Abstract
Biophysical cues from the cellular microenvironment are detected by mechanosensitive machineries that translate physical signals into biochemical signaling cascades. At the crossroads of extracellular space and cell interior are located several ion channel families, including TRP family proteins, that are triggered by mechanical stimuli and drive intracellular signaling pathways through spatio-temporally controlled Ca2+-influx. Mechanosensitive Ca2+-channels, therefore, act as critical components in the rapid transmission of physical signals into biologically compatible information to impact crucial processes during development, morphogenesis and regeneration. Given the mechanosensitive nature of many of the TRP family channels, they must also respond to the biophysical changes along the development of several pathophysiological conditions and have also been linked to cancer progression. In this review, we will focus on the TRPV, vanilloid family of TRP proteins, and their connection to cancer progression through their mechanosensitive nature.
1. Introduction
All tissues contain specific cellular machineries involved in sensing and converting physical cues into biological responses [1,2]. Mechanosensing hence allows cells to adopt their structure and functions according to the external stimuli, like changes in the composition of the matrix, pressure or shear forces, subsequently regulating all crucial cellular functions and through that the homeostasis of different tissues. Along cancer progression, biophysical properties of the stroma undergo drastic alterations [3,4]. Both stiffness and pressure within the transformed tissues are usually much higher and stromal composition is also commonly altered. Such mechanical cues are recognized by the mechanosensitive machineries and can promote cancer progression f.i. by triggering proliferation and cell migration through several intracellular cascades [5,6]. Additionally, mechanosensing machineries can undergo changes in their function along the neoplastic progression, impairing the ability of the cells to sense and respond normally to the changes in their microenvironment [7,8].
Every step along the metastatic progression, including EMT, invasive migration and angiogenesis, is directed by changes in Ca2+-homeostasis [9,10]. One of the central players in regulating Ca2+-homeostasis and transmitting information about the extracellular biophysical cues is represented by plasma membrane-embedded calcium channels. Therefore, many of the physical features, affecting cancer progression positively, may be mediated by mechanosensitive ion channels that through Ca2+ influx favor activation of specific downstream pathways [8,10,11,12,13,14]. Perhaps the most studied Ca2+-triggered pathways include calcium/calmodulin-dependent kinase II (CaMKII) and a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways that regulate cell cycle and apoptosis [15,16]. NF-κB protein complex controls the transcription of DNA upon various stresses and is involved in inflammatory and immune responses, regulation of proliferation and cell survival [17,18]. Other important calcium-dependent factors are calcium-dependent cysteine proteases, calpains and the calcium-dependent serine-threonine phosphatase, calcineurin that control f.i. cell cycle, apoptosis and cell migration [19,20,21]. Abnormal activity of mechanosensitive Ca2+-channels could therefore play a major role in cancer progression through these pathways or other less studied signaling cascades. Moreover, the expression of such channels is also known to be altered along the neoplastic progression [14,22,23]. The mechanisms behind the abnormal Ca2+-channel expression in cancerous tissues are still poorly known but they may be for example linked to the changes in the stromal composition or hormonal status [24,25,26,27].
One major group of cell membrane-associated Ca2+-channels are formed by the Transient receptor potential (TRP) family proteins that can respond to various extracellular cues, including biochemical compounds, pH, heat, osmolarity and physical stimuli, to trigger activation of specific intracellular cascades through subtle changes in ion influx [28,29]. TRP protein family is composed of over 30 different cationic ion channels that display vital functions in various tissues. This superfamily of proteins has been mostly studied in non-excitable cell types but possesses also important functions in the nervous system [30,31]. Based on the sequence homology, the TRP superfamily is further divided into seven sub-families, more specifically into the TRPV (vanilloid), TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPN (NOMPC) and TRPP (polycystin) families [32]. TRPV subfamilies of channel proteins are widely expressed in both non-sensory and sensory cells and display high sequence similarity across different species [33,34,35]. TRPV channels that are capable of sensing physical cues, act in concert with the other cell membrane-linked mechanosensitive structures, including integrin-based cell adhesions, and transmit mechanical signals into a cell readable format [36,37,38,39,40,41,42]. In addition to the extracellular cues, activation of TRPV channels is affected by intracellular signaling, post-translational modifications as well as lipid- and protein-protein interactions, constructing a complex regulatory network [31,43,44]. Upon activation, these channels may then alter membrane potential or concentration of intracellular Ca2+ that impacts cellular events. Therefore, deregulation of TRPV and other TRP family channels also plays a major role in the development of several pathophysiological conditions, including various cancers. Mechanosensitive TRPV channels have been linked to cancer progression at least through their ability to both sense and modify mechanically altered microenvironment [45,46,47,48,49], through their association with mechanosensitive Rho GTPases and actin cytoskeleton, leading to altered migratory features [50,51,52,53], as well as due to their role in angiogenesis [54,55,56,57] and cell proliferation [58,59,60]. Many of these channel proteins have also shown potential as therapeutical targets [61,62]. In this short review, we will focus on the unique characteristics of TRPV channels as mechanosensors and the possible connection of their mechanosensitive nature to the progression of various cancers through altered Ca2+-signaling.
2. TRPV Family—Expression and Biological Functions
TRPs were originally identified in photoreceptors of Drosophila [63] and are expressed in a range of species from yeast to humans. TRPs are expressed in a tissue specific manner and they all have a common membrane structure with six transmembrane segments, S1-S6, containing a TRP domain and a loop between S5 and S6, which defines the pore and selectivity of the channel filter [64]. Similar to the voltage-gated potassium channels, TRP tetramers form the functional unit of the cation channels and the ankyrin repeat domain (ARD) is involved in the oligomerizations. TRPV subfamily is constituted by the members TRPV1-6. These are further divided into two subgroups: TRPV1-4 and TRPV5-6. The first group, TRPV1-4, form homo-and heteromeric channels that display mild Ca2+-selectivity [20,65,66]. While TRPV5 and TRPV6 can form both homo- and heteromeric channels that are highly selective for Ca2+. TRPV channels are structurally similar to the other TRP channels, but they contain additional three to five ankyrin repeat domains in the N-terminus [31]. Despite the high sequence similarity, all of the TRPV family members display specific activation mechanisms and physiological functions.
Many of the TRPV proteins are triggered by multiple stimuli, suggesting that they can participate in the activation of several downstream cascades [33,67,68,69]. TRPV1 is highly expressed in sensory neurons and mainly localized at the plasma membrane [64,70,71]. The channel is activated by heat, pH and compounds, such as capsaicin [31,72] and involved in thermal nociception [41,72,73,74]. Accordingly, TRPV1 KO mice possess impaired heat-evoked pain sensation [41]. While TRPV1 is expressed at the plasma membrane, TRPV2 is mainly localized in the intracellular membranes [75,76]. It is found in the sensory and motor neurons as well as in many non-neuronal cell types [64,77,78,79]. TRPV2 has various physiological roles, from the perception of noxious stimuli to nociception and the importance of its expression has also been demonstrated in the normal function of distinct immune cell types [80,81]. TRPV2 can regulate intracellular calcium homeostasis by acting as a lipid-, thermo- and mechanosensor. Some growth factors, hormones, cytokines and endocannabinoids can also trigger the translocation of TRPV2 from the endosome to the plasma membrane [82]. Additionally, TRPV2 functions can be regulated by Phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphorylation in an extracellular signal-regulated kinase (ERK)-dependent manner [76,83]. Interestingly, TRPV2 is not activated in vivo by vanilloids [70,75,76].
TRPV3 is a Ca2+-permeable, non-selective cation channel and widely expressed throughout the tissues but especially abundant in various epithelial tissues, including the specialized epithelial cells, keratinocytes of the skin [84,85]. In the skin keratinocytes, TRPV3 can be activated at least by heat and its activation can regulate various downstream functions, including skin barrier formation, wound healing, sensing of temperature, itch and pain [20,71,86,87,88,89,90]. In general, TRPV3 seems to be important for the health of the skin, and loss of TRPV3 function leads to skin inflammations, dermatitis, itchiness and hair loss. Like the expression of TRPV3, the expression of TRPV4 is also very ubiquitous and more prominent in epithelial tissues, where it can induce calcium influx in response to various extracellular cues, such as heat, osmotic changes and mechanical stretching [91,92,93]. TRPV4-mediated Ca2+-influx directs various physiological functions in different cell types and has an important role in cell volume regulation [65,94,95,96]. TRPV4−/− mice also display drastic defects in osmoregulation [97,98]. In epithelial tissues, TRPV4 seems to be important for the maintenance of junctional permeability through its impact on adherens junction and tight junction proteins [99,100,101]. In mammary epithelial tissues, TRPV4 regulates the integrity of the cell-cell junctions specifically through the expression of tight junction proteins [102]. TRPV4-mediated calcium influx is also important for the formation of intact cell-cell junctions in skin keratinocytes, where it maintains intercellular barrier function [103]. Besides its important role in tissue integrity, TRPV4 has been connected to several other physiological functions, including its major role in the maintenance of vascular development, tone and permeability [104,105]. TRPV4 has also been linked to the central nervous system and nociceptive response in primary sensory neurons [106,107].
TRPV5 and TRPV6 display a high level of sequence similarity (75% amino acid identity) and are alike in many ways [108]. Both are highly Ca2+-selective, 1,25-dihydroxyvitamin D3 responsive and have major roles in the maintenance of Ca2+ homeostasis in higher organisms [109,110,111]. TRPV5 is mainly expressed in the kidney, more specifically in the distal convoluted tubules (DCT) and connecting tubules (CNT) [112,113,114], while TRPV6 exhibits a wider expression pattern and is expressed prominently f.i. in the kidney, pancreas, prostate, placenta, and breast [111,115,116]. In Ca2+-transporting epithelial tissues, these proteins are localized at the apical membrane and have an important role in intestinal and renal reabsorption [109,113,117]. TRPV6 seems to be constitutively active, and Ca2+-influx through this channel depends on the intracellular and extracellular Ca2+-balance [118]. The activity of this channel can also be tuned by hormones, that is, estrogen and progesterone, tamoxifen and vitamin D, leading to changes in cell proliferation and survival [119].
TRPV family channels are thus widely expressed in a tissue-specific manner and display numerous functions. The regulation of the activity of these channels is a complex process and impacted by various extracellular and intracellular cues. As demonstrated with TRPV4 [120], at least the N- and C-terminal cytoplasmic domains are important for channel gating. Additionally, processes that affect the oligomerization and trafficking of the TRPVs play a major role in the regulation of these channels and TRPV2, -4 and -5 membrane transportation seems to be dependent on the N-terminal site of the proteins, [121,122,123]. Alternative splice variants of TRPV2 mRNA can also inhibit the translocation of TRPV2, in this way affecting the activity of this channel protein [124]. Regulation of channel trafficking is clearly a complex process and reviewed in more detail f.i. by Doñate-Macián et al., 2019 [125].
3. Mechanosensitivity of TRPV Channels
3.1. Activation of Mechanosensitive Channels
As already mentioned, TRPV family channels are capable of sensing various extracellular cues to subsequently trigger specific cation-dependent intracellular signaling pathways [126,127]. Initially, the role of these channels in sensing mechanical signals was detected in Drosophila and Caenorhabditis elegans, displaying TRP gene mutants that had a defective response to the mechanical or osmotic stress [128,129,130,131]. Later, analogous studies were performed in the mammalian and prokaryotic model systems [11,132,133]. Currently, it is known that sensing of physical changes by the channel proteins can take place in two ways: By sensing ”force-from-lipids” or “force-from-filament”, that is, channels can directly respond to membrane stress/stretch/tension or can feel the force through their interaction with cytoskeletal/structural/adhesive components [134,135,136,137,138]. This leads to conformational changes and gating of the channel [139,140,141,142]. The channel activation upon force sensing is very rapid, arising in milliseconds and it insures fast transduction of mechanical stimuli into an ion flux [143]. In case the channels are activated by intracellular mechanosensitive signaling cascades, the activation process is indirect and slower.
3.2. Mechanical Stretching and Osmolarity
TRPV channels 1, 2 and 4 seem to be responsive to mechanical stretching and cell size changes due to hypo-osmotic swelling. The activation of TRPV1 by stretch or cell shrinkage has been linked to the mechanical nociception [144,145] and increased osmolarity has been shown to associate with increased Ca2+-influx through this channel [146]. The response of TRPV1 to stretch and osmotic stimuli is also dependent on the actin cytoskeleton [147,148]. Both TRPV2 and TRPV4 are also sensitive to membrane stretch [81,125]. In line with that, they are playing an important role in tissues, which are under high mechanical stress, like cardiac and skeletal muscle. In the cardiomyocytes, membrane stretching by hypotonic swelling triggers TRPV2-mediated Ca2+-influx [149]. Consequently, TRPV2 has an important role in the maintenance of the structure and synchronized contractility of cardiac muscle [150]. Stretch-dependent activation of TRPV2 has also a function in the regulation of neural circuit formation [77]. In developing neurons, TRPV2 binds actin and has been linked to the actin-dependent axonal outgrowth upon focal mechanical forces [151]. Upon mechanical stimulation, TRPV2 has been shown to rapidly accumulate at the site of the stress [151]. Clustering of TRPV2 at the sites of mechanical forces then leads to reorganization of the actin cytoskeleton, subsequently promoting axonal outgrowth. It seems that strict regulation of axonal out-growth also requires interplay between several other mechanosensitive structures [152]. As the ankyrin repeats of TRPV2 interact with many cytoskeletal proteins and the ankyrin repeats at the N-terminal region of TRPV proteins, are important for their mechanosensitive features, the association of TRPV2 with the cytoskeletal structures may be necessary for its function as a mechanosensor [77,151,153]. The stretch sensitivity of TRPV2 has also been studied f.i. in CHO and HEK293 cell lines. In both of them, altered Ca2+-response was detected upon stretching on elastic substrates [77,149,154]. Additionally, studies on rat lung alveolar epithelial type II, ATII, cells showed that the strain-induced, TRPV2-dependent Ca2+-influx was dependent on the focal adhesions and actomyosin structures [155], again linking the mechanosensitive activity of TRPV2 to the cytoskeleton. There is also strong evidence that TRPV2 acts as a mechanosensor in the circulatory organs and intestinal tract [78,81].
