A Runner’s High for New Neurons? Potential Role for Endorphins in Exercise Effects on Adult Neurogenesis
Abstract
1. Exercise Effects on Brain and Mental Health
2. Hippocampus and Adult Neurogenesis—Potential Involvement in Many of the Exercise-Related Effects
3. Exercise Effects on Adult Neurogenesis
4. Functional Role of Neurogenesis on Exercise-Induced Changes to Mental Functioning
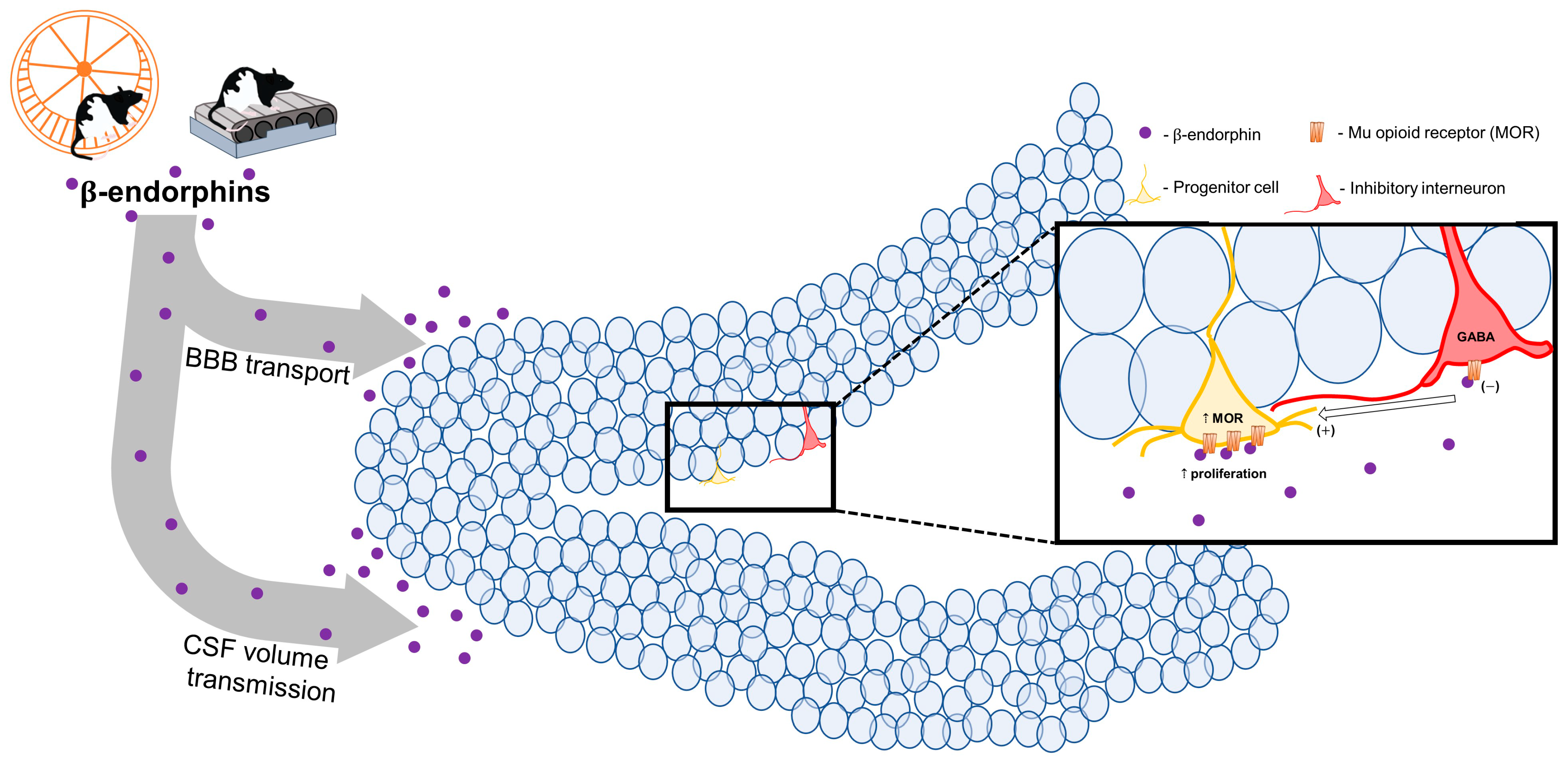
5. Potential Mechanisms Involved in Exercise Effects on Neurogenesis—Endorphins?
6. β-Endorphin: Just for the Periphery?
