Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics
Abstract
:1. Introduction
2. Methods
Subjects
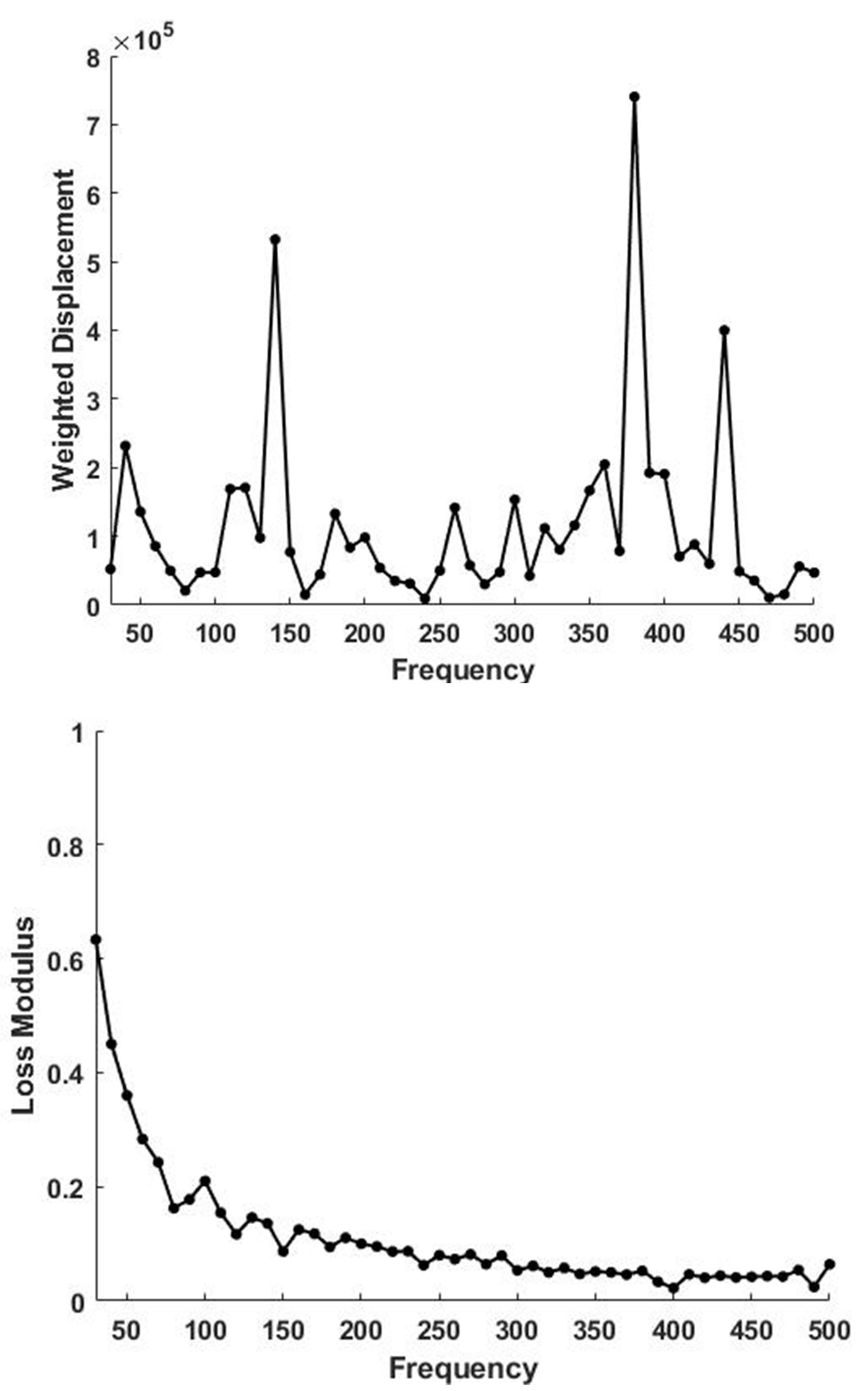
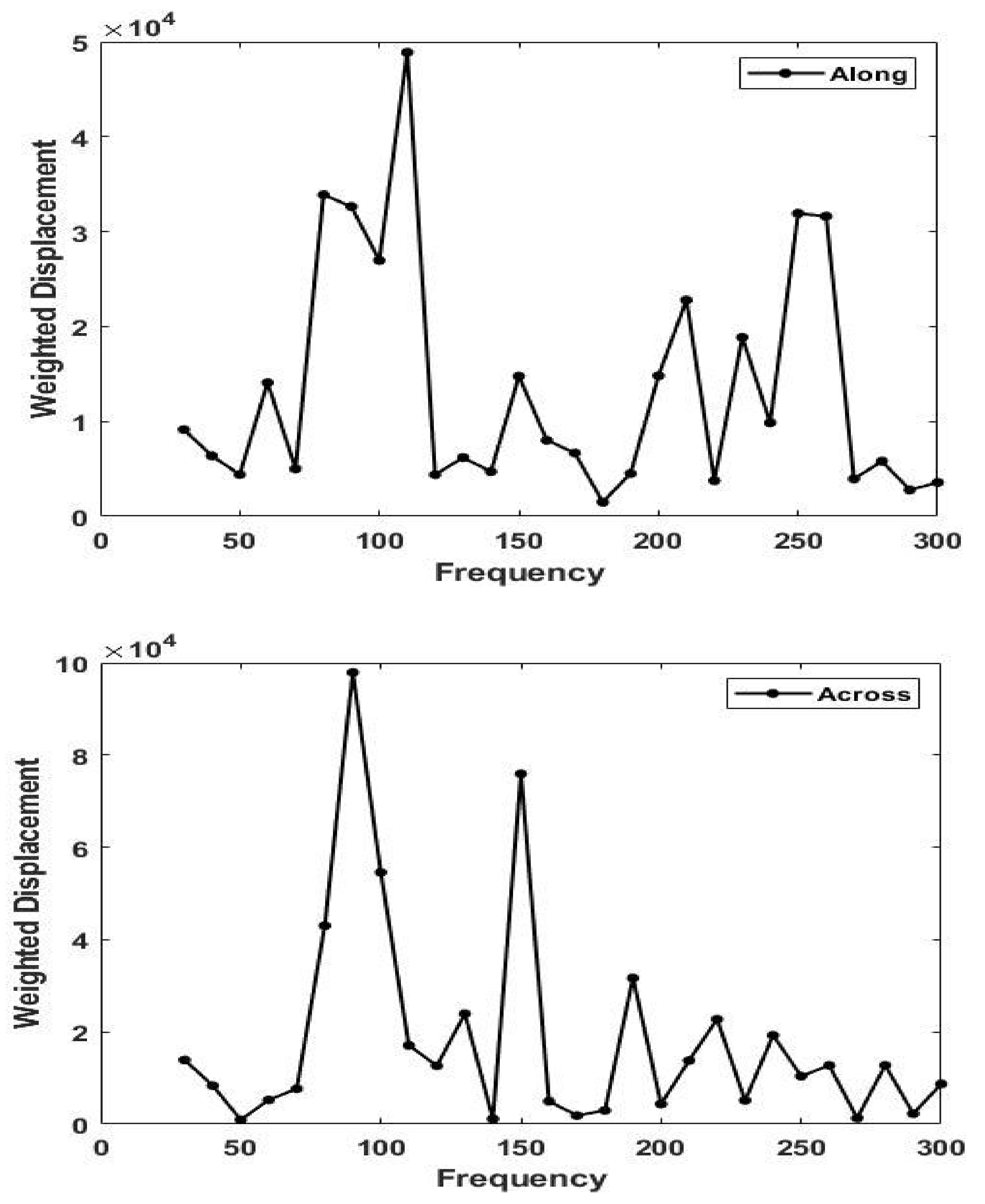
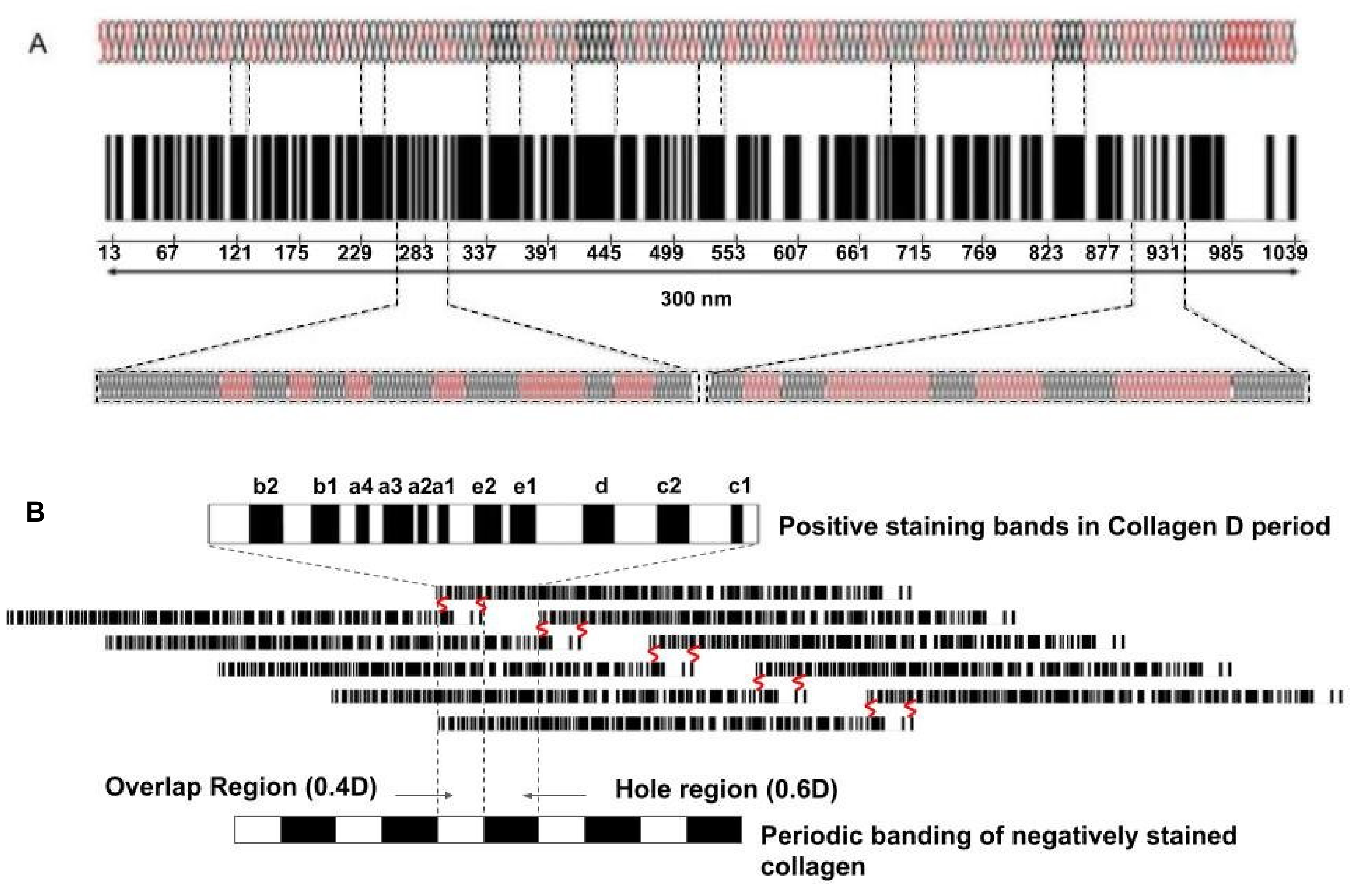
3. Results
4. Discussion
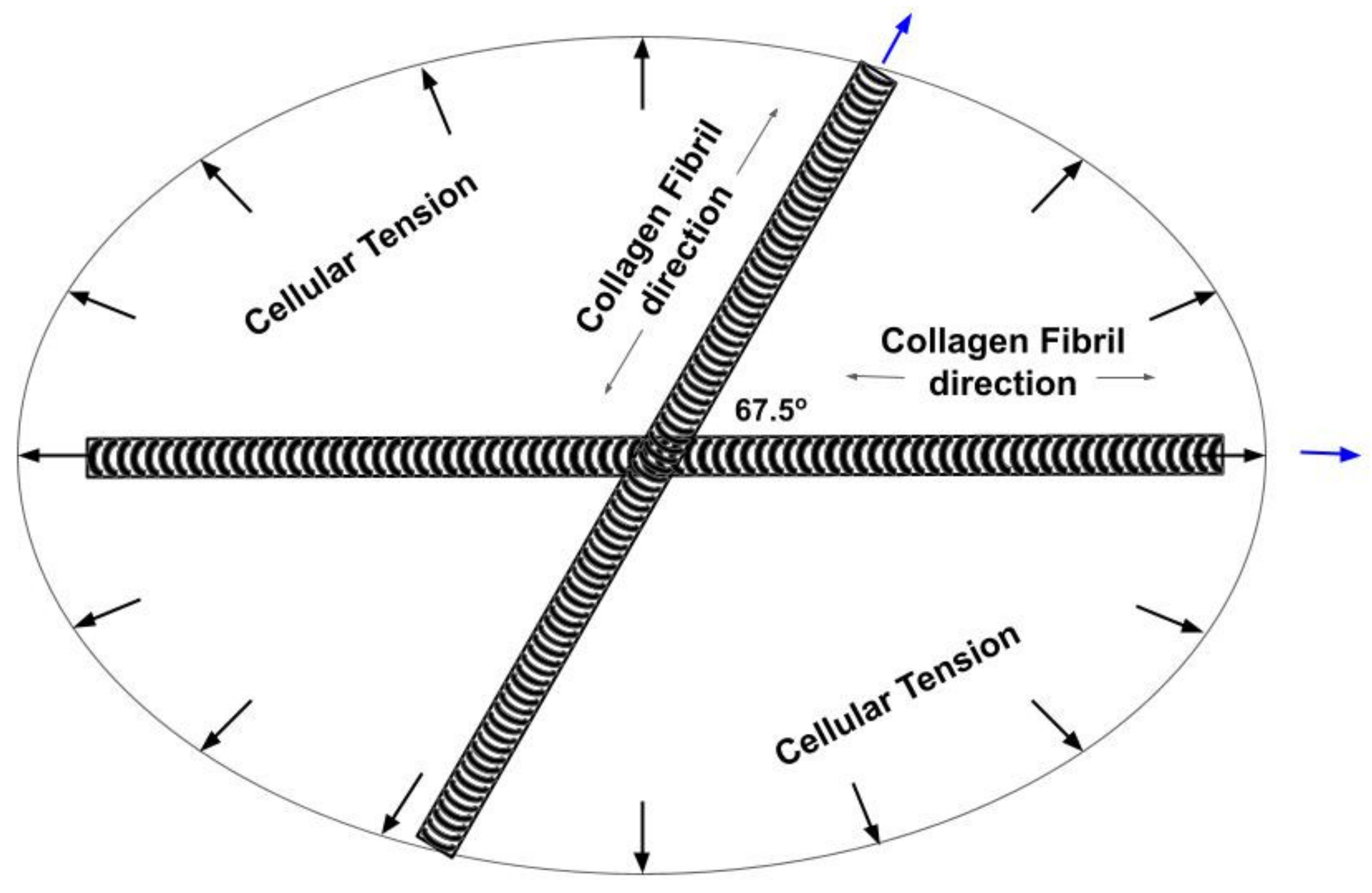
4.1. Langer’s Lines and the Direction of Collagen Fibers in Skin
4.2. Determination of the Orientation of the Collagen Fibers in Skin
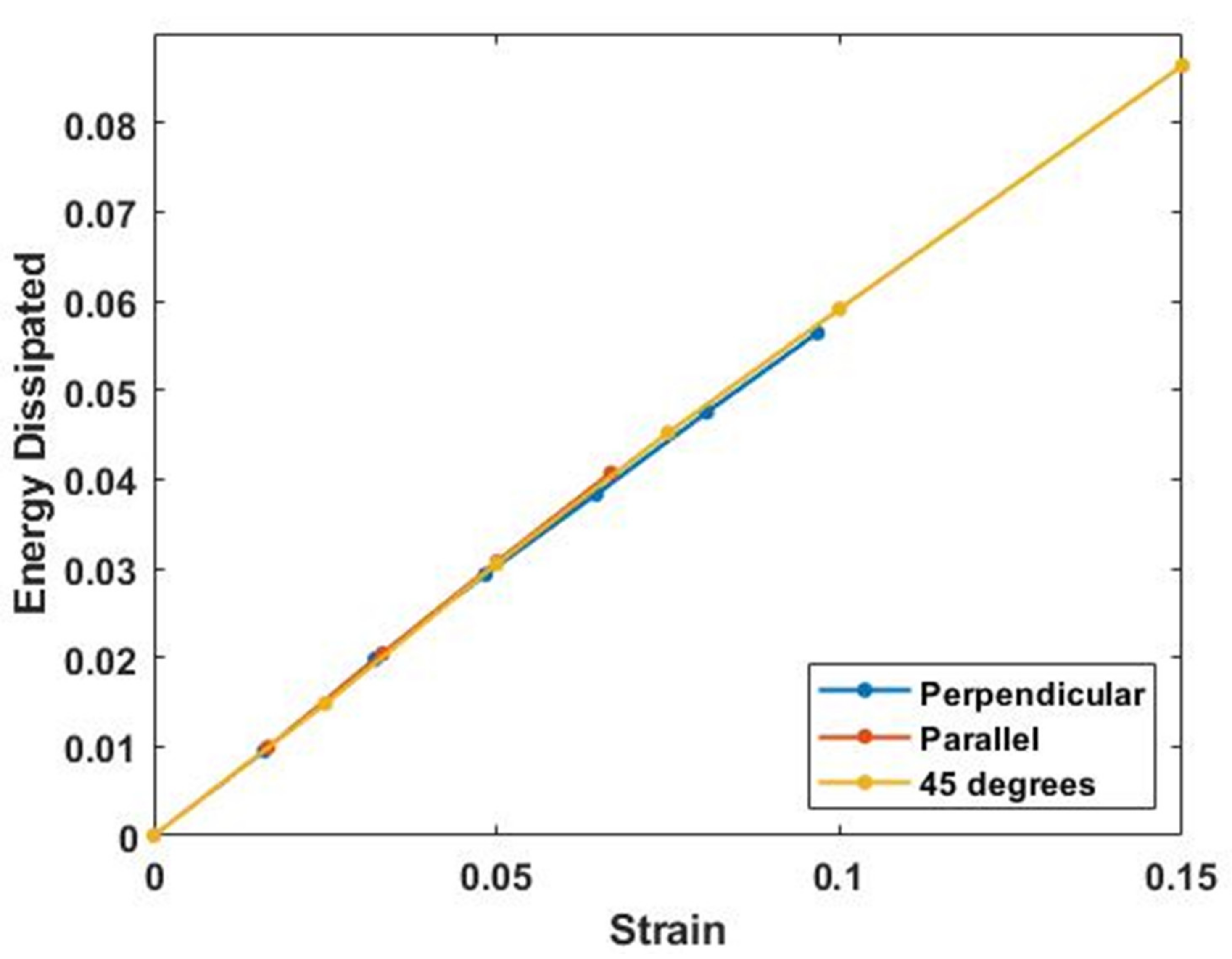
4.3. Energy Storage and Dissipation by Skin
4.4. Effects of Aging
4.5. Energy Storage and Dissipation in Tendon
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| PG | Proteoglycan |
| VOCT | vibrational optical coherence tomography |
| ACL | anterior cruciate ligament |
References
- Silver, F.H. Mechanosensing and Mechanochemical Transduction in Extracellular Matrix; Springer: New York, NY, USA, 2006. [Google Scholar]
- Franchi, M.; Trire, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen structure of tendon relates to function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef]
- Holmes, D.F.; Lu, Y.; Straborg, T.; Kadler, K.E. Collagen fibril assembly and function. Curr. Top. Dev. Biol. 2018, 130, 107–142. [Google Scholar]
- Silver, F.H.; Siperko, L.M. Mechanosensing and Mechanochemical Transduction. Crit. Rev. Biomed. Eng. 2003, 31, 255–331. [Google Scholar] [CrossRef]
- Roberts, T.J.; Marsh, R.L.; Weyand, P.G.; Taylor, C.R. Muscular force in running turkeys: The economy of minimizing work. Science 1997, 275, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.M. Animal Mechanics, 2nd ed.; Blackwell Scientific: Oxford, UK, 1983. [Google Scholar]