TRPV4 is probably the most studied TRPV family channel in respect of its mechanosensitivity. Its activation has been explored in various tissue types and based on these studies TRPV4 is clearly an important osmo-mechanosensitive channel, responding to variations in membrane stretching upon osmotic changes. Some of the first reports on TRPV4 –/– mice have shown the importance of TRPV4 in pressure sensation and a higher threshold for the response to strong noxious mechanical stimulation [93,97,156]. Early studies on TRPV4 have also shown its prominent expression in the kidney [91,157,158,159,160]. Kidney cells are constantly exposed to extracellular fluids/changes in the osmolarity and TRPV4 in the cortical collecting ductal cells has an important role in the cell volume regulation in an aquaporin-2 and cytoskeleton-dependent manner [161]. Studies in HEK293 or CHO-K1 cells have also shown the activation of TRPV4 by the application of hypotonic solutions [91,157,158] and in primary sensory neurons, hypotonic solutions activate TRPV4 to trigger nociceptors [106]. In line with the study of Galizia et al., 2012 [161], other studies have as well presented evidence for the importance of intact actin cytoskeleton in TRPV4-dependent cell volume regulation [162,163,164,165]. Furthermore, TRPV4 has an important role in the mechanical regulation of calcium homeostasis of the cardiomyocytes, subsequently affecting their contractility but also repair after cardiac infarction [166,167,168]. There are also indications that TRPV4-mediated Ca2+-influx regulates the contractility of airway smooth muscle cells and vascular endothelial cells [108,169]. Besides its expression in the artery endothelial cells, TRPV4 can be found in the vascular smooth muscle cells of some arteries [170]. While in these artery smooth muscle cells TRPV4 activation seems to be involved in the regulation of arterial dilation, its upregulation in pulmonary arterial smooth muscle cells has been reported to increase smooth muscle tone and cause pulmonary hypertension in chronically hypoxic rats [171]. Moreover, TRPV4-triggered Ca2+-influx has also been studied in urothelial cells, where this channel has been linked to ATP-release upon stretching, hypotonic solutions or intravesicular pressure changes [172,173,174]. Furthermore, recent studies have shown that some TRPV channels can also be triggered by chemical agonists through their ability to sense mechanical perturbations in the plasma membrane [175]. At least with TRPV4, this is linked to the regulation of innate defense mechanisms against bacterial infections [176,177,178].
3.3. Activation upon Shear Forces
In addition to membrane-stretching, TRPV channels are also responsive to shear forces. In the cardiovascular system, TRPV4 is prominently expressed in the endothelial cells of larger arteries and arterioles and mediates shear-stress-induced relaxation [179,180,181,182]. This response can be disrupted by specific TRPV4 inhibitors or downregulation of the channel protein. In contrast, TRPV4 activation leads to vasodilation [180]. Moreover, shear stresses have been shown to regulate the clustering and translocation of the TRPV4 channel from the adherens junctions to the basal side of the endothelial cells, in a focal adhesion kinase and integrin α5ß1-dependent manner [183]. Interestingly, both TRPV4 and TRPV6 have been functionally linked to the shear force-sensitive cilia and microvilli. Cilia can sense various mechanical stimuli in distinct organs and TRPV4 seems to be an important component of this mechanosensitive structure [184,185]. TRPV4 has been shown to be associated with the primary cilia, through which it is involved in sensing both hypo-osmotic solutions and mechanical cues, subsequently triggering Ca2+-response in ciliated epithelial tissues [186,187]. For instance, in the airway epithelium, TRPV4 is prominently expressed and responsive to the airflow-caused physical shear stresses [188]. Moreover, TRPV4-mediated Ca2+-influx can also couple changes in fluid viscosity to the changes in ciliar beat frequency [189], creating a regulatory feedback loop. TRPV6 on the other hand has been linked to microvilli, actin-based membrane protrusions that have an important role in the mechanotransduction within various epithelial tissues [190]. TRPV6 is responding to shear forces in human placental trophoblastic cells and the following Ca2+-influx promotes the formation of microvilli in an Akt/Ezrin-dependent manner [190]. TRPV6 could therefore have a major role in microvilli-mediated mechanosensitive functions in various epithelial tissues.
3.4. Stiffness-Sensing
Moreover, there are indications that some TRPV channels can act as stiffness-sensors. In the mouse epidermal keratinocytes, TRPV4 has been shown to play a major role in tissue stiffness-sensing and subsequently regulate YAP/TAZ localization [191,192]. On the other hand, TRPV4 itself mediates collagen matrix assembly, matrix stiffening and promoting fibrosis [193,194], in this way creating a feedback loop in both sensing and creating a mechanically altered cellular environment. Additionally, TRPV6 expression levels seem to be dependent on the stiffness of the underlying matrix, at least in the 2D mammary epithelial cell culture model [45].
3.5. Sensing Forces through Integrin-Based Adhesions
Integrin-based adhesions act as central sensors of the biophysical changes in the cellular microenvironment [195]. They respond to various physical cues, including stiffness, composition and applied external forces [196]. Integrins and cytoskeletal structures also directly form complexes with TRPV channel proteins and could in this way transmit information on the biophysical changes to subsequently trigger the opening of the ion channels [197]. TRPV4 interacts directly with α2-integrin and the Src tyrosine kinase Lyn [198]. TRPV4 is also known to be triggered through forces applied to β1-integrin [199]. Mechanistically, forces from the cytoplasmic tail of β1-integrin are transmitted through the CD98hc cytoplasmic tail to the TRPV4 ankyrin repeat domains to activate force-induced Ca2+-influx [200]. Besides TRPV4, at least TRPV1 is linked to integrins: TRPV1 is expressed together with integrin subunits that can bind fibronectin and TRPV1 translocation to the cell membrane takes place in a fibronectin-dependent manner, playing a role in primary sensory neuron sensitization [201]. Additionally, integrin-TRPV cooperation plays an important role in osmosensation [197]. Most likely, integrins and TRPV channels have many additional interconnections upon various external physical cues and their roles in mechanotransduction should be further investigated.
3.6. Are All the TRPV Channels Mechanosensitive?
While TRPV2 and 4 seem to be clearly mechanoresponsive, the other channels are less studied and there are no clear indications f.i. for the role of TRPV3 or TRPV5 in the mechanotransduction. Additionally, in the study by Higashikawa et al., TRPV3 was not found to be stimulated by mechanical stretching [202]. It has, however, been shown that TRPV3 forms a signaling complex with TGF-α and EGFR and that this complex plays a role in the mechanical skin barrier function [203]. TGF-α and EGFR are also known to regulate mechanotransduction pathways, involved in contractility and cell migration [204]. Furthermore, TRPV1, TRPV3 and TRPV4 ankyrin repeat domains bind ATP and calmodulin (CaM) [205]. CaM is also linked to the regulation of mechanosensitive cascades, affecting cell migration [206]. Besides these, there are other known TRPV channel interactions with unknown consequences and the functional significance of these associations for the cellular mechanotransduction events should be studied in the future.
4. Expression and Activity of TRPV Channels along Cancer Progression
Among other TRP family channels, TRPV channels have been associated with a wide variety of human cancers [58,59,207]. Altered expression of TRP channels has been linked to cancer progression through enhanced cell proliferation, changes in differentiation and impaired cell death, leading to the uncontrolled expansion of the transformed cells [58,59,60,124]. The major Ca2+-triggered pathways, affecting the above features, include CaMKII, NF-κB, calpains and calcineurin pathways [15,16,19,20,21]. Besides them, other less studied signaling cascades may affect cancer progression through TRP-triggered Ca2+-influx.
Changes in the expression of TRP channel proteins are thought to play a role in the later stages of various cancers—not in the actual carcinogenesis or initial phases in the cancer progression. Mutations in the TRP genes are not common, and mostly either the expression or activity of these channels is deregulated along the progression [12,16,124,208]. TRPV1 is abnormally expressed at least in breast, prostate and urothelial cancers as well as in human papillary thyroid carcinoma and gliomas [209,210,211,212,213,214]. The upregulation of TRPV1 in high-grade prostate and breast cancer samples correlates with the tumor grade [209,210], while downregulation of TRPV1 expression is associated with the progression of urothelial cancers [215]. In androgen-responsive NCaP prostate cancer cells, Capsaicin, a TRPV1 agonist, was found to enhance TRPV1-dependent cell proliferation through Akt and ERK pathways [216]. Cross-talk in between α1D-adrenoceptors and TRPV1 seems to enhance cell proliferation and targeting both TRPV1 and α1D-adrenoceptors could act as a therapeutical choice [217]. In another study, performed with PC3 prostate cancer cells, Capsaicin was, however, found to inhibit proliferation and induce apoptosis [218]. This took place via inhibition of coenzyme Q activity, leading to increased ROS generation and caspase-3 activation. Interestingly, in the MCF-7 breast cancer cell line, both TRPV1 agonists and antagonists significantly reduce cell growth with yet poorly known mechanisms [219]. Therefore, both too high and too low TRPV1 expression, through specific intracellular pathways in a cell type specific-manner, may provide an advantage for the expansion of cancer cells. Other than proliferation, TRPV1 has been associated with cell migration and in human hepatoblastoma HepG2 cells TRPV1-triggered Ca2+-influx promotes cell migration [220].
The link between abnormal TRPV2 expression and cancer progression has been widely studied and TRPV2 seems to exhibit oncogenic activity in cancers of the prostate and breast, as well as in esophageal squamous cell carcinoma, leukemia, multiple myeloma and glioblastomas [82,221,222,223]. Overexpression of TRPV2 in urothelial-, prostate-, esophageal squamous cell- and hepatocarcinoma tissues, as well as cell lines of the same cancer types, correlates with advanced disease and metastasis [224,225,226,227,228]. As with other TRP channels, changes in TRPV2-mediated signaling result in uncontrolled proliferation, impaired apoptosis and changes in the migratory features of the cells [82,221,229,230]. In the prostate carcinoma model, trafficking of TRPV2 to the plasma membrane correlated with enhanced cell migration through the phosphoinositide 3-kinase (PI3K) pathway [231]. In bladder cancer cells, TRPV2 also enhanced cell migration and invasion but did not affect cell proliferation in vitro [89,232,233]. In hepatoma and hepatocarcinoma models, TRPV2 may also increase drug resistance [234] and suppression of TRPV2 activity in nude mice xenografts reduced tumor growth and invasion [226]. On the other hand, in glioblastoma cells, stimulation of TRPV2 could sensitize cancer cells to cytotoxic chemotherapeutic agents [235]. Furthermore, Mizuno et al. 2014 reported that lower TRPV2 levels in bladder cancer cells are associated with increased proliferation, again indicating cell-type-specific differences in the role of this TRPV channel during cancer progression [233]. In contrast to TRPV2, TRPV3 has been much less studied but it is a known regulator of growth and survival of the skin cell populations and its overexpression has been associated at least with the proliferation of lung cancer cells [236,237]. As TRPV3 is present in the same signaling complex with EGFR and EGFR activity triggers TRPV3 [203], there is a clear functional interplay in between these factors and it potentially plays a role along cancer progression.
Abnormal TRPV4 expression is linked to at least gastric, liver, pancreatic, colorectal, lung and breast cancers [59,238,239]. A significant upregulation of TRPV4 has been detected in breast cancer cell lines with the potential to metastasize and its expression seems to increase with tumor grade and size, subsequently correlating with poor survival [240,241,242]. Additionally, TRPV4 has been linked to cell proliferation through the CaMKII pathway and regulation of apoptosis in distinct cancer models [243,244]. The expression of TRPV5 is more restricted, and this TRPV family protein is also less studied in comparison to highly similar TRPV6. The role of TRPV6 has been extensively investigated upon malignant transformation in various tissues and its expression is known to be elevated in many cancers [111,116]. Expression of TRPV6 is upregulated in prostate, breast, colon, esophageal and cervical tumor tissues, as well as in the corresponding tumor cell lines. Increased expression of TRPV6 stimulates the metastasis of cancer cells and confers chemotherapy resistance [116,245,246,247,248]. In at least prostate and breast cancer, high TRPV6 expression is linked to cellular proliferation and invasion through Ca2+-dependent pathways and its high levels have been proposed to act as a prognostic factor [119,249,250,251]. Additionally, in prostate cancer, TRPV6 contributes to cell survival and resistance to apoptosis [247]. Distinct TRPV channels thus act as central regulators of the hallmarks of cancer, but they also display clear functional differences in a cancer-type-dependent manner.
5. TRPVs and Cancer Progression—Links to Mechanosensitive Pathways
5.1. Interplay in between Small Rho GTPases and TRPVs
Deregulation of TRP channels and altered Ca2+ homeostasis have been directly linked to the hallmarks of cancers [60]. Spatio-temporal activation and duration of Ca2+-influx determine the activation of specific signaling cascades and transcription factors that guide various cellular processes but also play a role in cancer progression [21,252]. Besides the most studied Ca2+-triggered pathways, CaMKII, NF-κB, calpains and calcineurin pathways, also specific mechanosignaling routes may favor cancer progression through TRP-triggered Ca2+-influx.
Small GTPases (Rho- and Ras-like) that act as major regulators of the mechanical signaling are linked to cancer progression through their impact on actin dynamics, cell polarity, differentiation and proliferation [204,253,254,255]. Interestingly, there is interplay between TRPs and small GTPases along cancer progression: Many small GTPases interact with calcium-dependent signaling cascades and can trigger Ca2+-associated effectors, impact TRP trafficking to the plasma membrane or directly affect the gating and activity of certain TRP channels [50,51,52,53]. Conversely, the activity of small GTPases has a strong association with Ca2+-homeostasis [50], suggesting a reciprocal interaction between TRPs and small GTPases. TRPs are associated with Rho-dependent cytoskeletal reorganizations, cellular contractility and cell adhesion turnover, subsequently affecting migratory features of the cancer cells [256,257]. In breast cancer cells, treatment with Rho-kinase inhibitors leads to a reduction in TRPV2 levels [258], indicating a Rho-dependent regulation of TRPV2 activity. Additionally, Rac1 is able to dictate intracellular trafficking of TRPV2 in fibrosarcoma cells [259], implicating a central role of these Rho GTPases in TRPV2 regulation. While in some cancer cell lines TRPV2 seems to have a positive impact on cancer cell migration [223,226,260], the interplay between TRPV2 and RhoA/Rac1 seems to suppress invasion of Fibroblast-like Synoviocytes (FLS cells) [261]. In this model setup, TRPV2 stimulation caused a decrease in RhoA and Rac1 activity, consequently leading to a lower number of contractile actin bundles and cell adhesions as well as inhibition of lamellipodia formation [261]. As Activation of Rac1 through the PI3 pathway leads to TRPV2 translocation to the plasma membrane, subsequently resulting in increased Ca2+-triggered invasiveness in some cell types [259] and active TRPV2 seems to inhibit Rac1 [261], there may be a regulatory feedback loop in between these two factors and TRPV2 could display both invasion-promoting or inhibiting features in a cell-type-specific manner.