7. Direct Evidence for Endorphin Influence on Neurogenesis
8. Circumstantial Evidence for β-Endorphin Roles in Neurogenesis—A Need for More Behavioral Studies Utilizing Endogenous Opioids
9. Implications and Conclusions
Funding
Conflicts of Interest
References
- Jesus, I.; Vanhee, V.; Deramaudt, T.B.; Bonay, M. Promising effects of exercise on the cardiovascular, metabolic and immune system during COVID-19 period. J. Hum. Hypertens. 2021, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gorostegi-Anduaga, I.; Corres, P.; MartinezAguirre-Betolaza, A.; Pérez-Asenjo, J.; Aispuru, G.R.; Fryer, S.M.; Maldonado-Martín, S. Effects of different aerobic exercise programmes with nutritional intervention in sedentary adults with overweight/obesity and hypertension: EXERDIET-HTA study. Eur. J. Prev. Cardiol. 2018, 25, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Al Dahamsheh, Z.; Al Rashdan, K.; Al Hadid, A.; Jaradat, R.; Al Bakheet, M.; Bataineh, Z.S. The Impact of Aerobic Exercise on Female Bone Health Indicators. Med. Arch. 2019, 73, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Howard, V.J.; McDonnell, M.N. Physical Activity in Primary Stroke Prevention: Just Do It! Stroke 2015, 46, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Thomas, K.N.; Williams, M.J.A.; Atkinson, G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011, 77, 288–294. [Google Scholar] [CrossRef]
- Paillard, T.; Rolland, Y.; de Souto Barreto, P. Protective Effects of Physical Exercise in Alzheimer’s Disease. J. Clin. Neurol. 2015, 11, 212–219. [Google Scholar] [CrossRef]
- Briken, S.; Gold, S.M.; Patra, S.; Vettorazzi, E.; Harbs, D.; Tallner, A.; Ketels, G.; Schulz, K.H.; Heesen, C. Effects of exercise on fitness and cognition in progressive MS: A randomized, controlled pilot trial. Mult. Scler. J. 2014, 20, 382–390. [Google Scholar] [CrossRef]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Dilorenzo, T.M.; Bargman, E.P.; Stucky-Ropp, R.; Brassington, G.S.; Frensch, P.A.; LaFontaine, T. Long-term effects of aerobic exercise on psychological outcomes. Prev. Med. 1999, 28, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kandola, A.; Hendrikse, J.; Lucassen, P.J.; Yücel, M. Aerobic Exercise as a Tool to Improve Hippocampal Plasticity and Function in Humans: Practical Implications for Mental Health Treatment. Front. Hum. Neurosci. 2016, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Way, K.; Kannis-Dymand, L.; Lastella, M.; Lovell, G.P. Mental health practitioners’ reported barriers to prescription of exercise for mental health consumers. Ment. Health Phys. Act. 2018, 14, 52–60. [Google Scholar] [CrossRef]
- Bernstein, E.E.; McNally, R.J. Examining the Effects of Exercise on Pattern Separation and the Moderating Effects of Mood Symptoms. Behav. Ther. 2019, 50, 582–593. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Petruzzello, S.J.; Landers, D.M.; Hatfield, B.D.; Kubitz, K.A.; Salazar, W. A Meta-Analysis on the Anxiety-Reducing Effects of Acute and Chronic Exercise: Outcomes and Mechanisms. Sports Med. 1991, 11, 143–182. [Google Scholar] [CrossRef]
- Craft, L.L.; Landers, D.M. The Effect of Exercise on Clinical Depression and Depression Resulting from Mental Illness: A Meta-Analysis. J. Sport Exerc. Psychol. 1998, 20, 339–357. [Google Scholar] [CrossRef]
- Zschucke, E.; Renneberg, B.; Dimeo, F.; Wüstenberg, T.; Ströhle, A. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology 2015, 51, 414–425. [Google Scholar] [CrossRef]
- Masley, S.; Roetzheim, R.; Gualtieri, T. Aerobic exercise enhances cognitive flexibility. J. Clin. Psychol. Med. Settings 2009, 16, 186–193. [Google Scholar] [CrossRef]
- Cameron, H.A.; Glover, L.R. Adult Neurogenesis: Beyond Learning and Memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef]
- Gould, E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007, 8, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jimenez, E.P.; Terreros-Roncal, J.; Flor-Garcia, M.; Rabano, A.; Llorens-Martin, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Garthe, A.; Kempermann, G. An old test for new neurons: Refining the morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Neurosci. 2013, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.R.; Denny, C.A.; Hen, R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav. Neurosci. 2010, 124, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Clelland, C.D.; Choi, M.; Romberg, C.; Clemenson, G.D.; Fragniere, A.; Tyers, P.; Jessberger, S.; Saksida, L.M.; Barker, R.A.; Gage, F.H.; et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009, 325, 210–213. [Google Scholar] [CrossRef]
