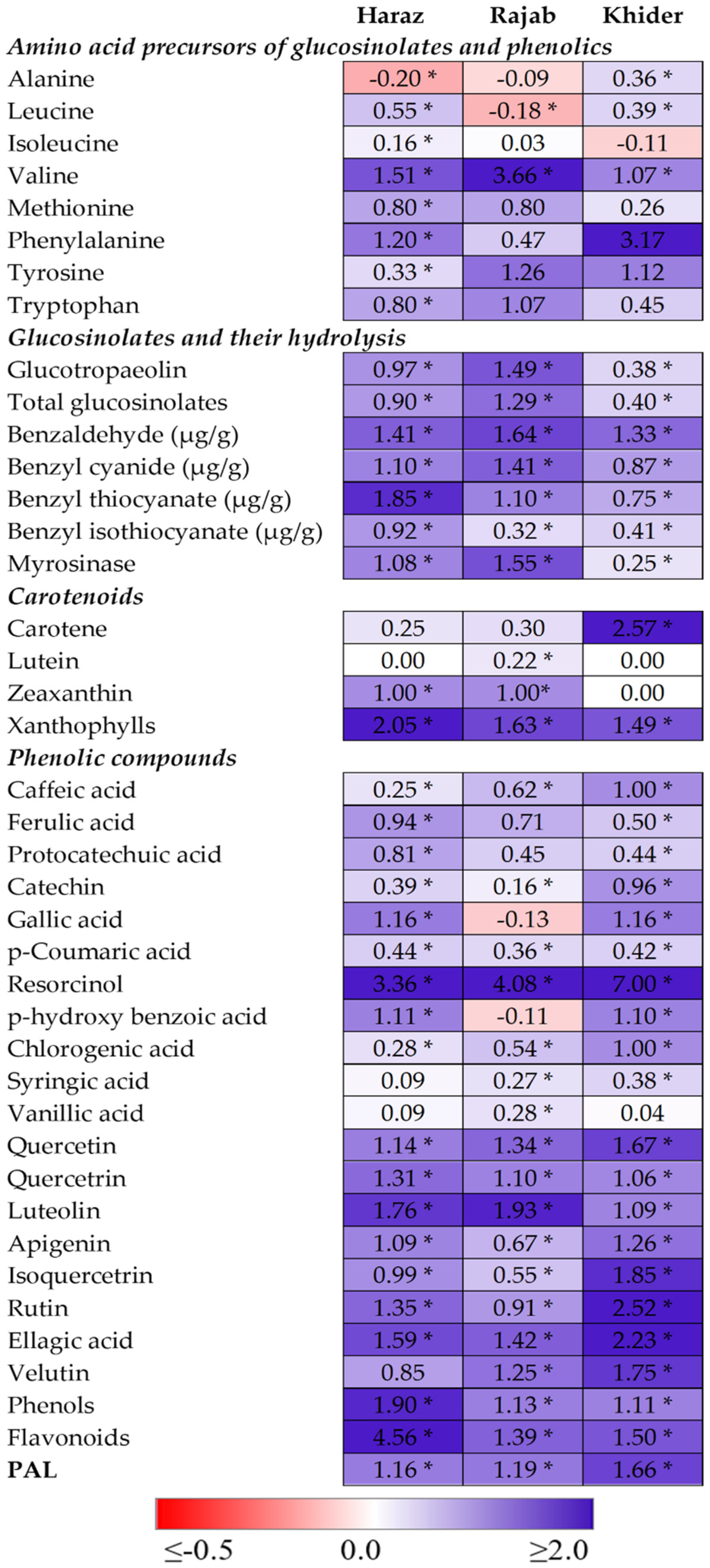
3.3. The Levels of Amino Acid Precursors for Glucosinolates and Phenolics Are Enhanced by eCO2
3.4. The Accumulation and Hydrolysis of Glucosinolates Are Induced by eCO2
3.5. Elevated CO2 Induces the Accumulation of Antioxidant Metabolites
3.6. The eCO2-Induced Accumulation of Antioxidants and Glucosinolates Is Accompanied by Promoted Bioactivity
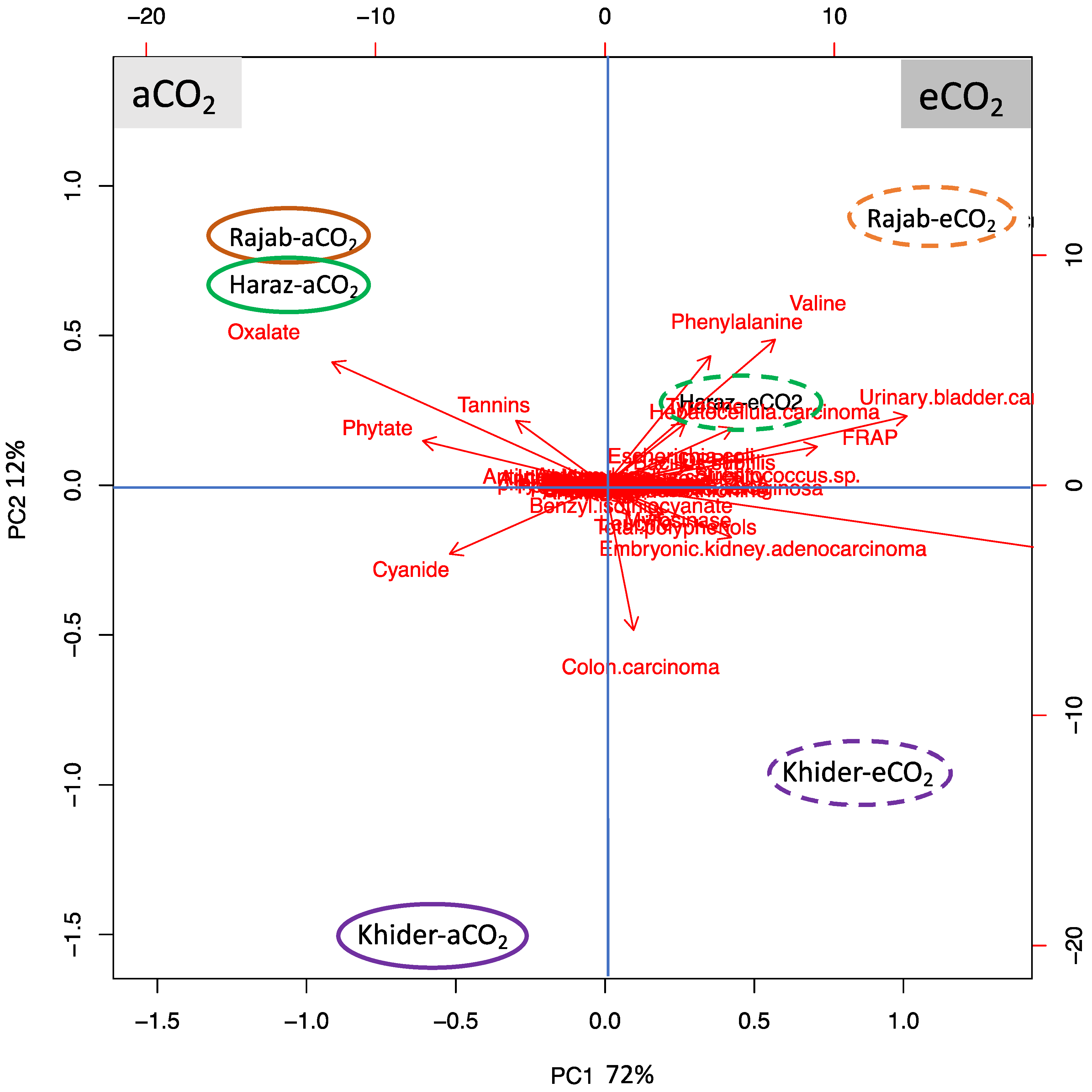
3.7. L. sativum Cultivar-Specific Response to eCO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramadan, M.F.; Oraby, H.F. Lepidium sativum Seeds: Therapeutic Significance and Health-Promoting Potential. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–289. [Google Scholar]
- Rafińska, K.; Pomastowski Pawełand Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Mennicke, W.H.; Görler, K.; Krumbiegel, G.; Lorenz, D.; Rittmann, N. Studies on the metabolism and excretion of benzyl isothiocyanate in man. Xenobiotica 1988, 18, 441–447. [Google Scholar] [CrossRef]
- Getahun, T.; Sharma, V.; Gupta, N. Chemical composition, antibacterial and antioxidant activities of oils obtained by different extraction methods from Lepidium sativum L. seeds. Ind. Crop. Prod. 2020, 156, 112876. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.A.; Khamis, G. Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Hassan, A.H.A.; Al Jaouni, S.K.; Alkhalifah, D.H.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H.; Khamis, G. Influence of elevated CO2 on nutritive value and health-promoting prospective of three genotypes of Alfalfa sprouts (Medicago Sativa). Food Chem. 2021, 340, 128147. [Google Scholar] [CrossRef]
- AlObaidi, L.A.H. Study the anticancer effect of Lepidium sativum leaves extract on squamous cell carcinoma (CAL-27) cell lines. J. Nat. Sci. Res. 2014, 4, 48–52. [Google Scholar]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health--Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Sosińska, E.; Obiedziński, M.W. Effect of processing on the content of glucobrassicin and its degradation products in broccoli and cauliflower. Food Control 2011, 22, 1348–1356. [Google Scholar] [CrossRef]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.W.; Botero-Omary, M.; Chung, F.-L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Dosz, E.B.; Sun, J.; Hoeflinger, J.L.; Van Tassell, M.L.; Chen, P.; Harnly, J.M.; Miller, M.J.; Jeffery, E.H. Myrosinase-dependent and -independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front. Plant Sci. