COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street?
Abstract
1. Introduction
2. Features of COVID-19 Vaccines and Thrombocytopenia
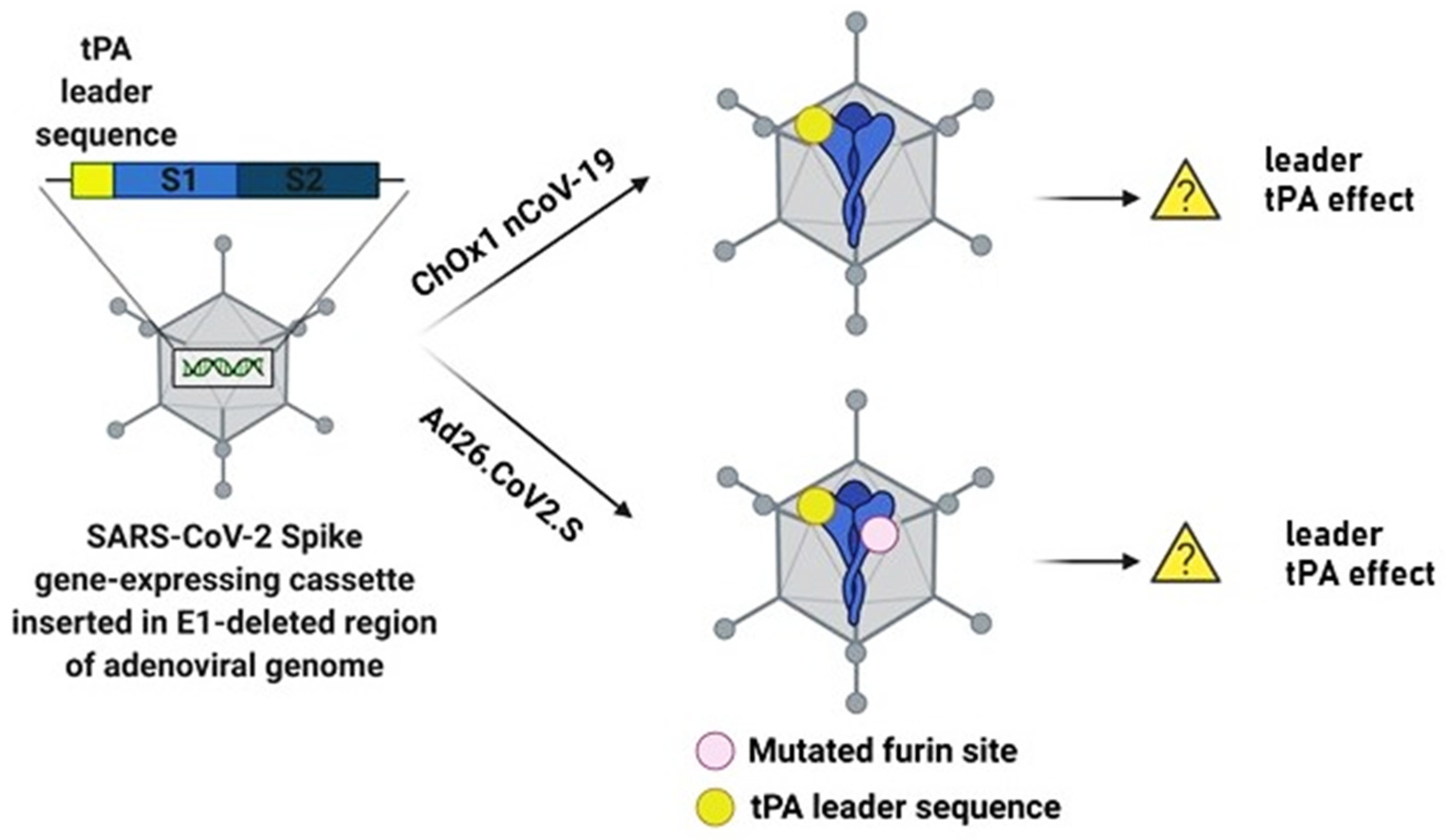
2.1. Tissue Plasminogen Activator (tPA) Leader Sequence and Thrombocytopenia Risk
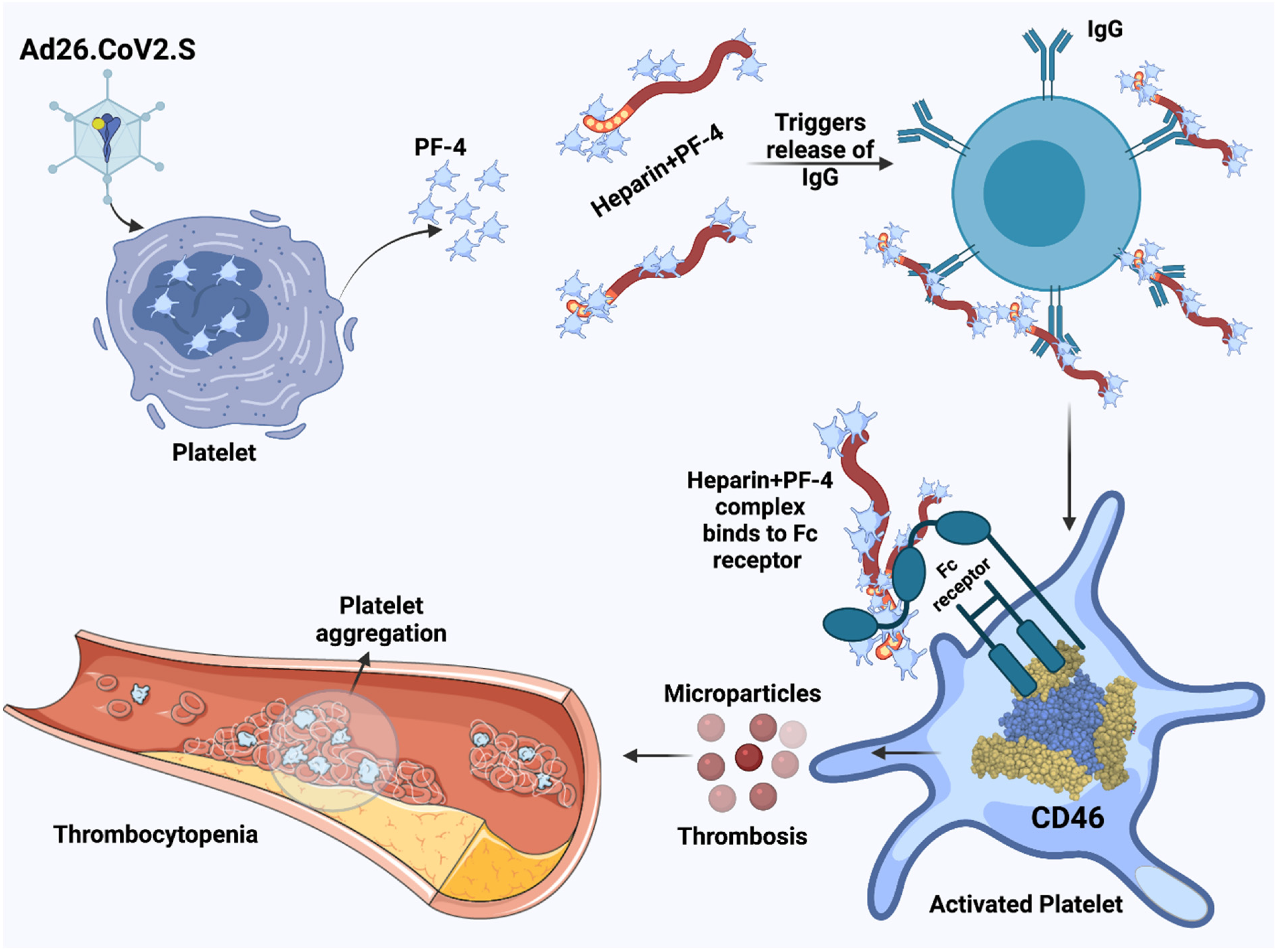
2.2. Adenovirus-Induced Thrombocytopenia
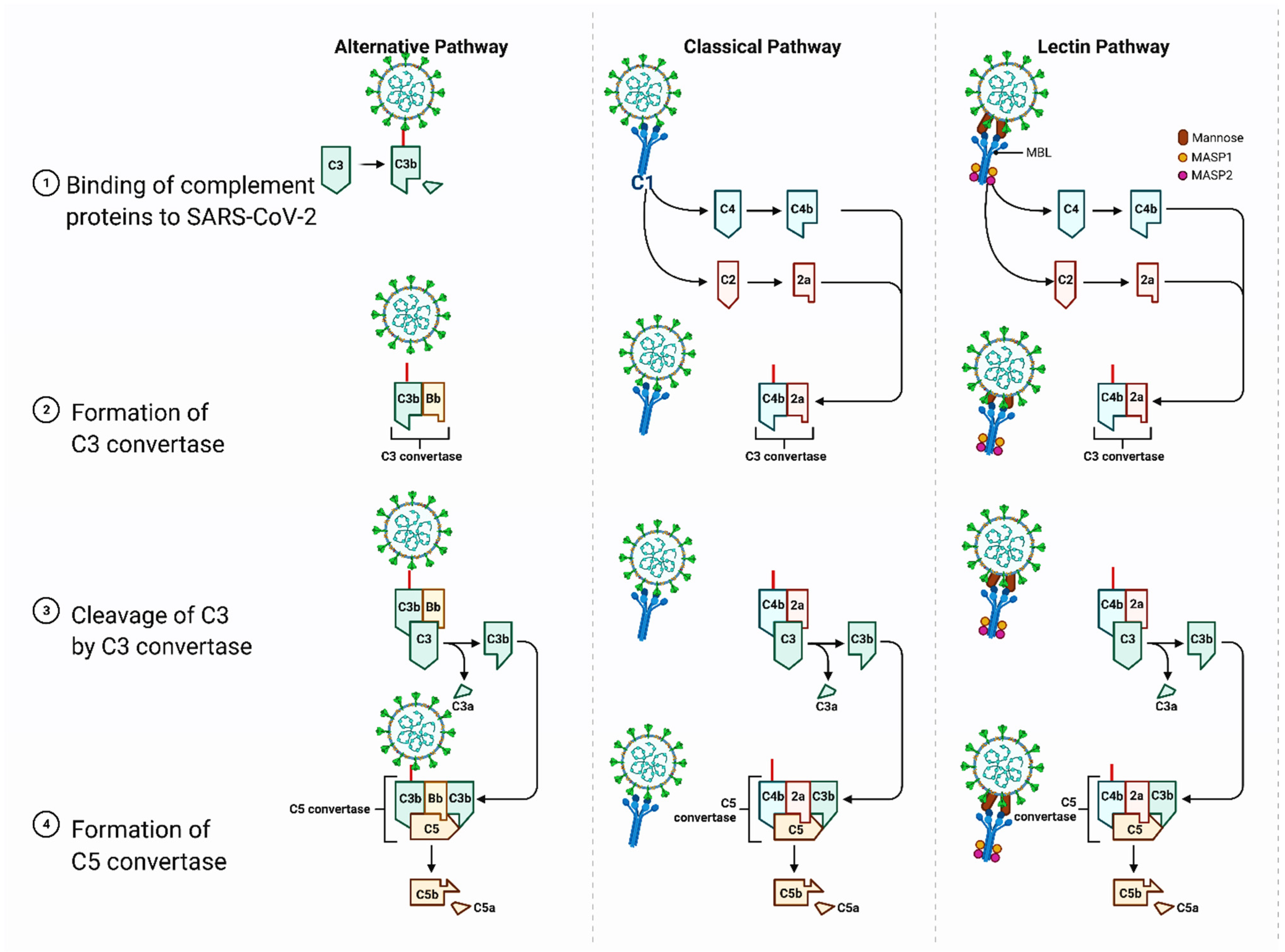
3. Structural and Biological Features of the Spike Protein and Thrombosis Risk
3.1. Heparin-Induced Thrombocytopenia and the Possible Role of Vaccines Containing the SARS-CoV-2 Spike Protein
3.2. Potential Role of the CLR DC-SIGN in the Development of Thrombosis
3.3. Potential Role of the CD147 Receptor in the Development of Thrombosis
4. Potential Risk Groups for Thrombosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Arashkia, A.; Jalilvand, S.; Mohajel, N.; Afchangi, A.; Azadmanesh, K.; Salehi-Vaziri, M.; Fazlalipour, M.; Pouriayevali, M.H.; Jalali, T.; Nasab, D.D.M.; et al. Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects. Rev. Med. Virol. 2020, e2183. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.E.; Paul, P.; MacCannell, D.R.; Johansson, M.A.; Brooks, J.T.; MacNeil, A.; Slayton, R.B.; Tong, S.; Silk, B.J.; Armstrong, G.L.; et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage—United States, 29 December 2020–12 January 2021. Morb. Mortal. Wkly Rep. 2021, 70, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.S.; Vihta, K.-D.; Gethings, O.; Pritchard, E.; Jones, J.; House, T.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; et al. Increased infections, but not viral burden, with new SARS-CoV variant. medRxiv 2021. [Google Scholar] [CrossRef]