- Weeden, C.S.S.; Mercurio, J.C.; Cameron, H.A. A role for hippocampal adult neurogenesis in shifting attention toward novel stimuli. Behav. Brain Res. 2019, 376. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Cameron, H.A. Adult neurogenesis and mental illness. Neuropsychopharmacology 2015, 40. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef]
- Brown, J.; Cooper-Kuhn, C.M.; Kempermann, G.; Van Praag, H.; Winkler, J.; Gage, F.H.; Kuhn, H.G. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003, 17, 2042–2046. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Rada, P.; Pieruzzini, P.R.; Hsueh, B.; Gould, E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J. Neurosci. 2013, 33. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.M.; Galea, L.A.M.; Mistlberger, R.E.; Kempermann, G. Adult Hippocampal Neurogenesis and Voluntary Running Activity: Circadian and Dose-Dependent Effects. J. Neurosci. Res. 2004, 76, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.S.; Jeffrey, S.; Girard, I.; Mitchell, G.S.; Van Praag, H.; Garland, T.; Gage, F.H. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav. Neurosci. 2003, 117, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Inoue, K.; Okamoto, M.; Liu, Y.F.; Matsui, T.; Yook, J.S.; Soya, H. Voluntary resistance running induces increased hippocampal neurogenesis in rats comparable to load-free running. Neurosci. Lett. 2013, 537, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Nguemeni, C.; McDonald, M.W.; Jeffers, M.S.; Livingston-Thomas, J.; Lagace, D.; Corbett, D. Short- and Long-term Exposure to Low and High Dose Running Produce Differential Effects on Hippocampal Neurogenesis. Neuroscience 2018, 369, 202–211. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Wu, C.; He, X.F.; Wu, D.; He, X.; Liang, F.Y.; Dai, G.Y.; Pei, Z.; Xu, G.Q.; Lan, Y. Effects of voluntary wheel-running types on hippocampal neurogenesis and spatial cognition in middle-aged mice. Front. Cell. Neurosci. 2018, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Huang, Z.; Jiang, Y.; Zhou, X.; Zhang, J.; Zhang, D.; Wang, B.; Hou, G. Strength exercise weakens aerobic exerciseinduced cognitive improvements in rats. PLoS ONE 2018, 13, e0205562. [Google Scholar] [CrossRef] [PubMed]
- Codina-Martínez, H.; Fernández-García, B.; Díez-Planelles, C.; Fernández, Á.F.; Higarza, S.G.; Fernández-Sanjurjo, M.; Díez-Robles, S.; Iglesias-Gutiérrez, E.; Tomás-Zapico, C. Autophagy is required for performance adaptive response to resistance training and exercise-induced adult neurogenesis. Scand. J. Med. Sci. Sports 2020, 30, 238–253. [Google Scholar] [CrossRef]
- Novaes Gomes, F.G.; Fernandes, J.; Vannucci Campos, D.; Cassilhas, R.C.; Viana, G.M.; D’Almeida, V.; de Moraes Rêgo, M.K.; Buainain, P.I.; Cavalheiro, E.A.; Arida, R.M. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 2014, 50, 106–117. [Google Scholar] [CrossRef]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef]
- Gremmelspacher, T.; Gerlach, J.; Hubbe, A.; Haas, C.A.; Häussler, U. Neurogenic processes are induced by very short periods of voluntary wheel-running in male mice. Front. Neurosci. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Glover, L.R.; Sanzone, K.M.; Kamhi, J.F.; Cameron, H.A. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus 2009, 19, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Sah, N.; Peterson, B.D.; Lubejko, S.T.; Vivar, C.; Van Praag, H. Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci. Rep. 2017, 7, 10903. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med. 2008, 10, 118–127. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Gould, E. Stress, stress hormones, and adult neurogenesis. Exp. Neurol. 2012, 233. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Khalil, D.; Gould, E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 2006, 9, 526–533. [Google Scholar] [CrossRef]
- Leasure, J.L.; Decker, L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus 2009, 19, 907–912. [Google Scholar] [CrossRef]