- Alexander, R.M. Elastic energy stores in running vertebrates. Acta Zool. 1984, 24, 85–94. [Google Scholar] [CrossRef]
- Wilson, A.M.; McGuigan, M.P.; Su, A.; van den Bogert, A.J. Horses damp the spring in their step. Nature 2001, 414, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.D.; Silver, F.H. Viscoelastic Behavior of Human Connective Tissues: Relative Contribution of Viscous and Elastic Components. Connect. Tissue Res. 1983, 12, 59–70. [Google Scholar] [CrossRef]
- Shah, R.G.; Devore, D.; Silver, F.H. Biomechanical analysis of decellularized dermis and skin: Initial in vivo observations using OCT and vibrational analysis. J. Biomed. Mat. Res. 2018, 106A, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.G.; Silver, F.H. Viscoelastic behavior of tissues and implant materials: Estimation of the elastic modulus and viscous contribution using optical coherence tomography and vibrational analysis. J. Biomed. Tech. Res. 2017, 3, 105–109. [Google Scholar]
- Hodge, A.J.; Petruska, J.A. Recent studies with the electron microscope on ordered aggregates of the tropocollagen macromolecule. In Aspects of Protein Structure; Ramachandran, G.N., Ed.; Academic Press: New York, NY, USA, 1963; pp. 289–300. [Google Scholar]
- Silver, F.H.; Horvath, I.; Foran, D.J. Mechanical implications of the domain structure of fibril forming collagens: Comparison of the molecular and fibrillar flexibility of α-chains found in types I, II and III collagens. J. Theor. Biol. 2002, 216, 243–254. [Google Scholar] [CrossRef]
- Fletcher, G.C. Dynamic light scattering from collagen solutions. I. Translational diffusion coefficient and aggregation effects. Biopolymers 1976, 15, 2201–2217. [Google Scholar] [CrossRef]