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The role of isothiocyanates as cancer chemo-preventive, chemo-therapeutic and anti-melanoma agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-J.J.; Gan, R.-Y.Y.; Li, S.; Zhou, Y.; Li, A.-N.N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J. The photoprotective role of carotenoids in higher plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Boo, H.-O.; Hwang, S.-J.; Bae, C.-S.; Park, S.-H.; Heo, B.-G.; Gorinstein, S. Extraction and characterization of some natural plant pigments. Ind. Crop. Prod. 2012, 40, 129–135. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef]
- Sosnowska, D.; Podsędek, A.; Redzynia, M.; Kucharska, A.Z. Inhibitory effect of black chokeberry fruit polyphenols on pancreatic lipase–Searching for most active inhibitors. J. Funct. Foods 2018, 49, 196–204. [Google Scholar] [CrossRef]
- Figueroa Pérez, M.G.; Rocha-Guzmán, N.E.; Mercado-Silva, E.; Loarca-Piña, G.; Reynoso-Camacho, R. Effect of chemical elicitors on peppermint (Mentha piperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem. 2014, 156, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hozzein, W.N.; Saleh, A.M.; Habeeb, T.H.; Wadaan, M.A.M.; AbdElgawad, H. CO2 treatment improves the hypocholesterolemic and antioxidant properties of fenugreek seeds. Food Chem. 2020, 308, 125661. [Google Scholar] [CrossRef]
- Saleh, A.M.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. CO2 enrichment can enhance the nutritional and health benefits of parsley (Petroselinum crispum L.) and dill (Anethum graveolens L.). Food Chem. 2018, 269, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224–225, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Murray, V.; Ebi, K.L. IPCC special report on managing the risks of extreme events and disasters to advance climate change adaptation (SREX). J. Epidemiol. Community Health 2012, 66, 759–760. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Saleh, A.M.; Sobrinho, R.L.; Sheteiwy, M.S.; El-sawah, A.M.; Mohammed, A.E.; Elgawad, H.A. Arbuscular Mycorrhizae Mitigate Aluminum Toxicity and Regulate Proline Metabolism in Plants Grown in Acidic Soil. J. Fungi 2021, 7, 531. [Google Scholar] [CrossRef]