- Hauser, T.; Klaus, F.; Lipp, H.P.; Amrein, I. No effect of running and laboratory housing on adult hippocampal neurogenesis in wild caught long-tailed wood mouse. BMC Neurosci. 2009, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, A.; Guan, F.; Fan, R.; Chi, L.; Yang, B. Regular treadmill running improves spatial learning and memory performance in young mice through increased hippocampal neurogenesis and decreased stress. Brain Res. 2013, 1531, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Okamoto, M.; Shibato, J.; Lee, M.C.; Matsui, T.; Rakwal, R.; Soya, H. Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS ONE 2015, 10, e0128720. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yamamura, Y.; Liu, Y.-F.; Min-Chul, L.; Matsui, T.; Shima, T.; Soya, M.; Takahashi, K.; Soya, S.; McEwen, B.S.; et al. Hormetic effects by exercise on hippocampal neurogenesis with glucocorticoid signaling. Brain Plast. 2016, 1, 149–158. [Google Scholar] [CrossRef]
- Clark, P.J.; Brzezinska, W.J.; Thomas, M.W.; Ryzhenko, N.A.; Toshkov, S.A.; Rhodes, J.S. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 2008, 155, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Becker, S.; Luu, P.; Rosenzweig, S.; Wojtowicz, J.M. Adult hippocampal neurogenesis and memory interference. Behav. Brain Res. 2012, 227, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.M.; Askew, M.L.; Winocur, G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur. J. Neurosci. 2008, 27, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Wong-Goodrich, S.J.E.; Pfau, M.L.; Flores, C.T.; Fraser, J.A.; Williams, C.L.; Jones, L.W. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer Res. 2010, 70, 9329–9338. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.F.; Majdak, P.; Miller, D.S.; Bucko, P.J.; Merritt, J.R.; Krebs, C.P.; Rhodes, J.S. Evaluation of a C57BL/6J x 129S1/SvImJ Hybrid Nestin-Thymidine Kinase Transgenic Mouse Model for Studying the Functional Significance of Exercise-Induced Adult Hippocampal Neurogenesis. Brain Plast. 2016, 1, 83–95. [Google Scholar] [CrossRef]
- Snyder, J.S.; Cahill, S.P.; Frankland, P.W. Running Promotes Spatial Bias Independently of Adult Neurogenesis. Hippocampus 2017, 27, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.Y.; Lau, B.W.M.; Bin Tong, J.; Wong, R.; Ching, Y.P.; Qiu, G.; Tang, S.W.; Lee, T.M.C.; So, K.F. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS ONE 2011, 6, e24263. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; McCausland, H.C.; Sonti, A.N.; Cameron, H.A. Anxiolytic Actions of Exercise in Absence of New Neurons. Hippocampus 2016, 26. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Y.Y.; Xu, L.C.; Ma, L.Y.; Liu, F.Y.; Cui, S.; Cai, J.; Liao, F.F.; Wan, Y.; Yi, M. Adult hippocampal neurogenesis along the dorsoventral axis contributes differentially to environmental enrichment combined with voluntary exercise in alleviating chronic inflammatory pain in mice. J. Neurosci. 2017, 37, 4145–4157. [Google Scholar] [CrossRef]
- Kim, J.J.; Foy, M.R.; Thompson, R.F. Behavioral stress modifies hippocampal plasticity through N-methyl-D-asparate receptor activation. Proc. Natl. Acad. Sci. USA 1996, 93, 4750–4753. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Kitamura, T.; Saitoh, Y.; Ohkawa, N.; Kondo, T.; Inokuchi, K. Adult neurogenesis conserves hippocampal memory capacity. J. Neurosci. 2018, 38, 6854–6863. [Google Scholar] [CrossRef]
- Patten, A.R.; Sickmann, H.; Hryciw, B.N.; Kucharsky, T.; Parton, R.; Kernick, A.; Christie, B.R. Long-term exercise is needed to enhance synaptic plasticity in the hippocampus. Learn. Mem. 2013, 20, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Eadie, B.D.; Redila, V.A.; Christie, B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005, 486, 39–47. [Google Scholar] [CrossRef]
- Biedermann, S.; Fuss, J.; Zheng, L.; Sartorius, A.; Falfán-Melgoza, C.; Demirakca, T.; Gass, P.; Ende, G.; Weber-Fahr, W. In vivo voxel based morphometry: Detection of increased hippocampal volume and decreased glutamate levels in exercising mice. NeuroImage 2012, 61, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Holsinger, R.M.D. Exercise-induced brain-derived neurotrophic factor expression: Therapeutic implications for Alzheimer’s dementia. Ageing Res. Rev. 2018, 48, 109–121. [Google Scholar] [CrossRef]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef]
- Rich, B.; Scadeng, M.; Yamaguchi, M.; Wagner, P.D.; Breen, E.C. Skeletal myofiber vascular endothelial growth factor is required for the exercise training-induced increase in dentate gyrus neuronal precursor cells. J. Physiol. 2017, 595, 5931–5943. [Google Scholar] [CrossRef]