- Jones, T.C.; Biele, G.; Mühlemann, B.; Veith, T.; Schneider, J.; Beheim-Schwarzbach, J.; Bleicker, T.; Tesch, J.; Schmidt, M.L.; Sander, L.E.; et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021, 373, eabi5273. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef]
- Gessler, F.; Schmitz, A.K.; Dubinski, D.; Bernstock, J.D.; Lehmann, F.; Won, S.-E.; Wittstock, M.; Güresir, E.; Hadjiathanasiou, A.; Zimmermann, J.; et al. Neurosurgical Considerations Regarding Decompressive Craniectomy for Intracerebral Hemorrhage after SASR-CoV-2-Vaccination in Vaccine Induced Thrombotic Thrombocytopenia—VITT. J. Clin. Med. 2021, 10, 2777. [Google Scholar] [CrossRef]
- Lee, E.-J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 95, 534–537. [Google Scholar] [CrossRef]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Harrington, R.A.; Sane, D.C.; Califf, R.M.; Sigmon, K.N.; Abbottsmith, C.W.; Candela, R.J.; Lee, K.L.; Topol, E.J. Clinical importance of thrombocytopenia occurring in the hospital phase after administration of thrombolytic therapy for acute myocardial infarction. The Thrombolysis and Angioplasty in Myocardial Infarction Study Group. J. Am. Coll. Cardiol. 1994, 23, 891–898. [Google Scholar] [CrossRef][Green Version]
- Bragin, I.; Chen, J.M. A Case Report of Recombinant Tissue Plasminogen Activator Use in a SPAN-100-Positive Geriatric Patient with Thrombocytopenia. Cureus 2017, 9, e1933. [Google Scholar] [CrossRef] [PubMed]