The role of TRPV4 in cancer metastases has been investigated in several model systems [239,262] and TRPV4 has also been most thoroughly studied with respect to its mechanosensitive character along cancer progression [240,262,263,264]. Like TRPV2, TRPV4 has also been connected to cancers through Rho GTPases that act as major mediators of mechanical signaling. TRPV4 has recently been found to form complexes with RhoA and the TRPV4-mediated Ca2+-influx positively affected RhoA activity, subsequently leading to cytoskeletal changes [265]. Additionally, upon growth factor stimulation, TRPV4-mediated Ca2+-influx leads to activation of RhoA/ROCK pathway and subsequently causes increased contractility of ECM-modifying fibroblasts [49,166,266,267]. On the other hand, in tumor endothelial cells, overexpression of TRPV4 seems to have an opposite effect on RhoA activity [57]. TRPV4 has also been associated with Rho/ROCK pathway, cytoskeletal changes and invasion in cancer models: In endometrial cancer, TRPV4 promotes metastasis by Rho/ROCK-induced cytoskeletal changes [268]. In line with this, breast cancer models have shown that TRPV4 over-expression impacts cancer cell migration by leading to the higher activity of ROCK-regulated cofilin that promotes actin filament depolymerization [240]. Such cells become mechanically more compliant, enabling transendothelial migration. Additionally, in endometrial cancers, TRPV4 regulates cancer cell invasion through RhoA/ROCK1-dependent cytoskeletal changes [268] and in glioma cancer cells TRPV4 promotes invasion through Akt/Rac1 signaling pathway [269]. Akt pathway is also involved in gastric cancer invasion through, TRPV4-triggered Ca2+ influx [264]. Akt itself has been suggested to play a role in the adhesiveness of metastasizing cancer cells in a force-triggered manner [270]. Furthermore, TRPV4 impacts cell-exerted forces in migratory cells and affects dynamics of the trailing adhesions, probably through its interactions with some Rho-responsive focal adhesion proteins and co-operation with other cation channels [271]. Moreover, there are indications that deregulation of TRPV4 could potentiate cancer invasion by affecting scattering of cancer cells: Adherens junctions seem to be regulated through TRPV4-mediated activation of Rho GTPases that induce reorganization of actin-based structures along junction formation [272]. High TRPV4 levels thus favor breast cancer metastasis by regulating cell-cell contacts, actin-dependent cell compliance, cell migration and extravasation through the AKT-E-cadherin signaling [240,241].
5.2. TRPV-Linked Epithelial Mesenchymal Transition and Stiffness of the Microenvironment
Loss of E-cadherin and intact cell-ell junctions are important features in the process of epithelial mesenchymal transition, EMT, eventually leading to invasion and metastases of the transformed epithelial cells [273]. As reviewed above, TRPV4 can possibly potentiate invasion of breast cancer cells through Ca2+-dependent activation of AKT that leads to changes in actin dynamics and downregulation of junctional E-cadherin [240,241]. Silencing or inhibition of TRPV4 by chemical compounds also clearly suppresses the migratory features of TRPV4-expressing 4T07 breast cancer cells, further confirming the role of TRPV4 in cancer metastasis [240]. The direct link between EMT and TRPV4 has been shown by other studies as well [191,192,274]. Furthermore, these studies revealed the connection of TRPV4 to the stiffness of the matrix, and TRPV4 itself has also been linked to the modulation of the ECM. TRPV4 is responsive to the biophysical changes of the surrounding matrix and can be triggered through signals transmitted through specific ECM polymers [46,47]. Consecutively, it can affect the remodeling of collagen in the ECM, affecting the mechanical features of the matrix [48,49]. In line with these observations, in breast cancer models, TRPV4 regulates ECM stiffness by affecting the expression of matrix proteins [241]. As fibrosis is very common in tumors, leading to tissue stiffening, such an environment creates a feedback loop through TRPV4 activity to further promote mechanical changes in the stroma. TRPV4 therefore seems to have a major role in several steps of the cancer progression through its mechanosensitive nature and ability to both respond and regulate the stiffness of the environment. Increased stiffness could also further alter the function of other mechanosensitive TRPV channels and at least TRPV6 has been shown to be responsive to elevated stiffness with increased expression in a breast epithelial cell model [45]. Increased levels of TRPV6 resulted in upregulation of EMT markers, possibly explaining the earlier findings on high TRPV6 levels in the invasive regions of mammary carcinomas [45].
5.3. Matrix Degradation
One of the features that makes cancer cells more invasive, is their ability to secrete matrix metalloproteinases (MMPs) through invadopodias, actin-rich cellular protrusions, to degrade the surrounding ECM [275]. Intriguingly, TRPs and Rho GTPase are also linked to the expression of MMPs. Rho GTPases and PI3K together with several other signaling factors regulate the formation of invadopodia core structure to enable MMP secretion [276,277]. Many of these players, regulating invadosome formation, are triggered in a mechanosensitive manner and MMP secretion is also dependent on the stiffness of the surrounding matrix [278,279,280]. Interestingly, the same signaling pathways (inc. PI3K/Rac1 pathway) that are responsible for MMP upregulation, also control TRPV2 trafficking to the plasma membrane [24,259,260,281,282,283,284]. Further, TRPV2 translocation is force-dependent and its expression leads to elevated levels of MMPs [226,259]. In contrast, depletion of TRPV2 by siRNAs led to lower levels of MMP-2 and MMP-9 in prostate tumors of nude mice xenografts [226]. It may be therefore hypothesized that stiffening of the matrix leads to TRPV2 translocation to the plasma membrane in a PI3K/Rac1-dependent manner and that TRPV2-mediated Ca2+- influx plays a role in MMP production through yet unknown mechanism, at least in prostate and ovarian cancer models [226,259,285]. Additionally, in bladder tumor models, high TRPV2 activity correlates with MMP-2 levels, playing an important role in the progression and invasion of this cancer type [232].
5.4. Angiogenesis
Besides matrix degradation, utilization of vasculature is extremely important for the spreading of cancer cells. Angiogenesis is a typical feature for the progressing tumors and the new vessels, induced by signaling within the cancerous tissue, are usually more fragile to allow intravasation of the invading cancer cells [286]. Mechanosensitive TRPV4 has been clearly linked with VEGF/VEGFR2 signaling and tumor angiogenesis [54,55], further indicating that TRPV4 can play a role in various processes that potentiate cancer cell invasion. Mechanical signaling, mediated by TRPV4, has been shown to regulate the vasculature: It is known to be essential for the shear-stress induced endothelial cell (EC) reorientation downstream of mechanosensitive integrins [47]. In tumor endothelial cells (TECs), TRPV4-mediated Ca2+-influx impacts actin dynamics and migration of TECs in a membrane-stretch-dependent manner [56]. In a lung carcinoma model, TECs seem to express lower TRPV4 levels and display abnormal mechanoresponse to ECM stiffness. This results in aberrant migratory features of the endothelial cells and TEC-promoted angiogenesis, consequently affecting tumor growth in a mouse model [57]. In contrast, stimulation of TRPV4 in the same model system leads to normalization of the vascular endothelium through restored mechanosensitivity and inhibits tumor growth [57]. It has also been noticed that such TRPV4-defective cells possess a lower expression of VE-cadherin, which possibly impacts the angiogenetic process and leads to vascular leakage [287]. Other than low TRPV4 levels, the upregulation of TRPV4 is also harmful to the intact endothelial junctions, as high TRPV4 activity in lung vasculature causes disruption of the intact endothelial walls [288]. This indicates that TRPV4 may possess both pro- and anti-angiogenic features, depending on the cancer type and genetic background or that up- or alternatively downregulation of TRPV4 impacts distinct mechanosensitive pathways.
5.5. Concerted Action of Various Biophysical Changes through TRPV Channels
Other than the above mechanisms involved in cancer progression through mechanosensitive TRPV channels, TRPVs may play a role in cancer progression through several other yet unidentified ways. As cancer progression involves various biophysical changes in the extracellular environment, including stiffness, composition, hydrostatic pressure, compression and stretching, it is expected that all the mechanosensitive TRPV channels undergo some functional alterations. Combinations of various extracellular cues and signaling through several channels result in complex cellular phenotypes that may be difficult to mimic in in vitro assays. Besides the direct impact on the channel activity, transformation in other mechanosensitive structures that regulate TRPVs can take place. For instance, altered expression of cilia has been linked to the mechanical regulation of cancer progression [289]. TRPV channels that are directly linked to these structures and sensing force changes through them are clearly not able to respond normally to the mechanical signals. Additionally, other mechanosensitive structures, including integrin-based adhesion and the associated actin cytoskeleton can undergo various changes along cancer progression, also leading to changes in the activity of Ca2+-channels, either directly or indirectly. The possible association of distinct TRPV channels with cancer progression through their mechanosensitive features is summarized in Table 1 and a hypothetical model on the role of mechanosensitive TRPV channels in cancer progression is drawn in Figure 1.

Table 1.
Mechanosensitive TRPV channels in cancer progression.
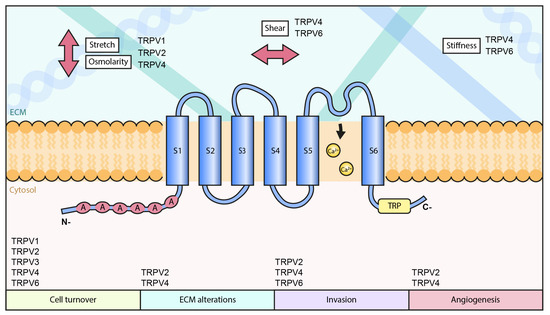
Figure 1.
Hypothetical role of TRPV channels in cancer progression.
6. Conclusions and Future Perspectives
Widely expressed mechanosensitive calcium channels are crucial mediators of physical signaling in various cellular processes and play a role in tumor pathophysiology. Alterations in cancer tissues, such as stiffness, composition, shear stress and pressure, act as physical stimuli that must impact the activity of these cationic channels. Although extensive studies have been performed on cancer-associated changes in mechanosensitive cell-adhesive and cytoskeletal structures, the membrane-embedded channels have obtained less attention and clearly require more studies in the future. What is still not well understood is how these mechanosensitive calcium channels handle forces, sensed through the plasma membrane, adhesive/cytoskeletal structures and the ECM fibers, as well as what extent of force changes are they dealing with. Many mechanisms that control f.i. cellular proliferation or apoptosis are extremely sensitive to modest changes in the Ca2+- homeostasis. Drastic changes in spatio-temporal calcium regulation through various channels in the transformed tissues may therefore reprogram cellular functions towards a more aggressive phenotype through several cascades. Although most of the TRP-mediated pathways involved in cancer progression are linked to the abnormal Ca2+ homeostasis, there are also indications for pore-independent functions for TRPs during metastatic development [290]. Clearly, this topic requires more studies in the future. One should also note that besides TRP channels, other mechanosensitive channels, like Piezo family channels, are important factors in sensing mechanical changes and in regulating mechanosensitive pathways that are linked to cancer progression [8,291,292]. These channels are also deregulated in various cancers and their cooperation with other ion channels along cancer progression should be investigated in the future.
Of the TRPV channel family, TRPV2 and TRPV4 have been mostly studied with respect to their altered mechanoresponsive features along cancer progression. However, it may well be that the other family members are as important in mediating abnormal mechanical signaling within transformed tissues. Hence, the mechanisms of how these distinct TRPV channels are responding to various biophysical changes in the tumor microenvironment, f.i. through gating or expression of the channel protein, needs more attention. Additionally, the intracellular signaling cascades downstream of these channels requires further studies and the exact mechanisms behind TRPV4-triggered alterations in contractile structures should be investigated. Moreover, the activation of the channels through direct and indirect mechanisms as well as the cross-talk in between these processes needs more attention. After all, these channels represent promising therapeutical targets that could be utilized in the management of advanced cancers.