- Fabel, K.; Fabel, K.; Tam, B.; Kaufer, D.; Baiker, A.; Simmons, N.; Kuo, C.J.; Palmer, T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003, 18, 2803–2812. [Google Scholar] [CrossRef]
- Mukuda, T.; Sugiyama, H. An angiotensin II receptor antagonist suppresses running-enhanced hippocampal neurogenesis in rat. Neurosci. Res. 2007, 58, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; LLorens-Martín, M.V.; Torres-Alemán, I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol. Cell. Neurosci. 2008, 37, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Hofer, M.; Pagliusi, S.R.; Pagliusi, S.R.; Hohn, A.; Hohn, A.; Leibrock, J.; Leibrock, J.; Barde, Y.; Barde, Y. Regional distribution. EMBO J. 1990, 9, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Akins, M.R.; Lee, F.S. BDNF control of adult SVZ neurogenesis. Dev. Psychobiol. 2012, 54, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Oliff, H.S.; Berchtold, N.C.; Isackson, P.; Cotman, C.W. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Mol. Brain Res. 1998, 61, 147–153. [Google Scholar] [CrossRef]
- Adlard, P.A.; Perreau, V.M.; Cotman, C.W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging 2005, 26, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef]
- Quesseveur, G.; David, D.J.; Gaillard, M.C.; Pla, P.; Wu, M.V.; Nguyen, H.T.; Nicolas, V.; Auregan, G.; David, I.; Dranovsky, A.; et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry 2013, 3. [Google Scholar] [CrossRef]
- Rossi, C.; Angelucci, A.; Costantin, L.; Braschi, C.; Mazzantini, M.; Babbini, F.; Fabbri, M.E.; Tessarollo, L.; Maffei, L.; Berardi, N.; et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 2006, 24, 1850–1856. [Google Scholar] [CrossRef]
- Li, Y.; Luikart, B.W.; Birnbaum, S.; Chen, J.; Kwon, C.H.; Kernie, S.G.; Bassel-Duby, R.; Parada, L.F. TrkB Regulates Hippocampal Neurogenesis and Governs Sensitivity to Antidepressive Treatment. Neuron 2008, 59, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Wildmann, J.; Kruger, A.; Schmole, M.; Niemann, J.; Matthaei, H. Increase of circulating beta-endorphin-like immunoreactivity correlates with the change in feeling of pleasantness after running. Life Sci. 1986, 38, 997–1003. [Google Scholar] [CrossRef]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108. [Google Scholar] [CrossRef]
- Guillemin, R.; Vargo, T.; Rossier, J.; Minick, S.; Ling, N.; Rivier, C.; Vale, W.; Bloom, F.; Smith, P. β-Endrophin and Adrenocorticotropin Are Secreted Concomitantly by the Pituitary Gland. Science 1977, 197, 1367–1369. [Google Scholar] [CrossRef]
- Parikh, D.; Hamid, A.; Friedman, T.C.; Nguyen, K.; Tseng, A.; Marquez, P.; Lutfy, K. Stress-induced analgesia and endogenous opioid peptides: The importance of stress duration. Eur. J. Pharmacol. 2011, 650, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Mutso, A.A.; Centeno, M.V.; Kan, L.; Wu, M.; Levinstein, M.; Banisadr, G.; Gobeske, K.T.; Miller, R.J.; Radulovic, J.; et al. Role of adult hippocampal neurogenesis in persistent pain. Pain 2016, 157, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Sauriyal, D.S.; Jaggi, A.S.; Singh, N. Extending pharmacological spectrum of opioids beyond analgesia: Multifunctional aspects in different pathophysiological states. Neuropeptides 2011, 45, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Bolijn, S.; Lucassen, P.J. How the Body Talks to the Brain; Peripheral Mediators of Physical Activity-Induced Proliferation in the Adult Hippocampus. Brain Plast. 2016, 1, 5–27. [Google Scholar] [CrossRef]
- Millan, M.J.; Przewlock, R.; Jerlicz, M.; Gramsch, C.; Höllt, V.; Herz, A. Stress-induced release of brain and pituitary β-endorphin: Major role of endorphins in generation of hyperthermia, not analgesia. Brain Res. 1981, 208, 325–338. [Google Scholar] [CrossRef]
- Xue, L.; Sun, J.; Zhu, J.; Ding, Y.; Chen, S.; Ding, M.; Pei, H. The patterns of exercise-induced β-endorphin expression in the central nervous system of rats. Neuropeptides 2020, 82. [Google Scholar] [CrossRef]
- Barfield, E.T.; Alexandra Moser, V.; Hand, A.; Grisel, J.E. ß-Endorphin Modulates the Effect of Stress on Novelty-Suppressed Feeding. Front. Behav. Neurosci. 2013, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.T.; Chavkin, C.; Milner, T.A. Opioid systems in the dentate gyrus. Prog. Brain Res. 2007, 163. [Google Scholar] [CrossRef]
- Padilla, S.L.; Reef, D.; Zeltser, L.M. Defining POMC neurons using transgenic reagents: Impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology 2012, 153, 1219–1231. [Google Scholar] [CrossRef]