- Morena, J.; May, C.; Strohm, T. Acute Thrombocytopenia after Tissue Plasminogen Activator for Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104865. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; Wu, L.; Luo, Y.; Jiang, C.; Gupta, S.; Huang, Z.; Everett, G. Safety of intravenous thrombolysis for acute ischemic stroke in patients with thrombocytopenia. Cerebrovasc. Dis. 2019, 48, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, S.D.; Schmidt, M.; Horváth-Puhó, E.; Thomsen, R.W.; Toft Sørensen, H.T. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: Side-effect or coincidence? Lancet 2021, 397, 1441–1443. [Google Scholar] [CrossRef]
- Baserga, M.; Chan, B. Hematochezia and thrombocytopenia in a 3-day-old infant: Congenital adenoviral infection. J. Neonatal Perinatal Med. 2018, 11, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Labelle, A.; Mazzetti, I.; Elbatarny, H.S.; Lillicrap, D. Adenovirus-induced thrombocytopenia: The role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 2007, 109, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Raddi, N.; Vigant, F.; Wagner-Ballon, O.; Giraudier, S.; Custers, J.; Hemmi, S.; Benihoud, K. Pseudotyping Serotype 5 Adenovirus with the Fiber from Other Serotypes Uncovers a Key Role of the Fiber Protein in Adenovirus 5-Induced Thrombocytopenia. Hum. Gene Ther. 2016, 27, 193–201. [Google Scholar] [CrossRef]
- Wolins, N.; Lozier, J.; Eggerman, T.L.; Jones, E.; Aguilar-Córdova, E.; Vostal, J.G. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br. J. Haematol. 2003, 123, 903–905. [Google Scholar] [CrossRef]
- Tapia, M.D.; Sow, S.O.; Ndiaye, B.P.; Mbaye, K.D.; Thiongane, A.; Ndour, C.T.; Mboup, S.; Ake, J.A.; Keshinro, B.; Akintunde, G.A.; et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in adults in Africa: A randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020, 20, 707–718. [Google Scholar] [CrossRef]