- Silver, F.H.; Langley, K.H.; Trelstad, R.L. Type I collagen fibrillogenesis: Initiation via a reversible linear growth step. Biopolymers 1979, 8, 2523–2535. [Google Scholar] [CrossRef]
- Silver, F.H.; Birk, D.E. Molecular structure of collagen in solution: Comparison of types I, II, III, and V. Int. J. Biol. Macromol. 1984, 6, 125–132. [Google Scholar] [CrossRef]
- Paterlini, M.G.; Nemethy, G.; Scheraga, H.A. The energy of formation of internal loops in triple-helical collagen polypeptides. Biopolymers 1995, 35, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Voss, T.; Kuhn, K.; Engle, J. Localization of flexible sites in thread-like molecules from electron micrographs: Comparison of interstitial, basement membrane and intima collagens. J. Mol. Biol. 1984, 172, 325–343. [Google Scholar] [CrossRef]
- Freeman, J.W.; Silver, F.H.; Woods, M.D.; Laurencin, C.T. The role of Type I collagen molecular structure in tendon elastic energy storage. Mater. Res. Soc. 2005, 874, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Mosler, E.; Folkhard, W.; Knorzer, E.; Nemetschek-Gansler, H.; Nemetschek, T.H.; Koch, M.H. Stress-induced molecular arrangement in tendon collagen. J. Mol. Biol. 1985, 182, 589–596. [Google Scholar] [CrossRef]
- Sasakai, N.; Odajima, S. Elongation mechanism of collagen fibrils and force-strain relationships of tendon at each level of structural hierarchy. J. Biomech. 1996, 9, 1131–1136. [Google Scholar] [CrossRef]