- Bloom, F.; Battenberg, E.; Rossier, J.; Ling, N.; Guillemin, R. Neurons containing β-endorphin in rat brain exist separately from those containing enkephalin: Immunocytochemical studies. Proc. Natl. Acad. Sci. USA 1978, 75, 1591–1595. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Zhao, Z.; Feng, Q.; Lin, R.; Sun, Y.; Ding, T.; Xu, F.; Luo, M.; Zhan, C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat. 2015, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.A.; Cavazzuti, M.; Baraldi, P.; Giuliani, D.; Panerai, A.E.; Corazza, R. CNS pattern of metabolic activity during tonic pain: Evidence for modulation by β-endorphin. Eur. J. Neurosci. 1999, 11, 874–888. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, H.; Ke, J.; Zhang, Z.; Wang, Y. Effects of L-655,708 on expression changes of GABA, glutamate, and beta-endorphin induced by propofol anesthesia in rats. Eur. J. Inflamm. 2018, 16. [Google Scholar] [CrossRef]
- Houghten, R.A.; Swann, R.W.; Li, C.H. beta-Endorphin: Stability, clearance behavior, and entry into the central nervous system after intravenous injection of the tritiated peptide in rats and rabbits. Proc. Natl. Acad. Sci. USA 1980, 77, 4588–4591. [Google Scholar] [CrossRef]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier-Abt, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar]
- Kumagai, A.K.; Eisenberg, J.B.; Pardridge, W.M. Absorptive-mediated endocytosis of cationized albumin and a β-endorphin-cationized albumin chimeric peptide by isolated brain capillaries. Model system of blood-brain barrier transport. J. Biol. Chem. 1987, 262, 15214–15219. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Blázquez, J.L.; Guerra, M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: The former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 2010, 31, 757–776. [Google Scholar] [CrossRef]
- Hoffmann, P.; Terenius, L.; Thoren, P. Cerebrospinal fluid immunoreactive fl-endorphin concentration is increased by voluntary exercise in the spontaneously hypertensive rat. Regul. Pept. 1990, 28, 233–239. [Google Scholar] [CrossRef]
- Veening, J.G.; Gerrits, P.O.; Barendregt, H.P. Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS 2012, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Leak, R.K.; Moore, R.Y. Innervation of ventricular and periventricular brain compartments. Brain Res. 2012, 1463, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ableitner, A.; Schulz, R. Neuroanatomical sites mediating the central actions of beta-endorphin as mapped by changes in glucose utilization: Involvement of mu opioid receptors. J. Pharmacol. Exp. Ther. 1992, 262, 415–423. [Google Scholar]
- Koehl, M.; Meerlo, P.; Gonzales, D.; Rontal, A.; Turek, F.W.; Abrous, D.N. Exercise-induced promotion of hippocampal cell proliferation requires β-endorphin. FASEB J. 2008, 22, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.I.; Naylor, A.S.; Jonsdottir, I.H.; Nyberg, F.; Eriksson, P.S.; Thorlin, T. Differential regulation of hippocampal progenitor proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur. J. Neurosci. 2004, 19, 1847–1855. [Google Scholar] [CrossRef]
- Nieto, S.J.; Quave, C.B.; Kosten, T.A. Naltrexone alters alcohol self-administration behaviors and hypothalamic-pituitary-adrenal axis activity in a sex-dependent manner in rats. Pharmacol. Biochem. Behav. 2018, 167, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.I.; Thorlin, T.; Bull, C.; Zarnegar, P.; Ekman, R.; Terenius, L.; Eriksson, P.S. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur. J. Neurosci. 2003, 17, 1159–1172. [Google Scholar] [CrossRef]
- Persson, A.I.; Thorlin, T.; Bull, C.; Eriksson, P.S. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol. Cell. Neurosci. 2003, 23, 360–372. [Google Scholar] [CrossRef]
- Zhang, H.; Torregrossa, M.M.; Jutkiewicz, E.M.; Shi, Y.G.; Rice, K.C.; Woods, J.H.; Watson, S.J.; Holden Ko, M.C. Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through δ- and μ-opioid receptors independent of antidepressant-like effects. Eur. J. Neurosci. 2006, 23, 984–994. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.S.R.; da Silva Fernandes, M.J.; Scorza, F.A.; Persike, D.S.; Scorza, C.A.; da Ponte, J.B.; de Albuquerque, M.; Cavalheiro, E.A.; Arida, R.M. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res. Bull. 2010, 83, 278–283. [Google Scholar] [CrossRef]