Funding
This research was funded by the Academy of Finland (S.T), grant number 1332258.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Discher, D.E. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating Force and Shape—The Rise of Mechanotransduction in Cell Biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Emon, B.; Bauer, J.; Jain, Y.; Jung, B.; Saif, T. Biophysics of Tumor Microenvironment and Cancer Metastasis—A Mini Review. Comput. Struct. Biotechnol. J. 2018, 16, 279–287. [Google Scholar] [CrossRef]
- Wei, S.C.; Yang, J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial–Mesenchymal Transition. Trends Cell Biol. 2016, 26, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Broders-Bondon, F.; Nguyen Ho-Bouldoires, T.H.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in Tumor Progression: The Dark Side of the Force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef]
- Fedorchak, G.R.; Kaminski, A.; Lammerding, J. Cellular Mechanosensing: Getting to the Nucleus of It All. Prog. Biophys. Mol. Biol. 2014, 115, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Pethő, Z.; Najder, K.; Bulk, E.; Schwab, A. Mechanosensitive Ion Channels Push Cancer Progression. Cell Calcium 2019, 80, 79–90. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Fels, B.; Bulk, E.; Pethő, Z.; Schwab, A. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals 2018, 11, 48. [Google Scholar] [CrossRef]
- Christensen, A.P.; Corey, D.P. TRP Channels in Mechanosensation: Direct or Indirect Activation? Nat. Rev. Neurosci. 2007, 8, 510–521. [Google Scholar] [CrossRef]
- Lehen’kyi, V.; Prevarskaya, N. Oncogenic TRP Channels. In Transient Receptor Potential Channels; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2011; Volume 704, pp. 929–945. ISBN 978-94-007-0264-6. [Google Scholar]
- Liu, C.; Montell, C. Forcing Open TRP Channels: Mechanical Gating as a Unifying Activation Mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef]
- Monteith, G.R.; Prevarskaya, N.; Roberts-Thomson, S.J. The Calcium–Cancer Signalling Nexus. Nat. Rev. Cancer 2017, 17, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Rokhlin, O.; Taghiyev, A.F.; Bayer, K.U.; Bumcrot, D.; Kotelianski, V.E.; Glover, R.A.; Cohen, M.B. Calcium/Calmodulin-Dependent Kinase II Plays an Important Role in Prostate Cancer Cell Survival. Cancer Biol. Ther. 2007, 6, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, G.; Ritaine, A.; Skryma, R.; Prevarskaya, N. Role of TRP Ion Channels in Cancer and Tumorigenesis. Semin. Immunopathol. 2016, 38, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Sée, V.; Rajala, N.K.M.; Spiller, D.G.; White, M.R.H. Calcium-Dependent Regulation of the Cell Cycle via a Novel MAPK–NF-ΚB Pathway in Swiss 3T3 Cells. J. Cell Biol. 2004, 166, 661–672. [Google Scholar] [CrossRef]
- Liu, F.; Bardhan, K.; Yang, D.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Liles, G.B.; Lee, J.R.; Liu, K. NF-ΚB Directly Regulates Fas Transcription to Modulate Fas-Mediated Apoptosis and Tumor Suppression. J. Biol. Chem. 2012, 287, 25530–25540. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The Calpain System. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.-P.; Ooi, L.; et al. TRPV3 Is a Temperature-Sensitive Vanilloid Receptor-like Protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef]
- Smedler, E.; Uhlén, P. Frequency Decoding of Calcium Oscillations. Biochim. Biophys. Acta BBA-Gen. Subj. 2014, 1840, 964–969. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Ouadid-Ahidouch, H.; Skryma, R.; Shuba, Y. Remodelling of Ca2+ Transport in Cancer: How It Contributes to Cancer Hallmarks? Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130097. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in Tumour Metastasis: New Roles for Known Actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef]
- Kanzaki, M.; Zhang, Y.-Q.; Mashima, H.; Li, L.; Shibata, H.; Kojima, I. Translocation of a Calcium-Permeable Cation Channel Induced by Insulin-like Growth Factor-I. Nat. Cell Biol. 1999, 1, 165–170. [Google Scholar] [CrossRef]
- Meyer, M.B.; Watanuki, M.; Kim, S.; Shevde, N.K.; Pike, J.W. The Human Transient Receptor Potential Vanilloid Type 6 Distal Promoter Contains Multiple Vitamin D Receptor Binding Sites That Mediate Activation by 1,25-Dihydroxyvitamin D3 in Intestinal Cells. Mol. Endocrinol. 2006, 20, 1447–1461. [Google Scholar] [CrossRef]
- Plant, T.D.; Strotmann, R. TRPV4: A Multifunctional Nonselective Cation Channel with Complex Regulation. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-4048-2. [Google Scholar]
- van Goor, M.K.C.; Hoenderop, J.G.J.; van der Wijst, J. TRP Channels in Calcium Homeostasis: From Hormonal Control to Structure-Function Relationship of TRPV5 and TRPV6. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2017, 1864, 883–893. [Google Scholar] [CrossRef]
- Vangeel, L.; Voets, T. Transient Receptor Potential Channels and Calcium Signaling. Cold Spring Harb. Perspect. Biol. 2019, 11, a035048. [Google Scholar] [CrossRef]
- Tsagareli, M.G.; Nozadze, I. An Overview on Transient Receptor Potential Channels Superfamily. Behav. Pharmacol. 2020, 31, 413–434. [Google Scholar] [CrossRef]
- Montell, C.; Birnbaumer, L.; Flockerzi, V. The TRP Channels, a Remarkably Functional Family. Cell 2002, 108, 595–598. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP Channels: An Overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef]
- Mutai, H.; Heller, S. Vertebrate and Invertebrate TRPV-like Mechanoreceptors. Cell Calcium 2003, 33, 471–478. [Google Scholar] [CrossRef]
- Niemeyer, B.A. Structure-Function Analysis of TRPV Channels. Naunyn. Schmiedebergs Arch. Pharmacol. 2005, 371, 285–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kojima, I.; Nagasawa, M. TRPV2. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 247–272. ISBN 978-3-642-54214-5. [Google Scholar]
- Hamill, O.P. Twenty Odd Years of Stretch-Sensitive Channels. Pflüg. Arch.-Eur. J. Physiol. 2006, 453, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Naeini, R.; Dedman, A.; Folgering, J.H.A.; Duprat, F.; Patel, A.; Nilius, B.; Honoré, E. TRP Channels and Mechanosensory Transduction: Insights into the Arterial Myogenic Response. Pflüg. Arch.-Eur. J. Physiol. 2008, 456, 529–540. [Google Scholar] [CrossRef]
- Nilius, B.; Honoré, E. Sensing Pressure with Ion Channels. Trends Neurosci. 2012, 35, 477–486. [Google Scholar] [CrossRef]
- Yin, J.; Kuebler, W.M. Mechanotransduction by TRP Channels: General Concepts and Specific Role in the Vasculature. Cell Biochem. Biophys. 2010, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sukharev, S.; Sachs, F. Molecular Force Transduction by Ion Channels–Diversity and Unifying Principles. J. Cell Sci. 2012, 125, 3075–3083. [Google Scholar] [CrossRef]
- Plant, T.D. TRPs in Mechanosensing and Volume Regulation. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2014; Volume 223, pp. 743–766. ISBN 978-3-319-05160-4. [Google Scholar]
- Pumroy, R.A.; Fluck, E.C.; Ahmed, T.; Moiseenkova-Bell, V.Y. Structural Insights into the Gating Mechanisms of TRPV Channels. Cell Calcium 2020, 87, 102168. [Google Scholar] [CrossRef]
- Dosey, T.L.; Wang, Z.; Fan, G.; Zhang, Z.; Serysheva, I.I.; Chiu, W.; Wensel, T.G. Structures of TRPV2 in Distinct Conformations Provide Insight into Role of the Pore Turret. Nat. Struct. Mol. Biol. 2019, 26, 40–49. [Google Scholar] [CrossRef]
- Yuan, P. Structural Biology of ThermoTRPV Channels. Cell Calcium 2019, 84, 102106. [Google Scholar] [CrossRef]
- Kärki, T.; Rajakylä, E.K.; Acheva, A.; Tojkander, S. TRPV6 Calcium Channel Directs Homeostasis of the Mammary Epithelial Sheets and Controls Epithelial Mesenchymal Transition. Sci. Rep. 2020, 10, 14683. [Google Scholar] [CrossRef]
- Ji, C.; McCulloch, C.A. TRPV4 Integrates Matrix Mechanosensing with Ca2+ Signaling to Regulate Extracellular Matrix Remodeling. FEBS J. 2020. [Google Scholar] [CrossRef]
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. TRPV4 Channels Mediate Cyclic Strain–Induced Endothelial Cell Reorientation Through Integrin-to-Integrin Signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Di Gregorio, M.; He, P.; McCulloch, C.A. TRPV4 Mediates the Calcium Influx Required for Flightless-Non-Muscle Myosin Interaction and Collagen Remodeling. J. Cell Sci. 2017, 130, 2196–2208. [Google Scholar] [CrossRef]
- Adapala, R.K.; Kanugula, A.K.; Paruchuri, S.; Chilian, W.M.; Thodeti, C.K. TRPV4 Deletion Protects Heart from Myocardial Infarction-Induced Adverse Remodeling via Modulation of Cardiac Fibroblast Differentiation. Basic Res. Cardiol. 2020, 115, 14. [Google Scholar] [CrossRef] [PubMed]
- Aspenström, P. Integration of Signalling Pathways Regulated by Small GTPases and Calcium. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2004, 1742, 51–58. [Google Scholar] [CrossRef]
- Bader, M.-F.; Doussau, F.; Chasserot-Golaz, S.; Vitale, N.; Gasman, S. Coupling Actin and Membrane Dynamics during Calcium-Regulated Exocytosis: A Role for Rho and ARF GTPases. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2004, 1742, 37–49. [Google Scholar] [CrossRef]
- Correll, R.N.; Pang, C.; Niedowicz, D.M.; Finlin, B.S.; Andres, D.A. The RGK Family of GTP-Binding Proteins: Regulators of Voltage-Dependent Calcium Channels and Cytoskeleton Remodeling. Cell. Signal. 2008, 20, 292–300. [Google Scholar] [CrossRef][Green Version]
- Chinigò, G.; Fiorio Pla, A.; Gkika, D. TRP Channels and Small GTPases Interplay in the Main Hallmarks of Metastatic Cancer. Front. Pharmacol. 2020, 11, 581455. [Google Scholar] [CrossRef]
- Thoppil, R.J.; Cappelli, H.C.; Adapala, R.K.; Kanugula, A.K.; Paruchuri, S.; Thodeti, C.K. TRPV4 Channels Regulate Tumor Angiogenesis via Modulation of Rho/Rho Kinase Pathway. Oncotarget 2016, 7, 25849–25861. [Google Scholar] [CrossRef]
- Kanugula, A.K.; Adapala, R.K.; Midha, P.; Cappelli, H.C.; Meszaros, J.G.; Paruchuri, S.; Chilian, W.M.; Thodeti, C.K. Novel Noncanonical Regulation of Soluble VEGF/VEGFR2 Signaling by Mechanosensitive Ion Channel TRPV4. FASEB J. 2019, 33, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Fiorio Pla, A.; Ong, H.L.; Cheng, K.T.; Brossa, A.; Bussolati, B.; Lockwich, T.; Paria, B.; Munaron, L.; Ambudkar, I.S. TRPV4 Mediates Tumor-Derived Endothelial Cell Migration via Arachidonic Acid-Activated Actin Remodeling. Oncogene 2012, 31, 200–212. [Google Scholar] [CrossRef]
- Adapala, R.K.; Thoppil, R.J.; Ghosh, K.; Cappelli, H.C.; Dudley, A.C.; Paruchuri, S.; Keshamouni, V.; Klagsbrun, M.; Meszaros, J.G.; Chilian, W.M.; et al. Activation of Mechanosensitive Ion Channel TRPV4 Normalizes Tumor Vasculature and Improves Cancer Therapy. Oncogene 2016, 35, 314–322. [Google Scholar] [CrossRef]
- Santoni, G.; Farfariello, V.; Amantini, C. TRPV Channels in Tumor Growth and Progression. In Transient Receptor Potential Channels; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2011; Volume 704, pp. 947–967. ISBN 978-94-007-0264-6. [Google Scholar]
- Yang, D.; Kim, J. Emerging Role of Transient Receptor Potential (TRP) Channels in Cancer Progression. BMB Rep. 2020, 53, 125–132. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP Channels in Cancer. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2007, 1772, 937–946. [Google Scholar] [CrossRef]
- Nilius, B.; Szallasi, A. Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef]
- Fu, S.; Hirte, H.; Welch, S.; Ilenchuk, T.T.; Lutes, T.; Rice, C.; Fields, N.; Nemet, A.; Dugourd, D.; Piha-Paul, S.; et al. First-in-Human Phase I Study of SOR-C13, a TRPV6 Calcium Channel Inhibitor, in Patients with Advanced Solid Tumors. Investig. New Drugs 2017, 35, 324–333. [Google Scholar] [CrossRef]
- Minke, B. Drosophila Mutant with a Transducer Defect. Biophys. Struct. Mech. 1977, 3, 59–64. [Google Scholar] [CrossRef]
- Perálvarez-Marín, A.; Doñate-Macian, P.; Gaudet, R. What Do We Know about the Transient Receptor Potential Vanilloid 2 (TRPV2) Ion Channel? FEBS J. 2013, 280, 5471–5487. [Google Scholar] [CrossRef]
- Garcia-Sanz, N. Identification of a Tetramerization Domain in the C Terminus of the Vanilloid Receptor. J. Neurosci. 2004, 24, 5307–5314. [Google Scholar] [CrossRef]