- Silver, F.H.; Christiansen, D.L.; Snowhill, P.B.; Chen, Y. Transition from viscous to elastic-based dependency of mechanical properties of self-assembled type I collagen fibers. J. Appl. Polym. Sci. 2001, 79, 134–142. [Google Scholar] [CrossRef]
- Pins, G.D.; Christiansen, D.L.; Patel, R.; Silver, F.H. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys. J. 1997, 73, 2164–2172. [Google Scholar] [CrossRef] [Green Version]
- Silver, F.H.; Freeman, J.; Seehra, G.P. Collagen self-assembly and development of matrix mechanical properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Liliana Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Kelsey, A.; Robinsona, M.S.; Carrie, E.; Barnuma, C.E.; Weiss, S.N.; Huegela, J.; Shetyea, S.S.; Linb, L.; Saezb, D.; Adams, S.M.; et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017, 64, 81–93. [Google Scholar]
- Dunkman, A.A.; Buckleya, M.R.; Mienaltowski, M.J.; Sheila, M.; Adams, S.M.; Thomas, S.J.; Satchella, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; et al. The tendon injury response is influenced by decorin and biglycan. Ann. Biomed. Eng. 2014, 42, 619–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Li, Q.; Wang, C.; Patel, P.; Adams, S.M.; Doyran, B.; Nia, H.T.; Oftadeh, R.; Zhou, S.; Li, C.Y.; et al. Decorin regulates the aggrecan network integrity and biomechanical functions of cartilage extracellular matrix. ACS Nano 2019, 13, 11320–11333. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Ateshian, G.A.; Lai, W.M.; Gu, W.Y. Effects of fixed charges on the stress-relaxation behavior of hydrated soft tissues in a confined compression problem. Int. J. Solids Struct. 1998, 35, 4945–4962. [Google Scholar] [CrossRef]