- Neumaier, J.F.; Mailheau, S.; Chavkin, C. Opioid receptor-mediated responses in the dentate gyrus and CA1 region of the rat hippocampus. J. Pharmacol. Exp. Ther. 1988, 244, 564–570. [Google Scholar] [PubMed]
- Drake, C.T.; Milner, T.A. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 1999, 849, 203–215. [Google Scholar] [CrossRef]
- Svoboda, K.R.; Adams, C.E.; Lupica, C.R. Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J. Neurosci. 1999, 19, 85–95. [Google Scholar] [CrossRef]
- Tozuka, Y.; Fukuda, S.; Namba, T.; Seki, T.; Hisatsune, T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 2005, 47, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Gould, E. Differential Effects of Stress and Glucocorticoids on Adult Neurogenesis; Springer: Berlin/Heidelberg, Germany, 2013; Volume 15. [Google Scholar]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation HHS Public Access. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef]
- Hill, A.S.; Sahay, A.; Hen, R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 2015, 40, 2368–2378. [Google Scholar] [CrossRef]
- Culig, L.; Surget, A.; Bourdey, M.; Khemissi, W.; Le Guisquet, A.M.; Vogel, E.; Sahay, A.; Hen, R.; Belzung, C. Increasing adult hippocampal neurogenesis in mice after exposure to unpredictable chronic mild stress may counteract some of the effects of stress. Neuropharmacology 2017, 126, 179–189. [Google Scholar] [CrossRef]
- Izquierdo, I.; Souza, D.O.; Carrasco, M.A.; Dias, R.D.; Perry, M.L.; Eisinger, S.; Elisabetsky, E.; Vendite, D.A. Beta-endorphin causes retrograde amnesia and is released from the rat brain by various forms of training and stimulation. Psychopharmacology 1980, 70, 173–177. [Google Scholar] [CrossRef]
- Heybach, J.P.; Vernikos, J. Naloxone inhibits and morphine potentiates the adrenal steroidogenic response to ACTH. Eur. J. Pharmacol. 1981, 75, 1–6. [Google Scholar] [CrossRef]
- Miller, B.R.; Hen, R. The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 2015, 30, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Barrot, M.; Schad, C.A.; Self, D.W.; Nestler, E.J. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl. Acad. Sci. USA 2000, 97, 7579–7584. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.A.; Heyser, C.J.; Spear, L.P. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: Reversal by naloxone. Psychopharmacology 1994, 114, 433–440. [Google Scholar] [CrossRef] [PubMed]
- degli Uberti, E.C.; Petraglia, F.; Bondanelli, M.; Guo, A.L.; Valentini, A.; Salvadori, S.; Criscuolo, M.; Nappi, R.E.; Genazzani, A.R. Involvement of μ-opioid receptors in the modulation of pituitary-adrenal axis in normal and stressed rats. J. Endocrinol. Investig. 1995, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.M.; Kim, H.; Jang, M.H.; Shin, M.C.; Lee, T.H.; Lim, B.V.; Kim, C.J.; Kim, E.H.; Kim, K.M.; Kim, S.S. Treadmill running and swimming increase cell proliferation in the hippocampal dentate gyrus of rats. Neurosci. Lett. 2002, 333, 123–126. [Google Scholar] [CrossRef]
- Hayward, M.D.; Pintar, J.E.; Low, M.J. Selective reward deficit in mice lacking β-endorphin and enkephalin. J. Neurosci. 2002, 22, 8251–8258. [Google Scholar] [CrossRef]
- Karlsson, R.M.; Wang, A.S.; Sonti, A.N.; Cameron, H.A. Adult neurogenesis affects motivation to obtain weak, but not strong, reward in operant tasks. Hippocampus 2018, 28, 512–522. [Google Scholar] [CrossRef]
- McGonigle, C.E.; Nentwig, T.B.; Wilson, D.E.; Rhinehart, E.M.; Grisel, J.E. Β-Endorphin Regulates Alcohol Consumption Induced By Exercise Restriction in Female Mice. Alcohol 2016, 53, 51–60. [Google Scholar] [CrossRef][Green Version]
- He, Y.; Lu, Y.; Shen, Y.; Wu, F.; Xu, X.; Kong, E.; Huang, Z.; Sun, Y.; Yu, W. Transgenic increase in the β-endorphin concentration in cerebrospinal fluid alleviates morphine-primed relapse behavior through the μ opioid receptor in rats. J. Med. Virol. 2019, 91, 1158–1167. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; Jamurtas, A.Z.; Kamimori, G.H.; Hegde, S.; Otterstetter, R.; Brown, D.A. Gender effect on beta-endorphin response to exercise. Med. Sci. Sports Exerc. 1998, 30, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, R.B.; Gerstein, A.V.; Wildman, R.P.; Mathes, W.F.; D’Anci, K.E. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol. Biochem. Behav. 1998, 61, 19–27. [Google Scholar] [CrossRef]