- Hellwig, N.; Albrecht, N.; Harteneck, C.; Schultz, G.; Schaefer, M. Homo- and Heteromeric Assembly of TRPV Channel Subunits. J. Cell Sci. 2005, 118, 917–928. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP Channels as Cellular Sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C. Regulation of TRP Channels via Lipid Second Messengers. Annu. Rev. Physiol. 2003, 65, 735–759. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. The TRP Superfamily of Cation Channels. Sci. Signal. 2005, 2005, re3. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A Capsaicin-Receptor Homologue with a High Threshold for Noxious Heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M. A Heat-Sensitive TRP Channel Expressed in Keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Caterina, M.J. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Cohen, M.R.; Huynh, K.W.; Cawley, D.; Moiseenkova-Bell, V.Y. Understanding the Cellular Function of TRPV2 Channel through Generation of Specific Monoclonal Antibodies. PLoS ONE 2013, 8, e85392. [Google Scholar] [CrossRef]
- Cohen, M.R.; Johnson, W.M.; Pilat, J.M.; Kiselar, J.; DeFrancesco-Lisowitz, A.; Zigmond, R.E.; Moiseenkova-Bell, V.Y. Nerve Growth Factor Regulates Transient Receptor Potential Vanilloid 2 via Extracellular Signal-Regulated Kinase Signaling To Enhance Neurite Outgrowth in Developing Neurons. Mol. Cell. Biol. 2015, 35, 4238–4252. [Google Scholar] [CrossRef]
- Shibasaki, K.; Murayama, N.; Ono, K.; Ishizaki, Y.; Tominaga, M. TRPV2 Enhances Axon Outgrowth through Its Activation by Membrane Stretch in Developing Sensory and Motor Neurons. J. Neurosci. 2010, 30, 4601–4612. [Google Scholar] [CrossRef]
- Mihara, H.; Boudaka, A.; Shibasaki, K.; Yamanaka, A.; Sugiyama, T.; Tominaga, M. Involvement of TRPV2 Activation in Intestinal Movement through Nitric Oxide Production in Mice. J. Neurosci. 2010, 30, 16536–16544. [Google Scholar] [CrossRef]
- Shibasaki, K.; Ishizaki, Y.; Mandadi, S. Astrocytes Express Functional TRPV2 Ion Channels. Biochem. Biophys. Res. Commun. 2013, 441, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, Z. Antiaging Gene Klotho Enhances Glucose-Induced Insulin Secretion by Up-Regulating Plasma Membrane Levels of TRPV2 in MIN6 β-Cells. Endocrinology 2012, 153, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K. Physiological Significance of TRPV2 as a Mechanosensor, Thermosensor and Lipid Sensor. J. Physiol. Sci. 2016, 66, 359–365. [Google Scholar] [CrossRef]
- Liberati, S.; Morelli, M.; Amantini, C.; Santoni, M.; Nabissi, M.; Cardinali, C.; Santoni, G. Advances in Transient Receptor Potential Vanilloid-2 Channel Expression and Function in Tumor Growth and Progression. Curr. Protein Pept. Sci. 2014, 15, 732–737. [Google Scholar] [CrossRef]
- Mercado, J.; Gordon-Shaag, A.; Zagotta, W.N.; Gordon, S.E. Ca2+-Dependent Desensitization of TRPV2 Channels Is Mediated by Hydrolysis of Phosphatidylinositol 4,5-Bisphosphate. J. Neurosci. 2010, 30, 13338–13347. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, Thyme and Clove-Derived Flavors and Skin Sensitizers Activate Specific TRP Channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, M.X. TRPV3. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 273–291. ISBN 978-3-642-54214-5. [Google Scholar]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 Is a Calcium-Permeable Temperature-Sensitive Cation Channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef]
- Yamamoto-Kasai, E.; Imura, K.; Yasui, K.; Shichijou, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Yoshioka, T. TRPV3 as a Therapeutic Target for Itch. J. Investig. Dermatol. 2012, 132, 2109–2112. [Google Scholar] [CrossRef]
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 Regulates Nitric Oxide Synthase-Independent Nitric Oxide Synthesis in the Skin. Nat. Commun. 2011, 2, 369. [Google Scholar] [CrossRef]
- Yamada, T.; Ueda, T.; Shibata, Y.; Ikegami, Y.; Saito, M.; Ishida, Y.; Ugawa, S.; Kohri, K.; Shimada, S. TRPV2 Activation Induces Apoptotic Cell Death in Human T24 Bladder Cancer Cells: A Potential Therapeutic Target for Bladder Cancer. Urology 2010, 76, 509.e1–509.e7. [Google Scholar] [CrossRef]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The Thermosensitive TRPV3 Channel Contributes to Rapid Wound Healing in Oral Epithelia. FASEB J. 2015, 29, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid Receptor–Related Osmotically Activated Channel (VR-OAC), a Candidate Vertebrate Osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired Pressure Sensation in Mice Lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K. TRPV4 Ion Channel as Important Cell Sensors. J. Anesth. 2016, 30, 1014–1019. [Google Scholar] [CrossRef]
- White, J.P.M.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F. TRPV4: A Physio and Pathophysiologically Significant Ion Channel. Int. J. Mol. Sci. 2020, 21, 3837. [Google Scholar] [CrossRef]
- Liedtke, W.; Friedman, J.M. Abnormal Osmotic Regulation in Trpv4−/− Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Fisslthaler, B.; Suzuki, M.; Janssens, A.; Voets, T.; Morisseau, C.; Hammock, B.D.; Fleming, I.; Busse, R.; et al. Modulation of the Ca2 Permeable Cation Channel TRPV4 by Cytochrome P450 Epoxygenases in Vascular Endothelium. Circ. Res. 2005, 97, 908–915. [Google Scholar] [CrossRef]
- Reiter, B.; Kraft, R.; Günzel, D.; Zeissig, S.; Schulzke, J.-D.; Fromm, M.; Harteneck, C. TRPV4-Mediated Regulation of Epithelial Permeability. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1802–1812. [Google Scholar] [CrossRef]
- Martínez-Rendón, J.; Sánchez-Guzmán, E.; Rueda, A.; González, J.; Gulias-Cañizo, R.; Aquino-Jarquín, G.; Castro-Muñozledo, F.; García-Villegas, R. TRPV4 Regulates Tight Junctions and Affects Differentiation in a Cell Culture Model of the Corneal Epithelium. J. Cell. Physiol. 2017, 232, 1794–1807. [Google Scholar] [CrossRef]
- Kitsuki, T.; Yoshimoto, R.U.; Aijima, R.; Hatakeyama, J.; Cao, A.-L.; Zhang, J.-Q.; Ohsaki, Y.; Mori, Y.; Kido, M.A. Enhanced Junctional Epithelial Permeability in TRPV4-Deficient Mice. J. Periodontal Res. 2020, 55, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Mizusawa, M.; Sharmin, M.M.; Hayashi, S.; Yonekura, S. TRPV4 Increases the Expression of Tight Junction Protein-Encoding Genes via XBP1 in Mammary Epithelial Cells. Animals 2020, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, Y.; Yuki, T.; Yoshida, H.; Sugiyama, Y.; Inoue, S. Activation of TRPV4 Strengthens the Tight-Junction Barrier in Human Epidermal Keratinocytes. Skin Pharmacol. Physiol. 2013, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Baylie, R.L.; Brayden, J.E. TRPV Channels and Vascular Function. Acta Physiol. Oxf. Engl. 2011, 203, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, M.; Lv, X.; Wang, Z.; Yang, J.; Li, Y.; Yu, F.; Wen, X.; Feng, L.; Zhou, T. Role of Transient Receptor Potential Vanilloid 4 in Vascular Function. Front. Mol. Biosci. 2021, 8, 677661. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Yeh, J.J.; Boyd, A.E.; Parada, C.A.; Chen, X.; Reichling, D.B.; Levine, J.D. Hypotonicity Induces TRPV4-Mediated Nociception in Rat. Neuron 2003, 39, 497–511. [Google Scholar] [CrossRef]
- Kanju, P.; Liedtke, W. Pleiotropic Function of TRPV4 Ion Channels in the Central Nervous System. Exp. Physiol. 2016, 101, 1472–1476. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, C.; Li, H.; Tang, C.; Kan, H.; Yang, Z.; Mao, A.; Ma, X. TRPV4 (Transient Receptor Potential Vanilloid 4) Mediates Endothelium-Dependent Contractions in the Aortas of Hypertensive Mice. Hypertension 2018, 71, 134–142. [Google Scholar] [CrossRef]
- Na, T.; Peng, J.-B. TRPV5: A Ca2+ Channel for the Fine-Tuning of Ca2+ Reabsorption. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 321–357. ISBN 978-3-642-54214-5. [Google Scholar]
- Fecher-Trost, C.; Weissgerber, P.; Wissenbach, U. TRPV6 Channels. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 359–384. ISBN 978-3-642-54214-5. [Google Scholar]
- Peng, J.-B.; Suzuki, Y.; Gyimesi, G.; Hediger, M.A. TRPV5 and TRPV6 Calcium-Selective Channels. In Calcium Entry Channels in Non-Excitable Cells; Kozak, J.A., Putney, J.W., Eds.; Methods in Signal Transduction Series; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 241–274. ISBN 978-1-315-15259-2. [Google Scholar]
- Hoenderop, J.G.J.; van der Kemp, A.W.C.M.; Hartog, A.; van de Graaf, S.F.J.; van Os, C.H.; Willems, P.H.G.M.; Bindels, R.J.M. Molecular Identification of the Apical Ca2+Channel in 1,25-Dihydroxyvitamin D3-Responsive Epithelia. J. Biol. Chem. 1999, 274, 8375–8378. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.J.; Nilius, B.; Bindels, R.J.M. Calcium Absorption Across Epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef] [PubMed]
- Bindels, R.J. Molecular Pathophysiology of Renal Calcium Handling. Kidney Blood Press. Res. 2000, 23, 183–184. [Google Scholar]
- Peng, J.-B.; Zhuang, L.; Berger, U.V.; Adam, R.M.; Williams, B.J.; Brown, E.M.; Hediger, M.A.; Freeman, M.R. CaT1 Expression Correlates with Tumor Grade in Prostate Cancer. Biochem. Biophys. Res. Commun. 2001, 282, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Peng, J.-B.; Tou, L.; Takanaga, H.; Adam, R.M.; Hediger, M.A.; Freeman, M.R. Calcium-Selective Ion Channel, CaT1, Is Apically Localized in Gastrointestinal Tract Epithelia and Is Aberrantly Expressed in Human Malignancies. Lab. Investig. 2002, 82, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Landowski, C.P.; Hediger, M.A. Mechanisms and Regulation of Epithelial Ca2+ Absorption in Health and Disease. Annu. Rev. Physiol. 2008, 70, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Bödding, M.; Flockerzi, V. Ca2+ Dependence of the Ca2+-Selective TRPV6 Channel. J. Biol. Chem. 2004, 279, 36546–36552. [Google Scholar] [CrossRef]
- Bolanz, K.A.; Hediger, M.A.; Landowski, C.P. The Role of TRPV6 in Breast Carcinogenesis. Mol. Cancer Ther. 2008, 7, 271–279. [Google Scholar] [CrossRef]
- Arniges, M.; Fernández-Fernández, J.M.; Albrecht, N.; Schaefer, M.; Valverde, M.A. Human TRPV4 Channel Splice Variants Revealed a Key Role of Ankyrin Domains in Multimerization and Trafficking. J. Biol. Chem. 2006, 281, 1580–1586. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Grimm, C.; Oshima, K.; D’hoedt, D.; Nilius, B.; Mensenkamp, A.R.; Bindels, R.J.M.; Plomann, M.; Heller, S. PACSINs Bind to the TRPV4 Cation Channel. J. Biol. Chem. 2006, 281, 18753–18762. [Google Scholar] [CrossRef] [PubMed]
- de Groot, T.; van der Hagen, E.A.E.; Verkaart, S.; te Boekhorst, V.A.M.; Bindels, R.J.M.; Hoenderop, J.G.J. Role of the Transient Receptor Potential Vanilloid 5 (TRPV5) Protein N Terminus in Channel Activity, Tetramerization, and Trafficking. J. Biol. Chem. 2011, 286, 32132–32139. [Google Scholar] [CrossRef]
- Doñate-Macian, P.; Bañó-Polo, M.; Vazquez-Ibar, J.-L.; Mingarro, I.; Perálvarez-Marín, A. Molecular and Topological Membrane Folding Determinants of Transient Receptor Potential Vanilloid 2 Channel. Biochem. Biophys. Res. Commun. 2015, 462, 221–226. [Google Scholar] [CrossRef]
- Gkika, D.; Prevarskaya, N. Molecular Mechanisms of TRP Regulation in Tumor Growth and Metastasis. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2009, 1793, 953–958. [Google Scholar] [CrossRef]
- Doñate-Macián, P.; Enrich-Bengoa, J.; Dégano, I.R.; Quintana, D.G.; Perálvarez-Marín, A. Trafficking of Stretch-Regulated TRPV2 and TRPV4 Channels Inferred Through Interactomics. Biomolecules 2019, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The Transient Receptor Potential Family of Ion Channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. AN INTRODUCTION TO TRP CHANNELS. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef]
- Colbert, H.A.; Smith, T.L.; Bargmann, C.I. OSM-9, A Novel Protein with Structural Similarity to Channels, Is Required for Olfaction, Mechanosensation, and Olfactory Adaptation in Caenorhabditis Elegans. J. Neurosci. 1997, 17, 8259–8269. [Google Scholar] [CrossRef]
- Walker, R.G.; Willingham, A.T.; Zuker, C.S. A Drosophila Mechanosensory Transduction Channel. Science 2000, 287, 2229–2234. [Google Scholar] [CrossRef]
- Kim, J.; Chung, Y.D.; Park, D.; Choi, S.; Shin, D.W.; Soh, H.; Lee, H.W.; Son, W.; Yim, J.; Park, C.-S.; et al. A TRPV Family Ion Channel Required for Hearing in Drosophila. Nature 2003, 424, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.D.; Wilson, R.I.; Laurent, G.; Benzer, S. Painless, a Drosophila Gene Essential for Nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef]
- Sukharev, S.; Corey, D.P. Mechanosensitive Channels: Multiplicity of Families and Gating Paradigms. Sci. Signal. 2004, 2004, re4. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, R.G.; Heller, S. The Mechanosensitive Nature of TRPV Channels. Pflüg. Arch.-Eur. J. Physiol. 2005, 451, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B. The Ion Channels to Cytoskeleton Connection as Potential Mechanism of Mechanosensitivity. Biochim. Biophys. Acta BBA-Biomembr. 2014, 1838, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Katta, S.; Krieg, M.; Goodman, M.B. Feeling Force: Physical and Physiological Principles Enabling Sensory Mechanotransduction. Annu. Rev. Cell Dev. Biol. 2015, 31, 347–371. [Google Scholar] [CrossRef]