- Silver, F.H.; Bradica, G.; Tria, A. Elastic energy storage in human articular cartilage: Estimation of the elastic spring constant for type II collagen and changes associated with osteoarthritis. Matrix Biol. 2002, 21, 129–137. [Google Scholar] [CrossRef]
- Silver, F.H.; Horvath, I.; Kelkar, N.; Deshmukh, T.; Shah, R. In vivo biomechanical analysis of human tendon using vibrational optical coherence tomography: Preliminary results. J. Clin. Cases Rep. 2020, 4, 12–19. [Google Scholar]
- Silver, F.H.; Shah, R.G.; Silver, L.L. The use of vibrational optical coherence tomography in matching host tissue and implant mechanical properties. Biomater. Med. Appl. 2018, 2. [Google Scholar] [CrossRef]
- Silver, F.H.; Shah, R.G.; Richard, M.; Benedetto, D. Comparative “virtual biopsies” of normal skin and skin lesions using vibrational optical coherence tomography. Ski. Res. Tech. 2019, 25, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Kelkar, N.; Desmukh, T.; Horvath, I.; Shah, R.G. Mechano-Vibrational Spectroscopy of Tissues and Materials Using Vibrational Optical Coherence Tomography: A New Non-Invasive and Non-Destructive Technique. Recent Prog. Mater. 2020, 2. [Google Scholar] [CrossRef]
- Silver, F.H.; De Vore, D.; Shah, R. Biochemical, biophysical, and mechanical characterization of decellularized dermal implants. Mat. Sci. Appl. 2017, 8, 873–888. [Google Scholar] [CrossRef] [Green Version]
- Silver, F.H.; Kato, Y.P.; Ohno, M.; Wasserman, A.J. Analysis of mammalian connective tissue: Relationship between hierarchical structures and mechanical properties. J. Long Term Eff. Med. Implants. 1992, 2, 165–198. [Google Scholar] [PubMed]