- Tapia, M.D.; Sow, S.O.; Mbaye, K.D.; Thiongane, A.; Ndiaye, B.P.; Ndour, C.T.; Mboup, S.; Keshinro, B.; Kinge, T.N.; Vernet, G.; et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: A randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020, 20, 719–730. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Warkentin, T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemost. 2017, 15, 2099–2114. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Li, H.; Rhee, G.; Masek-Hammerman, K.; Teigler, J.E.; Abbink, P.; Barouch, D.H. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J. Virol. 2021, 86, 10862–10865. [Google Scholar] [CrossRef]
- Gupalo, E.; Buriachkovskaia, L.; Othman, M. Human platelets express CAR with localization at the sites of intercellular interaction. Virol. J. 2011, 8, 456. [Google Scholar] [CrossRef]
- Seyran, M.; Takayama, K.; Uversky, V.N.; Lundstrom, K.; Palù, G.; Sherchan, S.P.; Attrish, D.; Rezaei, N.; Aljabali, A.A.A.; Ghosh, S.; et al. The structural basis of accelerated host cell entry by SARS-CoV-2. FEBS J. 2020. [Google Scholar] [CrossRef]
- Ahmetaj-Shala, B.; Vaja, R.; Atanur, S.S.; George, P.M.; Kirkby, N.S.; Mitchell, J.A. Cardiorenal Tissues Express SARS-CoV-2 Entry Genes and Basigin (BSG/CD147) Increases with Age in Endothelial Cells. JACC Basic Transl. Sci. 2020, 5, 1111–1123. [Google Scholar] [CrossRef]
- Carli, G.; Nichele, I.; Ruggeri, M.; Barra, S.; Tosetto, A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med. 2021, 16, 803–804. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, X.; Chen, H.; Chaturvedi, S.; Braunstein, E.M.; Brodsky, R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 2020, 136, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Benigni, A.; Remuzzi, G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020, 98, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Mdkhana, B.; Al Heialy, S.; Alsafar, H.S.; Hamoudi, R.; Hamid, Q.; Halwani, R. Enhanced expression of immune checkpoint receptors during SARS-CoV-2 viral infection. Mol. Ther. Methods Clin. Dev. 2021, 20, 109–121. [Google Scholar] [CrossRef]
- Ma, L.; Sahu, S.K.; Cano, M.; Kuppuswamy, V.; Bajwa, J.; McPhatter, J.; Pine, A.; Meizlish, M.; Goshua, G.; Chang, C.H.; et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kowarz, E.; Krutzke, L.; Reis, J.; Bracharz, S.; Kochanek, S.; Marschalek, R. “Vaccine-Induced Covid-19 Mimicry” Syndrome: Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Res. Square 2021. [Google Scholar] [CrossRef]
- Jaax, M.E.; Krauel, K.; Marschall, T.; Brandt, S.; Gansler, J.; Fürll, B.; Appel, B.; Fischer, S.; Block, S.; Helm, C.A.; et al. Complex formation with nucleic acids and aptamers alters the antigenic properties of platelet factor 4. Blood 2013, 122, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Liu, Y.; Shayakhmetov, D.; Li, Z.-Y.; Ni, S.; Lieber, A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J. Virol. 2007, 81, 4866–4871. [Google Scholar] [CrossRef]
- U.S. Agencies Push for Pause of J&J COVID-19 Vaccine After Patients Develop Rare Blood Clots. Available online: https://www.clinicalomics.com/topics/patient-care/therapeutics/vaccines/u-s-agencies-push-for-pause-of-jj-covid-19-vaccine-after-patients-develop-rare-blood-clots/?utm_medium=newsletter&utm_source=Clinical+OMICs+Update&utm_content=01&utm_campaign=Clinical+OMICs+Update_20210413&oly_enc_id=8353J6956023D7S (accessed on 10 May 2021).