- Teng, J.; Loukin, S.; Anishkin, A.; Kung, C. The Force-from-Lipid (FFL) Principle of Mechanosensitivity, at Large and in Elements. Pflüg. Arch.-Eur. J. Physiol. 2015, 467, 27–37. [Google Scholar] [CrossRef]
- Bavi, O.; Cox, C.; Vossoughi, M.; Naghdabadi, R.; Jamali, Y.; Martinac, B. Influence of Global and Local Membrane Curvature on Mechanosensitive Ion Channels: A Finite Element Approach. Membranes 2016, 6, 14. [Google Scholar] [CrossRef]
- Cox, C.D.; Bavi, N.; Martinac, B. Biophysical Principles of Ion-Channel-Mediated Mechanosensory Transduction. Cell Rep. 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Gustin, M.; Zhou, X.; Martinac, B.; Kung, C. A Mechanosensitive Ion Channel in the Yeast Plasma Membrane. Science 1988, 242, 762–765. [Google Scholar] [CrossRef]
- Sachs, F. Mechanical Transduction in Biological Systems. Crit. Rev. Biomed. Eng. 1988, 16, 141–169. [Google Scholar]
- Sokabe, M.; Sachs, F.; Jing, Z.Q. Quantitative Video Microscopy of Patch Clamped Membranes Stress, Strain, Capacitance, and Stretch Channel Activation. Biophys. J. 1991, 59, 722–728. [Google Scholar] [CrossRef]
- Sachs, F. Stretch-Activated Ion Channels: What Are They? Physiology 2010, 25, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B.; Cox, C.D. Mechanosensory Transduction: Focus on Ion Channels. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809633-8. [Google Scholar]
- Jones, R.C.W. The Mechanosensitivity of Mouse Colon Afferent Fibers and Their Sensitization by Inflammatory Mediators Require Transient Receptor Potential Vanilloid 1 and Acid-Sensing Ion Channel 3. J. Neurosci. 2005, 25, 10981–10989. [Google Scholar] [CrossRef] [PubMed]
- Nozadze, I.; Tsiklauri, N.; Gurtskaia, G.; Tsagareli, M.G. Role of Thermo TRPA1 and TRPV1 Channels in Heat, Cold, and Mechanical Nociception of Rats. Behav. Pharmacol. 2016, 27, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, E.; Hiyama, T.Y.; Noda, M. Osmosensitivity of Transient Receptor Potential Vanilloid 1 Is Synergistically Enhanced by Distinct Activating Stimuli Such as Temperature and Protons. PLoS ONE 2011, 6, e22246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kindrat, A.N.; Sharif-Naeini, R.; Bourque, C.W. Actin Filaments Mediate Mechanical Gating during Osmosensory Transduction in Rat Supraoptic Nucleus Neurons. J. Neurosci. 2007, 27, 4008–4013. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Schmidt, H.; Hucho, F. TRPV1 at Nerve Endings Regulates Growth Cone Morphology and Movement through Cytoskeleton Reorganization: Regulation of Growth Cone Motility by TRPV1. FEBS J. 2007, 274, 760–772. [Google Scholar] [CrossRef]
- Muraki, K.; Iwata, Y.; Katanosaka, Y.; Ito, T.; Ohya, S.; Shigekawa, M.; Imaizumi, Y. TRPV2 Is a Component of Osmotically Sensitive Cation Channels in Murine Aortic Myocytes. Circ. Res. 2003, 93, 829–838. [Google Scholar] [CrossRef]
- Katanosaka, Y.; Iwasaki, K.; Ujihara, Y.; Takatsu, S.; Nishitsuji, K.; Kanagawa, M.; Sudo, A.; Toda, T.; Katanosaka, K.; Mohri, S.; et al. TRPV2 Is Critical for the Maintenance of Cardiac Structure and Function in Mice. Nat. Commun. 2014, 5, 3932. [Google Scholar] [CrossRef] [PubMed]
- Sugio, S.; Nagasawa, M.; Kojima, I.; Ishizaki, Y.; Shibasaki, K. Transient Receptor Potential Vanilloid 2 Activation by Focal Mechanical Stimulation Requires Interaction with the Actin Cytoskeleton and Enhances Growth Cone Motility. FASEB J. 2017, 31, 1368–1381. [Google Scholar] [CrossRef]
- Kerstein, P.C.; Nichol IV, R.H.; Gomez, T.M. Mechanochemical Regulation of Growth Cone Motility. Front. Cell. Neurosci. 2015, 9, 244. [Google Scholar] [CrossRef]
- Jin, X.; Touhey, J.; Gaudet, R. Structure of the N-Terminal Ankyrin Repeat Domain of the TRPV2 Ion Channel. J. Biol. Chem. 2006, 281, 25006–25010. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Katanosaka, Y.; Arai, Y.; Komamura, K.; Miyatake, K.; Shigekawa, M. A Novel Mechanism of Myocyte Degeneration Involving the Ca2+-Permeable Growth Factor–Regulated Channel. J. Cell Biol. 2003, 161, 957–967. [Google Scholar] [CrossRef]
- Fois, G.; Wittekindt, O.; Zheng, X.; Felder, E.T.; Miklavc, P.; Frick, M.; Dietl, P.; Felder, E. An Ultra Fast Detection Method Reveals Strain-Induced Ca2+ Entry via TRPV2 in Alveolar Type II Cells. Biomech. Model. Mechanobiol. 2012, 11, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Horn, N.A.; Huynh, T.; Kelava, L.; Lansman, J.B. Evidence TRPV4 Contributes to Mechanosensitive Ion Channels in Mouse Skeletal Muscle Fibers. Channels 2012, 6, 246–254. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a Nonselective Cation Channel That Confers Sensitivity to Extracellular Osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Wissenbach, U.; Bödding, M.; Freichel, M.; Flockerzi, V. Trp12, a Novel Trp Related Protein from Kidney. FEBS Lett. 2000, 485, 127–134. [Google Scholar] [CrossRef]
- Tian, W.; Salanova, M.; Xu, H.; Lindsley, J.N.; Oyama, T.T.; Anderson, S.; Bachmann, S.; Cohen, D.M. Renal Expression of Osmotically Responsive Cation Channel TRPV4 Is Restricted to Water-Impermeant Nephron Segments. Am. J. Physiol.-Ren. Physiol. 2004, 287, F17–F24. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Zaika, O.; O’Neil, R.G.; Mamenko, M. Novel Insights into TRPV4 Function in the Kidney. Pflüg. Arch.-Eur. J. Physiol. 2013, 465, 177–186. [Google Scholar] [CrossRef]
- Galizia, L.; Pizzoni, A.; Fernandez, J.; Rivarola, V.; Capurro, C.; Ford, P. Functional Interaction between AQP2 and TRPV4 in Renal Cells. J. Cell. Biochem. 2012, 113, 580–589. [Google Scholar] [CrossRef]
- Liu, X.; Bandyopadhyay, B.; Nakamoto, T.; Singh, B.; Liedtke, W.; Melvin, J.E.; Ambudkar, I. A Role for AQP5 in Activation of TRPV4 by Hypotonicity. J. Biol. Chem. 2006, 281, 15485–15495. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Bereiter-Hahn, J.; Jendrach, M. Functional Interaction of the Cation Channel Transient Receptor Potential Vanilloid 4 (TRPV4) and Actin in Volume Regulation. Eur. J. Cell Biol. 2009, 88, 141–152. [Google Scholar] [CrossRef]
- Prager-Khoutorsky, M.; Bourque, C.W. Osmosensation in Vasopressin Neurons: Changing Actin Density to Optimize Function. Trends Neurosci. 2010, 33, 76–83. [Google Scholar] [CrossRef]
- Prager-Khoutorsky, M.; Bourque, C.W. Mechanical Basis of Osmosensory Transduction in Magnocellular Neurosecretory Neurones of the Rat Supraoptic Nucleus. J. Neuroendocrinol. 2015, 27, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Adapala, R.K.; Thoppil, R.J.; Luther, D.J.; Paruchuri, S.; Meszaros, J.G.; Chilian, W.M.; Thodeti, C.K. TRPV4 Channels Mediate Cardiac Fibroblast Differentiation by Integrating Mechanical and Soluble Signals. J. Mol. Cell. Cardiol. 2013, 54, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Peana, D.; Veteto, A.B.; Lambert, M.D.; Nourian, Z.; Karasseva, N.G.; Hill, M.A.; Lindman, B.R.; Baines, C.P.; Krenz, M.; et al. TRPV4 Increases Cardiomyocyte Calcium Cycling and Contractility yet Contributes to Damage in the Aged Heart Following Hypoosmotic Stress. Cardiovasc. Res. 2019, 115, 46–56. [Google Scholar] [CrossRef]
- Veteto, A.B.; Peana, D.; Lambert, M.D.; McDonald, K.S.; Domeier, T.L. Transient Receptor Potential Vanilloid-4 Contributes to Stretch-Induced Hypercontractility and Time-Dependent Dysfunction in the Aged Heart. Cardiovasc. Res. 2020, 116, 1887–1896. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Varty, L.; Rizzo, C.A.; Yang, R.; Correll, C.C.; Phelps, P.T.; Egan, R.W.; Hey, J.A. Functional TRPV4 Channels Are Expressed in Human Airway Smooth Muscle Cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L272–L278. [Google Scholar] [CrossRef]
- Earley, S.; Heppner, T.J.; Nelson, M.T.; Brayden, J.E. TRPV4 Forms a Novel Ca2+ Signaling Complex With Ryanodine Receptors and BK Ca Channels. Circ. Res. 2005, 97, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-R.; Lin, A.H.Y.; Hughes, J.M.; Flavahan, N.A.; Cao, Y.-N.; Liedtke, W.; Sham, J.S.K. Upregulation of Osmo-Mechanosensitive TRPV4 Channel Facilitates Chronic Hypoxia-Induced Myogenic Tone and Pulmonary Hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L555–L568. [Google Scholar] [CrossRef]
- Birder, L.; Kullmann, F.A.; Lee, H.; Barrick, S.; de Groat, W.; Kanai, A.; Caterina, M. Activation of Urothelial Transient Receptor Potential Vanilloid 4 by 4α-Phorbol 12,13-Didecanoate Contributes to Altered Bladder Reflexes in the Rat. J. Pharmacol. Exp. Ther. 2007, 323, 227–235. [Google Scholar] [CrossRef]
- Gevaert, T.; Vriens, J.; Segal, A.; Everaerts, W.; Roskams, T.; Talavera, K.; Owsianik, G.; Liedtke, W.; Daelemans, D.; Dewachter, I.; et al. Deletion of the Transient Receptor Potential Cation Channel TRPV4 Impairs Murine Bladder Voiding. J. Clin. Investig. 2007, 117, 3453–3462. [Google Scholar] [CrossRef]
- Mochizuki, T.; Sokabe, T.; Araki, I.; Fujishita, K.; Shibasaki, K.; Uchida, K.; Naruse, K.; Koizumi, S.; Takeda, M.; Tominaga, M. The TRPV4 Cation Channel Mediates Stretch-Evoked Ca2+ Influx and ATP Release in Primary Urothelial Cell Cultures. J. Biol. Chem. 2009, 284, 21257–21264. [Google Scholar] [CrossRef]
- Startek, J.; Boonen, B.; Talavera, K.; Meseguer, V. TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 371. [Google Scholar] [CrossRef]
- Alpizar, Y.A.; Boonen, B.; Sanchez, A.; Jung, C.; López-Requena, A.; Naert, R.; Steelant, B.; Luyts, K.; Plata, C.; De Vooght, V.; et al. TRPV4 Activation Triggers Protective Responses to Bacterial Lipopolysaccharides in Airway Epithelial Cells. Nat. Commun. 2017, 8, 1059. [Google Scholar] [CrossRef]
- Boonen, B.; Alpizar, Y.A.; Sanchez, A.; López-Requena, A.; Voets, T.; Talavera, K. Differential Effects of Lipopolysaccharide on Mouse Sensory TRP Channels. Cell Calcium 2018, 73, 72–81. [Google Scholar] [CrossRef]
- Boonen, B.; Alpizar, Y.; Meseguer, V.; Talavera, K. TRP Channels as Sensors of Bacterial Endotoxins. Toxins 2018, 10, 326. [Google Scholar] [CrossRef]
- Hartmannsgruber, V.; Heyken, W.-T.; Kacik, M.; Kaistha, A.; Grgic, I.; Harteneck, C.; Liedtke, W.; Hoyer, J.; Köhler, R. Arterial Response to Shear Stress Critically Depends on Endothelial TRPV4 Expression. PLoS ONE 2007, 2, e827. [Google Scholar] [CrossRef]
- Köhler, R.; Heyken, W.-T.; Heinau, P.; Schubert, R.; Si, H.; Kacik, M.; Busch, C.; Grgic, I.; Maier, T.; Hoyer, J. Evidence for a Functional Role of Endothelial Transient Receptor Potential V4 in Shear Stress–Induced Vasodilatation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1495–1502. [Google Scholar] [CrossRef]
- Loot, A.E.; Popp, R.; Fisslthaler, B.; Vriens, J.; Nilius, B.; Fleming, I. Role of Cytochrome P450-Dependent Transient Receptor Potential V4 Activation in Flow-Induced Vasodilatation. Cardiovasc. Res. 2008, 80, 445–452. [Google Scholar] [CrossRef]
- Mendoza, S.A.; Fang, J.; Gutterman, D.D.; Wilcox, D.A.; Bubolz, A.H.; Li, R.; Suzuki, M.; Zhang, D.X. TRPV4-Mediated Endothelial Ca 2+ Influx and Vasodilation in Response to Shear Stress. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H466–H476. [Google Scholar] [CrossRef]
- Baratchi, S.; Knoerzer, M.; Khoshmanesh, K.; Mitchell, A.; McIntyre, P. Shear Stress Regulates TRPV4 Channel Clustering and Translocation from Adherens Junctions to the Basal Membrane. Sci. Rep. 2017, 7, 15942. [Google Scholar] [CrossRef]
- Köttgen, M.; Buchholz, B.; Garcia-Gonzalez, M.A.; Kotsis, F.; Fu, X.; Doerken, M.; Boehlke, C.; Steffl, D.; Tauber, R.; Wegierski, T.; et al. TRPP2 and TRPV4 Form a Polymodal Sensory Channel Complex. J. Cell Biol. 2008, 182, 437–447. [Google Scholar] [CrossRef]
- Mamenko, M.; Zaika, O.; Boukelmoune, N.; O’Neil, R.G.; Pochynyuk, O. Deciphering Physiological Role of the Mechanosensitive TRPV4 Channel in the Distal Nephron. Am. J. Physiol-Ren. Physiol. 2015, 308, F275–F286. [Google Scholar] [CrossRef]
- Lorenzo, I.M.; Liedtke, W.; Sanderson, M.J.; Valverde, M.A. TRPV4 Channel Participates in Receptor-Operated Calcium Entry and Ciliary Beat Frequency Regulation in Mouse Airway Epithelial Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 12611–12616. [Google Scholar] [CrossRef]
- Phan, M.N.; Leddy, H.A.; Votta, B.J.; Kumar, S.; Levy, D.S.; Lipshutz, D.B.; Lee, S.H.; Liedtke, W.; Guilak, F. Functional Characterization of TRPV4 as an Osmotically Sensitive Ion Channel in Porcine Articular Chondrocytes. Arthritis Rheum. 2009, 60, 3028–3037. [Google Scholar] [CrossRef]