- Kalath, S.; Tsipouras, P.; Silver, F.H. Non-invasive assessment of aortic mechanical properties. Ann. Biomed. Eng. 1986, 14, 513–524. [Google Scholar] [CrossRef]
- Kalath, S.; Tsipouras, P.; Silver, F.H. Increased aortic root stiffness associated with osteogenesis imperfecta. Ann. Biomed. Eng. 1987, 15, 91–99. [Google Scholar] [CrossRef]
- Silver, F.H.; Siperko, L.M.; Seehra, G.P. Mechanobiology of force transduction in dermis. Skin Res. Technol. 2002, 8, 1–21. [Google Scholar]
- Dunn, M.G.; Silver, F.H.; Swann, D.A. Mechanical analysis of hypertrophic scar tissue: Structural basis for apparent increased rigidity. J. Invest. Derm. 1985, 84, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. Photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Lavker, R. Cutaneous aging: Chronologic versus photoaging. In Photodamage; Gilchrest, B., Ed.; Blackwell: Cambridge, UK, 1995; Volume 1, pp. 123–135. [Google Scholar]
- Fore, J. A Review of skin and the effects of aging on skin structure and function. Wound Manag. Prev. 2006, 52, 1–9. [Google Scholar]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced expression of connective tissue growth factor (ctgf/ccn2) mediates collagen loss in chronologically aged human skin. J. Invest. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung-Won Shin, J.-W.; Kwon, S.-W.; Choi, J.-I.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, S.P.; Kjaer, M. The impact of loading, unloading, ageing and injury on the human tendon. J. Physiol. 2019, 597, 1283–1298. [Google Scholar] [CrossRef] [Green Version]
- Nagy, I.Z.; Von Hahn, H.P.; Verzar, F. Age-related alterations in the cell nuclei and the DNA content of rat tail tendon. Gerontologia 1969, 15, 258–264. [Google Scholar] [CrossRef]