- Hare, B.D.; Beierle, J.A.; Toufexis, D.J.; Hammack, S.E.; Falls, W.A. Exercise-associated changes in the corticosterone response to acute restraint stress: Evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology 2014, 39, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- White-Welkley, J.E.; Bunnell, B.N.; Mougey, E.H.; Meyerhoff, J.L.; Dishman, R.K. Treadmill exercise training and estradiol differentially modulate hypothalamic-pituitary-adrenal cortical responses to acute running and immobilization. Physiol. Behav. 1995, 57, 533–540. [Google Scholar] [CrossRef]
- Rahimi, S.; Peeri, M.; Azarbayjani, M.A.; Anoosheh, L.; Ghasemzadeh, E.; Khalifeh, N.; Noroozi-Mahyari, S.; Deravi, S.; Saffari-Anaraki, S.; Hemat Zangeneh, F.; et al. Long-term exercise from adolescence to adulthood reduces anxiety- and depression-like behaviors following maternal immune activation in offspring. Physiol. Behav. 2020, 226. [Google Scholar] [CrossRef]
- Duman, C.H.; Schlesinger, L.; Russell, D.S.; Duman, R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008, 1199, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Falck, R.S.; Davis, J.C.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in aerobic exercise efficacy to improve cognition: A systematic review and meta-analysis of studies in older rodents. Front. Neuroendocrinol. 2017, 46, 86–105. [Google Scholar] [CrossRef]
- Ma, X.; Hamadeh, M.J.; Christie, B.R.; Foster, J.A.; Tarnopolsky, M.A. Impact of treadmill running and sex on hippocampal neurogenesis in the mouse model of amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e36048. [Google Scholar] [CrossRef]
- Cahill, S.P.; Cole, J.D.; Yu, R.Q.; Clemans-Gibbon, J.; Snyder, J.S. Differential Effects of Extended Exercise and Memantine Treatment on Adult Neurogenesis in Male and Female Rats. Neuroscience 2018, 390, 241–255. [Google Scholar] [CrossRef]
- Pluchino, N.; Drakopoulos, P.; Casarosa, E.; Freschi, L.; Petignat, P.; Yaron, M.; Genazzani, A.R. Effect of estetrol on beta-endorphin level in female rats. Steroids 2015, 95, 104–110. [Google Scholar] [CrossRef]
- Bernardi, F.; Pluchino, N.; Pieri, M.; Begliuomini, S.; Lenzi, E.; Puccetti, S.; Casarosa, E.; Luisi, S.; Genazzani, A.R. Progesterone and medroxyprogesterone acetate effects on central and peripheral allopregnanolone and beta-endorphin levels. Neuroendocrinology 2006, 83, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.R.; Muoio, D.; Hackney, T.C. Effect of sex steroids on β-endorphin levels at rest and during submaximal treadmill exercise in anovulatory and ovulatory runners. Fertil. Steril. 1999, 71, 1085–1091. [Google Scholar] [CrossRef]
- Schneider, A.M.; Simson, P.E.; Spiller, K.; Adelstein, J.; Vacharat, A.; Short, K.R.; Kirby, L.G. Stress-dependent enhancement and impairment of retention by naloxone: Evidence for an endogenous opioid-based modulatory system protective of memory. Behav. Brain Res. 2009, 205, 290–293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holmes, M.M.; Galea, L.A.M. Defensive behavior and hippocampal cell proliferation: Differential modulation by naltrexone during stress. Behav. Neurosci. 2002, 116, 160–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brito, R.G.; Rasmussen, L.A.; Sluka, K.A. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. Pain Rep. 2017, 2, e618. [Google Scholar] [CrossRef] [PubMed]
- Glasper, E.R.; Schoenfeld, T.J.; Gould, E. Adult neurogenesis: Optimizing hippocampal function to suit the environment. Behav. Brain Res. 2012, 227. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Schoenfeld, T.J. Behavioral and structural adaptations to stress. Front. Neuroendocrinol. 2018, 49, 106–113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoenfeld, T.J.; Swanson, C. A Runner’s High for New Neurons? Potential Role for Endorphins in Exercise Effects on Adult Neurogenesis. Biomolecules 2021, 11, 1077. https://doi.org/10.3390/biom11081077
Schoenfeld TJ, Swanson C. A Runner’s High for New Neurons? Potential Role for Endorphins in Exercise Effects on Adult Neurogenesis. Biomolecules. 2021; 11(8):1077. https://doi.org/10.3390/biom11081077
Chicago/Turabian StyleSchoenfeld, Timothy J., and Chance Swanson. 2021. "A Runner’s High for New Neurons? Potential Role for Endorphins in Exercise Effects on Adult Neurogenesis" Biomolecules 11, no. 8: 1077. https://doi.org/10.3390/biom11081077
APA StyleSchoenfeld, T. J., & Swanson, C. (2021). A Runner’s High for New Neurons? Potential Role for Endorphins in Exercise Effects on Adult Neurogenesis. Biomolecules, 11(8), 1077. https://doi.org/10.3390/biom11081077