- Sidhaye, V.K.; Schweitzer, K.S.; Caterina, M.J.; Shimoda, L.; King, L.S. Shear Stress Regulates Aquaporin-5 and Airway Epithelial Barrier Function. Proc. Natl. Acad. Sci. USA 2008, 105, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Andrade, Y.N.; Fernandes, J.; Vázquez, E.; Fernández-Fernández, J.M.; Arniges, M.; Sánchez, T.M.; Villalón, M.; Valverde, M.A. TRPV4 Channel Is Involved in the Coupling of Fluid Viscosity Changes to Epithelial Ciliary Activity. J. Cell Biol. 2005, 168, 869–874. [Google Scholar] [CrossRef]
- Miura, S.; Sato, K.; Kato-Negishi, M.; Teshima, T.; Takeuchi, S. Fluid Shear Triggers Microvilli Formation via Mechanosensitive Activation of TRPV6. Nat. Commun. 2015, 6, 8871. [Google Scholar] [CrossRef]
- Sharma, S.; Goswami, R.; Zhang, D.X.; Rahaman, S.O. TRPV 4 Regulates Matrix Stiffness and TGF Β1-induced Epithelial-mesenchymal Transition. J. Cell. Mol. Med. 2019, 23, 761–774. [Google Scholar] [CrossRef]
- Sharma, S.; Goswami, R.; Rahaman, S.O. The TRPV4-TAZ Mechanotransduction Signaling Axis in Matrix Stiffness- and TGFβ1-Induced Epithelial-Mesenchymal Transition. Cell. Mol. Bioeng. 2019, 12, 139–152. [Google Scholar] [CrossRef]
- Zhan, L.; Li, J. The Role of TRPV4 in Fibrosis. Gene 2018, 642, 1–8. [Google Scholar] [CrossRef]
- Gilchrist, C.L.; Leddy, H.A.; Kaye, L.; Case, N.D.; Rothenberg, K.E.; Little, D.; Liedtke, W.; Hoffman, B.D.; Guilak, F. TRPV4-Mediated Calcium Signaling in Mesenchymal Stem Cells Regulates Aligned Collagen Matrix Formation and Vinculin Tension. Proc. Natl. Acad. Sci. USA 2019, 116, 1992–1997. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane Crosstalk between the Extracellular Matrix and the Cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef]
- Moore, S.W.; Roca-Cusachs, P.; Sheetz, M.P. Stretchy Proteins on Stretchy Substrates: The Important Elements of Integrin-Mediated Rigidity Sensing. Dev. Cell 2010, 19, 194–206. [Google Scholar] [CrossRef]
- Jiao, R.; Cui, D.; Wang, S.C.; Li, D.; Wang, Y.-F. Interactions of the Mechanosensitive Channels with Extracellular Matrix, Integrins, and Cytoskeletal Network in Osmosensation. Front. Mol. Neurosci. 2017, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Joseph, E.K.; Reichling, D.B.; Levine, J.D. Interaction of Transient Receptor Potential Vanilloid 4, Integrin, and Src Tyrosine Kinase in Mechanical Hyperalgesia. J. Neurosci. 2008, 28, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.D.; Thodeti, C.K.; Tytell, J.D.; Mammoto, A.; Overby, D.R.; Ingber, D.E. Ultra-Rapid Activation of TRPV4 Ion Channels by Mechanical Forces Applied to Cell Surface Β1 Integrins. Integr. Biol. 2010, 2, 435. [Google Scholar] [CrossRef] [PubMed]
- Potla, R.; Hirano-Kobayashi, M.; Wu, H.; Chen, H.; Mammoto, A.; Matthews, B.D.; Ingber, D.E. Molecular Mapping of Transmembrane Mechanotransduction through the Β1 Integrin-CD98hc-TRPV4 Axis. J. Cell Sci. 2020, 133, jcs248823. [Google Scholar] [CrossRef] [PubMed]
- Jeske, N.A.; Patwardhan, A.M.; Henry, M.A.; Milam, S.B. Fibronectin Stimulates TRPV1 Translocation in Primary Sensory Neurons. J. Neurochem. 2009, 108, 591–600. [Google Scholar] [CrossRef]
- Higashikawa, A.; Kojima, Y.; Sato, M.; Kimura, M.; Ogura, K.; Mochizuki, H.; Sase, T.; Shinya, A.; Kobune, K.; Furuya, T.; et al. Transient Receptor Potential Cation Channel Subfamily Vanilloid Member 3 Is Not Involved in Plasma Membrane Stretch-Induced Intracellular Calcium Signaling in Merkel Cells. Bull. Tokyo Dent. Coll. 2015, 56, 259–262. [Google Scholar] [CrossRef]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.; Huang, S.M.; et al. TRP Channel Regulates EGFR Signaling in Hair Morphogenesis and Skin Barrier Formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef]
- Ungefroren, H.; Witte, D.; Lehnert, H. The Role of Small GTPases of the Rho/Rac Family in TGF-β-Induced EMT and Cell Motility in Cancer: ROLE OF SMALL GTPASES IN CANCER. Dev. Dyn. 2018, 247, 451–461. [Google Scholar] [CrossRef]
- Phelps, C.B.; Wang, R.R.; Choo, S.S.; Gaudet, R. Differential Regulation of TRPV1, TRPV3, and TRPV4 Sensitivity through a Conserved Binding Site on the Ankyrin Repeat Domain. J. Biol. Chem. 2010, 285, 731–740. [Google Scholar] [CrossRef]
- Villalobo, A.; Berchtold, M.W. The Role of Calmodulin in Tumor Cell Migration, Invasiveness, and Metastasis. Int. J. Mol. Sci. 2020, 21, 765. [Google Scholar] [CrossRef]
- Bödding, M. TRP Proteins and Cancer. Cell. Signal. 2007, 19, 617–624. [Google Scholar] [CrossRef]
- Bernardini, M.; Fiorio Pla, A.; Prevarskaya, N.; Gkika, D. Human Transient Receptor Potential (TRP) Channel Expression Profiling in Carcinogenesis. Int. J. Dev. Biol. 2015, 59, 399–406. [Google Scholar] [CrossRef]
- Weber, L.V.; Al-Refae, K.; Wölk, G.; Bonatz, G.; Altmüller, J.; Becker, C.; Gisselmann, G.; Hatt, H. Expression and Functionality of TRPV1 in Breast Cancer Cells. Breast Cancer Targets Ther. 2016, 8, 243–252. [Google Scholar] [CrossRef]
- Czifra, G.; Varga, A.; Nyeste, K.; Marincsák, R.; Tóth, B.I.; Kovács, I.; Kovács, L.; Bíró, T. Increased Expressions of Cannabinoid Receptor-1 and Transient Receptor Potential Vanilloid-1 in Human Prostate Carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 507–514. [Google Scholar] [CrossRef]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-Induced Apoptosis of Glioma Cells Is Mediated by TRPV1 Vanilloid Receptor and Requires P38 MAPK Activation: Role of TRPV1 in the Glioma Cell Apoptosis. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef]
- Amantini, C.; Ballarini, P.; Caprodossi, S.; Nabissi, M.; Morelli, M.B.; Lucciarini, R.; Cardarelli, M.A.; Mammana, G.; Santoni, G. Triggering of Transient Receptor Potential Vanilloid Type 1 (TRPV1) by Capsaicin Induces Fas/CD95-Mediated Apoptosis of Urothelial Cancer Cells in an ATM-Dependent Manner. Carcinogenesis 2009, 30, 1320–1329. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Cheng, X.; Yu, H.; Bao, J.; Lu, R. Capsaicin Inhibits the Metastasis of Human Papillary Thyroid Carcinoma BCPAP Cells through the Modulation of the TRPV1 Channel. Food Funct. 2018, 9, 344–354. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity—Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Kalogris, C.; Caprodossi, S.; Amantini, C.; Lambertucci, F.; Nabissi, M.; Morelli, M.B.; Farfariello, V.; Filosa, A.; Emiliozzi, M.C.; Mammana, G.; et al. Expression of Transient Receptor Potential Vanilloid-1 (TRPV1) in Urothelial Cancers of Human Bladder: Relation to Clinicopathological and Molecular Parameters: TRPV1 as Prognostic Marker for Human Ucs. Histopathology 2010, 57, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Malagarie-Cazenave, S.; Olea-Herrero, N.; Vara, D.; Díaz-Laviada, I. Capsaicin, a Component of Red Peppers, Induces Expression of Androgen Receptor via PI3K and MAPK Pathways in Prostate LNCaP Cells. FEBS Lett. 2009, 583, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Amantini, C.; Nabissi, M.; Liberati, S.; Cardinali, C.; Farfariello, V.; Tomassoni, D.; Quaglia, W.; Piergentili, A.; Bonifazi, A.; et al. Cross-Talk between Alpha1D-Adrenoceptors and Transient Receptor Potential Vanilloid Type 1 Triggers Prostate Cancer Cell Proliferation. BMC Cancer 2014, 14, 921. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Sánchez, M.G.; Malagarie-Cazenave, S.; Olea, N.; Díaz-Laviada, I. Induction of Apoptosis in Prostate Tumor PC-3 Cells and Inhibition of Xenograft Prostate Tumor Growth by the Vanilloid Capsaicin. Apoptosis 2006, 11, 89–99. [Google Scholar] [CrossRef]
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Racca, S.; Abbadessa, G.; Piccione, F.; Re, G. Transient Receptor Potential Vanilloid 1 Expression and Functionality in MCF-7 Cells: A Preliminary Investigation. J. Breast Cancer 2014, 17, 332. [Google Scholar] [CrossRef]
- Waning, J.; Vriens, J.; Owsianik, G.; Stüwe, L.; Mally, S.; Fabian, A.; Frippiat, C.; Nilius, B.; Schwab, A. A Novel Function of Capsaicin-Sensitive TRPV1 Channels: Involvement in Cell Migration. Cell Calcium 2007, 42, 17–25. [Google Scholar] [CrossRef]
- Liberati, S.; Morelli, M.; Amantini, C.; Farfariello, V.; Santoni, M.; Conti, A.; Nabissi, M.; Cascinu, S.; Santoni, G. Loss of TRPV2 Homeostatic Control of Cell Proliferation Drives Tumor Progression. Cells 2014, 3, 112–128. [Google Scholar] [CrossRef]
- Santoni, G.; Amantini, C.; Maggi, F.; Marinelli, O.; Santoni, M.; Nabissi, M.; Morelli, M.B. The TRPV2 Cation Channels: From Urothelial Cancer Invasiveness to Glioblastoma Multiforme Interactome Signature. Lab. Investig. 2020, 100, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Nizamuddin, P.B.; Uddin, S.; Al-Thani, M.; Frenneaux, M.P.; Janahi, I.A.; Steinhoff, M.; Azizi, F. TRPV2: A Cancer Biomarker and Potential Therapeutic Target. Dis. Markers 2020, 2020, 8892312. [Google Scholar] [CrossRef] [PubMed]
- Caprodossi, S.; Lucciarini, R.; Amantini, C.; Nabissi, M.; Canesin, G.; Ballarini, P.; Di Spilimbergo, A.; Cardarelli, M.A.; Servi, L.; Mammana, G.; et al. Transient Receptor Potential Vanilloid Type 2 (TRPV2) Expression in Normal Urothelium and in Urothelial Carcinoma of Human Bladder: Correlation with the Pathologic Stage. Eur. Urol. 2008, 54, 612–620. [Google Scholar] [CrossRef]
- Liu, G.; Xie, C.; Sun, F.; Xu, X.; Yang, Y.; Zhang, T.; Deng, Y.; Wang, D.; Huang, Z.; Yang, L.; et al. Clinical Significance of Transient Receptor Potential Vanilloid 2 Expression in Human Hepatocellular Carcinoma. Cancer Genet. Cytogenet. 2010, 197, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Monet, M.; Lehen’kyi, V.; Gackiere, F.; Firlej, V.; Vandenberghe, M.; Roudbaraki, M.; Gkika, D.; Pourtier, A.; Bidaux, G.; Slomianny, C.; et al. Role of Cationic Channel TRPV2 in Promoting Prostate Cancer Migration and Progression to Androgen Resistance. Cancer Res. 2010, 70, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, S.-S.; Yan, Y.; Zhao, S. Overexpression of Transient Receptor Potential Vanilloid 2 Is Associated with Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma. Med. Oncol. 2014, 31, 17. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, F.; Yang, Y.; Ma, W.; Lin, Z.; Cheng, N.; Long, Y.; Deng, S.; Li, Z. Recurrent Activations of Transient Receptor Potential Vanilloid-1 and Vanilloid-4 Promote Cellular Proliferation and Migration in Esophageal Squamous Cell Carcinoma Cells. FEBS Open Bio 2019, 9, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Oishi, Y.; Doi, I.; Shibata, H.; Kojima, I. Inhibition of Proliferation of MCF-7 Breast Cancer Cells by a Blocker of Ca2+-Permeable Channel. Cell Calcium 1997, 22, 75–82. [Google Scholar] [CrossRef]
- Kojima, I.; Nagasawa, M. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Frontiers in neuroscience; CRC/Taylor & Francis: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-4048-2. [Google Scholar]
- Oulidi, A.; Bokhobza, A.; Gkika, D.; Vanden Abeele, F.; Lehen’kyi, V.; Ouafik, L.; Mauroy, B.; Prevarskaya, N. TRPV2 Mediates Adrenomedullin Stimulation of Prostate and Urothelial Cancer Cell Adhesion, Migration and Invasion. PLoS ONE 2013, 8, e64885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X. Effect of TRPV2 Cation Channels on the Proliferation, Migration and Invasion of 5637 Bladder Cancer Cells. Exp. Ther. Med. 2013, 6, 1277–1282. [Google Scholar] [CrossRef]
- Mizuno, H.; Suzuki, Y.; Watanabe, M.; Sokabe, T.; Yamamoto, T.; Hattori, R.; Gotoh, M.; Tominaga, M. Potential Role of Transient Receptor Potential (TRP) Channels in Bladder Cancer Cells. J. Physiol. Sci. 2014, 64, 305–314. [Google Scholar] [CrossRef]
- Ma, W.; Li, C.; Yin, S.; Liu, J.; Gao, C.; Lin, Z.; Huang, R.; Huang, J.; Li, Z. Novel Role of TRPV2 in Promoting the Cytotoxicity of H2O2-Mediated Oxidative Stress in Human Hepatoma Cells. Free Radic. Biol. Med. 2015, 89, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 Channel by Cannabidiol Sensitizes Glioblastoma Cells to Cytotoxic Chemotherapeutic Agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef]
- Nilius, B.; Bíró, T. TRPV3: A ‘More than Skinny’ Channel. Exp. Dermatol. 2013, 22, 447–452. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Fan, K.; Li, B.; Li, H.; Qi, H.; Guo, J.; Cao, Y.; Sun, H. Overexpression of TRPV3 Correlates with Tumor Progression in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2016, 17, 437. [Google Scholar] [CrossRef] [PubMed]
- Bahari, N.N.; Jamaludin, S.Y.N.; Jahidin, A.H.; Zahary, M.N.; Mohd Hilmi, A.B.; Bakar, N.H.A.; Ali, A.M. The Emerging Roles of TRPV4 in Cancer. Biomed. Pharmacol. J. 2017, 10, 1757–1764. [Google Scholar] [CrossRef]