- Langberg, H.; Rosendal, L.; Kjaer, M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J. Physiol. 2001, 534, 297–302. [Google Scholar] [CrossRef]
- Gardner, K.; Arnoczky, S.P.; Caballero, O.; Lavagnino, M. The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: An in vitro experimental study. Disabil. Rehabil. 2008, 30, 1523–1529. [Google Scholar] [CrossRef]
- Archambault, J.M.; Hart, D.A.; Herzog, W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect. Tissue Res. 2001, 42, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Tian, T.; Lavagnino, M.; Gradner, K.; Schuler, P.; Morse, P. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: The effects of strain frequency, strain magnitude, and cytosolic calcium. J. Orthop Res. 2002, 20, 947–952. [Google Scholar] [CrossRef]





| Tissue | Resonant Frequency (Hz) {SD} | Modulus E (MPa) {SD} |
|---|---|---|
| Bone | ||
| Lamellar Bone | 990 {10.00} | 173 {20} |
| Subchondral Bone | 586 {26.07} | 67.81 {11.11} |
| Ear and Lower Nasal cartilage | 290 {14.14} | 16.2 {1.74} |
| Upper Nasal Cartilage | 380 {14.14} | 30.4 {5.89} |
| Fat, Epidermal Cells | 40–70 {12.90} | 1.110 {0.25} |
| Fibrotic Tissue | 210 {10} | 10.84 {2.48} |
| Ligament | ||
| Anterior Cruciate Ligament (ACL) | 525 {7.07} | 53.9 {2.25} |
| Meniscus | 430 {14.14} | 31.4 {3.37} |
| Muscles | ||
| Bicep Muscle | 378 {16.02} | 29.6 {2.62} |
| Quadriceps Muscle | 365 {21.21} | 20.5 {2.32} |
| Nerve | ||
| Nerve | 266 {11.54} | 15.86 {2.24} |
| Normal Skin | 110 {7.38} | 2.15 {0.29} |
| Ocular | ||
| Cornea, Sclera | 140 {14.14} | 2.4 {0.14} |