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Arepally, G.M.; Ortel, T.L. Heparin-induced thrombocytopenia. Annu. Rev. Med. 2010, 61, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Sachs, U.J.; Cooper, N.; Czwalinna, A.; Müller, J.; Pötzsch, B.; Tiede, A.; Althaus, K. PF4-dependent immunoassays in patients with vaccine-induced immune thrombotic thrombocytopenia (VITT): Results of an inter-laboratory comparison. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Samuelson Bannow, B.; Warad, D.M.; Jones, C.G.; Pechauer, S.M.; Curtis, B.R.; Bougie, D.W.; Sharma, R.; Grill, D.E.; Redman, M.W.; Khalighi, P.R.; et al. A prospective, blinded study of a PF4-dependnet assay for hit diagnosis. Blood 2021, 137, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Amraie, R.; Napoleon, M.A.; Yin, W.; Jones, C.G.; Pechauer, S.M.; Curtis, B.R.; Bougie, D.W.; Sharma, R.; Grill, D.E.; Redman, M.W.; et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chaipan, C.; Soilleux, E.J.; Simpson, P.; Hofmann, H.; Gramberg, T.; Marzi, A.; Geier, M.; Stewart, E.A.; Eisemann, J.; Steinkasserer, A.; et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J. Virol. 2006, 80, 8951–8960. [Google Scholar] [CrossRef]
- Mahase, E. AstraZeneca vaccine: Blood clots are “extremely rare” and benefits outweigh risks, regulators conclude. Br. Med. J. 2021, 373, n931. [Google Scholar] [CrossRef]
- Suh, J.; Hong, H.; Ohana, M.; Bompard, F.; Revel, M.P.; Valle, C.; Gervaise, A.; Poissy, J.; Susen, S.; Hékimian, G.; et al. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology 2021, 298, E70–E80. [Google Scholar] [CrossRef]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-Astra Zeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. Br. Med. J. 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-J.; Liu, Z.-H.; Yao, F.-J.; Zeng, W.T.; Zheng, D.D.; Dong, Y.G.; Wu, S.H. Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis. PLoS Med. 2013, 10, e1001515. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Molina, A.; Monreal, M. Venous thromboembolism in women taking hormonal contraceptives. Expert Rev. Cardiovasc. Ther. 2010, 8, 211–215. [Google Scholar] [CrossRef]
- Stegeman, B.H.; de Bastos, M.; Rosendaal, F.R.; van Hylckama Vlieg, A.; Helmerhorst, F.M.; Stijnen, T.; Dekkers, O.M. Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. Br. Med. J. 2013, 347, f5298. [Google Scholar] [CrossRef]
- Marcucci, R.; Marietta, M. Vaccine-induced thrombotic thrombocytopenia: The elusive link between thrombosis and adenovirus-based SARS-CoV-2 vaccines. Intern Emerg. Med. 2021, 30, 1–7. [Google Scholar] [CrossRef]
- Furie, K.L.; Cushman, M.; Elkind, M.S.V.; Lyden, P.D.; Saposnik, G.; American Heart Association/American Stroke Association Stroke Council Leadership. Diagnosis and Management of Cerebral Venous Sinus Thrombosis with Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke 2021, 52, 2478–2482. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundstrom, K.; Barh, D.; Uhal, B.D.; Takayama, K.; Aljabali, A.A.A.; Abd El-Aziz, T.M.; Lal, A.; Redwan, E.M.; Adadi, P.; Chauhan, G.; et al. COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street? Biomolecules 2021, 11, 1020. https://doi.org/10.3390/biom11071020
Lundstrom K, Barh D, Uhal BD, Takayama K, Aljabali AAA, Abd El-Aziz TM, Lal A, Redwan EM, Adadi P, Chauhan G, et al. COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street? Biomolecules. 2021; 11(7):1020. https://doi.org/10.3390/biom11071020
Chicago/Turabian StyleLundstrom, Kenneth, Debmalya Barh, Bruce D. Uhal, Kazuo Takayama, Alaa A. A. Aljabali, Tarek Mohamed Abd El-Aziz, Amos Lal, Elrashdy M. Redwan, Parise Adadi, Gaurav Chauhan, and et al. 2021. "COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street?" Biomolecules 11, no. 7: 1020. https://doi.org/10.3390/biom11071020
APA StyleLundstrom, K., Barh, D., Uhal, B. D., Takayama, K., Aljabali, A. A. A., Abd El-Aziz, T. M., Lal, A., Redwan, E. M., Adadi, P., Chauhan, G., Sherchan, S. P., Azad, G. K., Rezaei, N., Serrano-Aroca, Á., Bazan, N. G., Hassan, S. S., Panda, P. K., Pal Choudhury, P., Pizzol, D., ... Uversky, V. N. (2021). COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street? Biomolecules, 11(7), 1020. https://doi.org/10.3390/biom11071020




