- Yu, S.; Huang, S.; Ding, Y.; Wang, W.; Wang, A.; Lu, Y. Transient Receptor Potential Ion-Channel Subfamily V Member 4: A Potential Target for Cancer Treatment. Cell Death Dis. 2019, 10, 497. [Google Scholar] [CrossRef]
- Lee, W.H.; Choong, L.Y.; Mon, N.N.; Lu, S.; Lin, Q.; Pang, B.; Yan, B.; Krishna, V.S.R.; Singh, H.; Tan, T.Z.; et al. TRPV4 Regulates Breast Cancer Cell Extravasation, Stiffness and Actin Cortex. Sci. Rep. 2016, 6, 27903. [Google Scholar] [CrossRef]
- Lee, W.H.; Choong, L.Y.; Jin, T.H.; Mon, N.N.; Chong, S.; Liew, C.S.; Putti, T.; Lu, S.Y.; Harteneck, C.; Lim, Y.P. TRPV4 Plays a Role in Breast Cancer Cell Migration via Ca2+-Dependent Activation of AKT and Downregulation of E-Cadherin Cell Cortex Protein. Oncogenesis 2017, 6, e338. [Google Scholar] [CrossRef]
- So, C.L.; Milevskiy, M.J.G.; Monteith, G.R. Transient Receptor Potential Cation Channel Subfamily V and Breast Cancer. Lab. Investig. 2020, 100, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Olivan-Viguera, A.; Garcia-Otin, A.L.; Lozano-Gerona, J.; Abarca-Lachen, E.; Garcia-Malinis, A.J.; Hamilton, K.L.; Gilaberte, Y.; Pueyo, E.; Köhler, R. Pharmacological Activation of TRPV4 Produces Immediate Cell Damage and Induction of Apoptosis in Human Melanoma Cells and HaCaT Keratinocytes. PLoS ONE 2018, 13, e0190307. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Kishida, S.; Kido, M.A.; Yoshikawa, H.; Ozeki, S.; et al. The TRPV4-AKT Axis Promotes Oral Squamous Cell Carcinoma Cell Proliferation via CaMKII Activation. Lab. Investig. J. Tech. Methods Pathol. 2020, 100, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Shen, Y.-X.; Yuan, Y.-F. Expression and Prognostic Roles of TRPV5 and TRPV6 in Non-Small Cell Lung Cancer after Curative Resection. Asian Pac. J. Cancer Prev. 2014, 15, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Huhn, S.; Bevier, M.; Pardini, B.; Naccarati, A.; Vodickova, L.; Novotny, J.; Vodicka, P.; Hemminki, K.; Försti, A. Colorectal Cancer Risk and Patients’ Survival: Influence of Polymorphisms in Genes Somatically Mutated in Colorectal Tumors. Cancer Causes Control 2014, 25, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Raphaël, M.; Lehen’kyi, V.; Vandenberghe, M.; Beck, B.; Khalimonchyk, S.; Vanden Abeele, F.; Farsetti, L.; Germain, E.; Bokhobza, A.; Mihalache, A.; et al. TRPV6 Calcium Channel Translocates to the Plasma Membrane via Orai1-Mediated Mechanism and Controls Cancer Cell Survival. Proc. Natl. Acad. Sci. USA 2014, 111, E3870–E3879. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Xie, X.; Wen, J.; Luo, K.-J.; Liu, Q.; Yang, H.; Hu, Y.; Fu, J.-H. TRPV6 Plays a New Role in Predicting Survival of Patients with Esophageal Squamous Cell Carcinoma. Diagn. Pathol. 2016, 11, 14. [Google Scholar] [CrossRef]
- Wissenbach, U.; Niemeyer, B.A.; Fixemer, T.; Schneidewind, A.; Trost, C.; Cavalié, A.; Reus, K.; Meese, E.; Bonkhoff, H.; Flockerzi, V. Expression of CaT-like, a Novel Calcium-Selective Channel, Correlates with the Malignancy of Prostate Cancer. J. Biol. Chem. 2001, 276, 19461–19468. [Google Scholar] [CrossRef]
- Fixemer, T.; Wissenbach, U.; Flockerzi, V.; Bonkhoff, H. Expression of the Ca2+-Selective Cation Channel TRPV6 in Human Prostate Cancer: A Novel Prognostic Marker for Tumor Progression. Oncogene 2003, 22, 7858–7861. [Google Scholar] [CrossRef]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High Expression of Transient Receptor Potential Channels in Human Breast Cancer Epithelial Cells and Tissues: Correlation with Pathological Parameters. Cell. Physiol. Biochem. 2011, 28, 813–822. [Google Scholar] [CrossRef]
- Parekh, A.B. Decoding Cytosolic Ca2+ Oscillations. Trends Biochem. Sci. 2011, 36, 78–87. [Google Scholar] [CrossRef]
- Shields, J.M.; Pruitt, K.; McFall, A.; Shaub, A.; Der, C.J. Understanding Ras: ‘It Ain’t over ’til It’s over’. Trends Cell Biol. 2000, 10, 147–154. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. RHO–GTPases and Cancer. Nat. Rev. Cancer 2002, 2, 133–142. [Google Scholar] [CrossRef]
- Clayton, N.S.; Ridley, A.J. Targeting Rho GTPase Signaling Networks in Cancer. Front. Cell Dev. Biol. 2020, 8, 222. [Google Scholar] [CrossRef]
- Fiorio Pla, A.; Gkika, D. Emerging Role of TRP Channels in Cell Migration: From Tumor Vascularization to Metastasis. Front. Physiol. 2013, 4, 311. [Google Scholar] [CrossRef]
- Canales, J.; Morales, D.; Blanco, C.; Rivas, J.; Díaz, N.; Angelopoulos, I.; Cerda, O. A TR(i)P to Cell Migration: New Roles of TRP Channels in Mechanotransduction and Cancer. Front. Physiol. 2019, 10, 757. [Google Scholar] [CrossRef]
- Gogebakan, B.; Bayraktar, R.; Suner, A.; Balakan, O.; Ulasli, M.; Izmirli, M.; Oztuzcu, S.; Camci, C. Do Fasudil and Y-27632 Affect the Level of Transient Receptor Potential (TRP) Gene Expressions in Breast Cancer Cell Lines? Tumor Biol. 2014, 35, 8033–8041. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Kojima, I. Translocation of TRPV2 Channel Induced by Focal Administration of Mechanical Stress. Physiol. Rep. 2015, 3, e12296. [Google Scholar] [CrossRef] [PubMed]
- Monet, M.; Gkika, D.; Lehen’kyi, V.; Pourtier, A.; Abeele, F.V.; Bidaux, G.; Juvin, V.; Rassendren, F.; Humez, S.; Prevarsakaya, N. Lysophospholipids Stimulate Prostate Cancer Cell Migration via TRPV2 Channel Activation. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2009, 1793, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Laragione, T.; Harris, C.; Gulko, P.S. TRPV2 Suppresses Rac1 and RhoA Activation and Invasion in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Int. Immunopharmacol. 2019, 70, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.M.; Greenlee, J.D.; King, M.R. Mechanosensitive Ion Channels: TRPV4 and P2X7 in Disseminating Cancer Cells. Cancer J. 2018, 24, 84–92. [Google Scholar] [CrossRef]
- Vriens, J.; Janssens, A.; Prenen, J.; Nilius, B.; Wondergem, R. TRPV Channels and Modulation by Hepatocyte Growth Factor/Scatter Factor in Human Hepatoblastoma (HepG2) Cells. Cell Calcium 2004, 36, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Xu, J.; Xiao, Y.; Wu, J.; Wan, H.; Tang, B.; Liu, J.; Fan, Y.; Wang, S.; Wu, Y.; et al. Calcium Promotes Human Gastric Cancer via a Novel Coupling of Calcium-Sensing Receptor and TRPV4 Channel. Cancer Res. 2017, 77, 6499–6512. [Google Scholar] [CrossRef] [PubMed]
- McCray, B.A.; Diehl, E.; Sullivan, J.M.; Aisenberg, W.H.; Zaccor, N.W.; Lau, A.R.; Rich, D.J.; Goretzki, B.; Hellmich, U.A.; Lloyd, T.E.; et al. Neuropathy-Causing TRPV4 Mutations Disrupt TRPV4-RhoA Interactions and Impair Neurite Extension. Nat. Commun. 2021, 12, 1444. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Vaughan, M.B.; Kropp, B.P.; Gabbiani, G.; Martin, M.D.; Haaksma, C.J.; Hinz, B. Contraction of Myofibroblasts in Granulation Tissue Is Dependent on Rho/Rho Kinase/Myosin Light Chain Phosphatase Activity: Contraction of Myofibroblasts Is Dependent on Phosphatase Activity. Wound Repair Regen. 2006, 14, 313–320. [Google Scholar] [CrossRef]
- Thodeti, C.K.; Paruchuri, S.; Meszaros, J.G. A TRP to Cardiac Fibroblast Differentiation. Channels 2013, 7, 211–214. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 Promote Metastasis by Regulating Cytoskeleton through the RhoA/ROCK1 Pathway in Endometrial Cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Ou-yang, Q.; Li, B.; Xu, M.; Liang, H. TRPV4 Promotes the Migration and Invasion of Glioma Cells via AKT/Rac1 Signaling. Biochem. Biophys. Res. Commun. 2018, 503, 876–881. [Google Scholar] [CrossRef]
- Basson, M.D. An Intracellular Signal Pathway That Regulates Cancer Cell Adhesion in Response to Extracellular Forces. Cancer Res. 2008, 68, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Mrkonjić, S.; Garcia-Elias, A.; Pardo-Pastor, C.; Bazellières, E.; Trepat, X.; Vriens, J.; Ghosh, D.; Voets, T.; Vicente, R.; Valverde, M.A. TRPV4 Participates in the Establishment of Trailing Adhesions and Directional Persistence of Migrating Cells. Pflüg. Arch.-Eur. J. Physiol. 2015, 467, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Sokabe, T.; Tominaga, M. The TRPV4 Cation Channel: A Molecule Linking Skin Temperature and Barrier Function. Commun. Integr. Biol. 2010, 3, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Deng, S.; Gopal, V.; Yap, K.C.-H.; Halim, C.E.; Lye, M.L.; Ong, M.S.; Tan, T.Z.; Sethi, G.; Hooi, S.C.; et al. Cytoskeletal Dynamics in Epithelial-Mesenchymal Transition: Insights into Therapeutic Targets for Cancer Metastasis. Cancers 2021, 13, 1882. [Google Scholar] [CrossRef]
- Azimi, I.; Robitaille, M.; Armitage, K.; So, C.L.; Milevskiy, M.J.G.; Northwood, K.; Lim, H.F.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Activation of the Ion Channel TRPV4 Induces Epithelial to Mesenchymal Transition in Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9417. [Google Scholar] [CrossRef]
- Conlon, G.A.; Murray, G.I. Recent Advances in Understanding the Roles of Matrix Metalloproteinases in Tumour Invasion and Metastasis. J. Pathol. 2019, 247, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, D.; Branch, K.M.; Weaver, A.M. Signaling Inputs to Invadopodia and Podosomes. J. Cell Sci. 2013, 216, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- Peláez, R.; Pariente, A.; Pérez-Sala, Á.; Larrayoz, I.M. Integrins: Moonlighting Proteins in Invadosome Formation. Cancers 2019, 11, 615. [Google Scholar] [CrossRef]
- Alexander, N.R.; Branch, K.M.; Parekh, A.; Clark, E.S.; Iwueke, I.C.; Guelcher, S.A.; Weaver, A.M. Extracellular Matrix Rigidity Promotes Invadopodia Activity. Curr. Biol. 2008, 18, 1295–1299. [Google Scholar] [CrossRef]
- Haage, A.; Schneider, I.C. Cellular Contractility and Extracellular Matrix Stiffness Regulate Matrix Metalloproteinase Activity in Pancreatic Cancer Cells. FASEB J. 2014, 28, 3589–3599. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Boels, K.; Glassmeier, G.; Herrmann, D.; Riedel, I.B.; Hampe, W.; Kojima, I.; Schwarz, J.R.; Schaller, H.C. The Neuropeptide Head Activator Induces Activation and Translocation of the Growth-Factor-Regulated Ca(2+)-Permeable Channel GRC. J. Cell Sci. 2001, 114, 3599–3606. [Google Scholar] [CrossRef]
- Zhuge, Y.; Xu, J. Rac1 Mediates Type I Collagen-Dependent MMP-2 Activation. J. Biol. Chem. 2001, 276, 16248–16256. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, J.F.; Kocić, J.; Fabra, A.; Cano, A.; Quintanilla, M. Rac1 Modulates TGF-Β1-Mediated Epithelial Cell Plasticity and MMP9 Production in Transformed Keratinocytes. FEBS Lett. 2010, 584, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Kojima, I. Translocation of Calcium-Permeable TRPV2 Channel to the Podosome: Its Role in the Regulation of Podosome Assembly. Cell Calcium 2012, 51, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Hao, W.; Liu, J.; Li, Y.; Wang, B.; Zu, X.; Xue, H. Study on the Clinical Significance of TRPV2 and MMP2 Expressions in Ovarian Cancer. BIOCELL 2021, 45, 521–526. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Cappelli, H.C.; Kanugula, A.K.; Adapala, R.K.; Amin, V.; Sharma, P.; Midha, P.; Paruchuri, S.; Thodeti, C.K. Mechanosensitive TRPV4 Channels Stabilize VE-Cadherin Junctions to Regulate Tumor Vascular Integrity and Metastasis. Cancer Lett. 2019, 442, 15–20. [Google Scholar] [CrossRef]
- Wandall-Frostholm, C.; Dalsgaard, T.; Bajoriūnas, V.; Oliván-Viguera, A.; Sadda, V.; Beck, L.; Mogensen, S.; Stankevicius, E.; Simonsen, U.; Köhler, R. Genetic Deficit of K Ca 3.1 Channels Protects against Pulmonary Circulatory Collapse Induced by TRPV4 Channel Activation: Pulmonary K Ca 3.1 and Circulatory Collapse. Br. J. Pharmacol. 2015, 172, 4493–4505. [Google Scholar] [CrossRef]
- Gradilone, S.A.; Pisarello, M.J.; LaRusso, N.F. Primary Cilia in Tumor Biology: The Primary Cilium as a Therapeutic Target in Cholangiocarcinoma. Curr. Drug Targets 2017, 18, 958–963. [Google Scholar] [CrossRef]
- Vrenken, K.S.; Jalink, K.; van Leeuwen, F.N.; Middelbeek, J. Beyond Ion-Conduction: Channel-Dependent and -Independent Roles of TRP Channels during Development and Tissue Homeostasis. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2016, 1863, 1436–1446. [Google Scholar] [CrossRef]
- Piddini, E. Epithelial Homeostasis: A Piezo of the Puzzle. Curr. Biol. CB 2017, 27, R232–R234. [Google Scholar] [CrossRef]
- De Felice, D.; Alaimo, A. Mechanosensitive Piezo Channels in Cancer: Focus on Altered Calcium Signaling in Cancer Cells and in Tumor Progression. Cancers 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).