| Tendon | ||
| Achilles Tendon | 440 {10.00} | 34.0 {5.98} |
| Flexor Digitorum Profundus Tendon | 370 {14.14} | 22.7 {9.42} |
| Patellar Tendon | 430 {5.77} | 33.8 {4.62} |
| Vascular | ||
| Carotid Artery | 136 {11.54} | 4.64 {0.98} |
| Radial Artery | 155 {11.98} | 3.66 {0.65} |
| Vein | 165 {7.07} | 4.84 {0.025} |
| Condition | Average Modulus Value | Standard Deviation | Sample Size | p Value | Significance |
|---|---|---|---|---|---|
| Control (No strain) | 2.34 × 106 | 6.91 × 105 | 17 | NA | NA |
| Strain Direction: 0 degree (5 Percent Strain) | 3.55 × 106 | 2.56 × 105 | 5 | 1.88 × 10−4 * | Significant |
| Strain direction: 67.5 degree (5 Percent Strain) | 3.03 × 106 | 4.07 × 105 | 6 | 1.31 × 10−2 * | Significant |
| Strain direction along 22.5, 45, 90, −67.5, −45, −22.5 degrees (5 Percent strain) | 2.54 × 106 | 3.12 × 105 | 27 | 1.56 × 10−1 | Not Significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, F.H.; Kelkar, N.; Deshmukh, T. Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics. Biomolecules 2021, 11, 1018. https://doi.org/10.3390/biom11071018
Silver FH, Kelkar N, Deshmukh T. Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics. Biomolecules. 2021; 11(7):1018. https://doi.org/10.3390/biom11071018
Chicago/Turabian StyleSilver, Frederick H., Nikita Kelkar, and Tanmay Deshmukh. 2021. "Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics" Biomolecules 11, no. 7: 1018. https://doi.org/10.3390/biom11071018
APA StyleSilver, F. H., Kelkar, N., & Deshmukh, T. (2021). Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics. Biomolecules, 11(7), 1018. https://doi.org/10.3390/biom11071018