- Moustakas, M.; Eleftheriou, E.P.; Ouzounidou, G. Short-term effects of aluminium at alkaline pH on the structure and function of the photosynthetic apparatus. Photosynthetica 1998, 34, 169–177. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; El-Maksoud, A.; Ahmed, A.; Alharbi, S.A.; Alfarraj, S.; Mohamed, M.S.M. Fortification of Acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties. Molecules 2021, 26, 3876. [Google Scholar] [CrossRef] [PubMed]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.M.; Hassan, Y.M.; Habeeb, T.H.; Alkhalaf, A.A.; Hozzein, W.N.; Selim, S.; Abdelgawad, H. Interactive effects of mercuric oxide nanoparticles and future climate CO2 on maize plant. J. Hazard. Mater. 2021, 401, 721402. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Hassan, Y.M.; Alotaibi, M.O.; Mohammed, A.E.; Saleh, A.M. C3 and C4 plant systems respond differently to the concurrent challenges of mercuric oxide nanoparticles and future climate CO2. Sci. Total Environ. 2020, 749, 142356. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.A.; Runion, G.B.; Rogers, H.H.; Torbert, H.A. Effects of atmospheric CO2 enrichment on crop nutrient dynamics under no-till conditions. J. Plant Nutr. 2008, 31, 758–773. [Google Scholar] [CrossRef] [Green Version]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Neumann, N.; Fitzgerald, G.J.; Seneweera, S. Elevated CO2 alters grain quality of two bread wheat cultivars grown under different environmental conditions. Agric. Ecosyst. Environ. 2014, 185, 24–33. [Google Scholar] [CrossRef]
- Weaver, C.M.; Kannan, S. Phytate and mineral bioavailability. In Food Phytates; CRC Press: Boca Raton, FL, USA, 2001; pp. 227–240. [Google Scholar]
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 2007, 137, 135–141. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Callaway, R.M.; Thomas, E.M.; Schlesinger, W.H. Mechanisms of phosphorus acquisition for ponderosa pine seedlings under high CO2 and temperature. Ann. Bot. 1997, 79, 111–120. [Google Scholar] [CrossRef]
- Watt, M.; Evans, J.R. Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiol. 1999, 120, 705–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- La, G.; Fang, P.; Teng, Y.; Li, Y.; Lin, X. Effect of CO2 enrichment on the glucosinolate contents under different nitrogen levels in bolting stem of Chinese kale (Brassica alboglabra L.). J. Zhejiang Univ. Sci. B 2009, 10, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.M.; Hassan, Y.M.; Selim, S.; AbdElgawad, H. NiO-nanoparticles induce reduced phytotoxic hazards in wheat (Triticum aestivum L.) grown under future climate CO2. Chemosphere 2019, 220, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Jones, C.G.; Couper, G.C.; Jones, T.H. Biosynthesis of plant phenolic compounds in elevated atmospheric CO2. Glob. Chang. Biol. 2000, 6, 497–506. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]

| Antinutrients | aCO2 | eCO2 | ||||
|---|---|---|---|---|---|---|
| Haraz | Rajab | Khider | Haraz | Rajab | Khider | |
| Tannins | 32.1 ± 1.1 c | 27.3 ± 1.2 b | 19.3 ± 2.4 a | 22.6 ± 2.4 B * | 18.2 ± 2.1 AB * | 15.4 ± 5.1 A |
| Phytate | 87.4 ± 1.1 a | 91.4 ± 4.7 a | 86.7 ± 5.2 a | 77.1 ± 4.0 A * | 72.1 ± 9.2 A * | 61.8 ± 9.0 A * |
| Cyanide | 69.1 ± 2.7 a | 70.8 ± 4.1 a | 85.4 ± 2.5 b | 72.1 ± 4.2 B | 55.1 ± 2.2 A * | 51.8 ± 1.9 A * |
| Oxalate | 112.7 ± 11.6 b | 123.7 ± 8.7 b | 91.5 ± 5.1 a | 101.2 ± 8.2 B | 77.5 ± 4.7 A * | 84.1 ± 5.1 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.O.; Khamis, G.; AbdElgawad, H.; Mohammed, A.E.; Sheteiwy, M.S.; Elobeid, M.M.; Saleh, A.M. Lepidium sativum Sprouts Grown under Elevated CO2 Hyperaccumulate Glucosinolates and Antioxidants and Exhibit Enhanced Biological and Reduced Antinutritional Properties. Biomolecules 2021, 11, 1174. https://doi.org/10.3390/biom11081174
Alotaibi MO, Khamis G, AbdElgawad H, Mohammed AE, Sheteiwy MS, Elobeid MM, Saleh AM. Lepidium sativum Sprouts Grown under Elevated CO2 Hyperaccumulate Glucosinolates and Antioxidants and Exhibit Enhanced Biological and Reduced Antinutritional Properties. Biomolecules. 2021; 11(8):1174. https://doi.org/10.3390/biom11081174
Chicago/Turabian StyleAlotaibi, Modhi O., Galal Khamis, Hamada AbdElgawad, Afrah E. Mohammed, Mohamed S. Sheteiwy, Mudawi M. Elobeid, and Ahmed M. Saleh. 2021. "Lepidium sativum Sprouts Grown under Elevated CO2 Hyperaccumulate Glucosinolates and Antioxidants and Exhibit Enhanced Biological and Reduced Antinutritional Properties" Biomolecules 11, no. 8: 1174. https://doi.org/10.3390/biom11081174
APA StyleAlotaibi, M. O., Khamis, G., AbdElgawad, H., Mohammed, A. E., Sheteiwy, M. S., Elobeid, M. M., & Saleh, A. M. (2021). Lepidium sativum Sprouts Grown under Elevated CO2 Hyperaccumulate Glucosinolates and Antioxidants and Exhibit Enhanced Biological and Reduced Antinutritional Properties. Biomolecules, 11(8), 1174. https://doi.org/10.3390/biom11081174








