Chemistry of Tropical Eucheumatoids: Potential for Food and Feed Applications
Abstract
1. Introduction
2. Materials and Methods
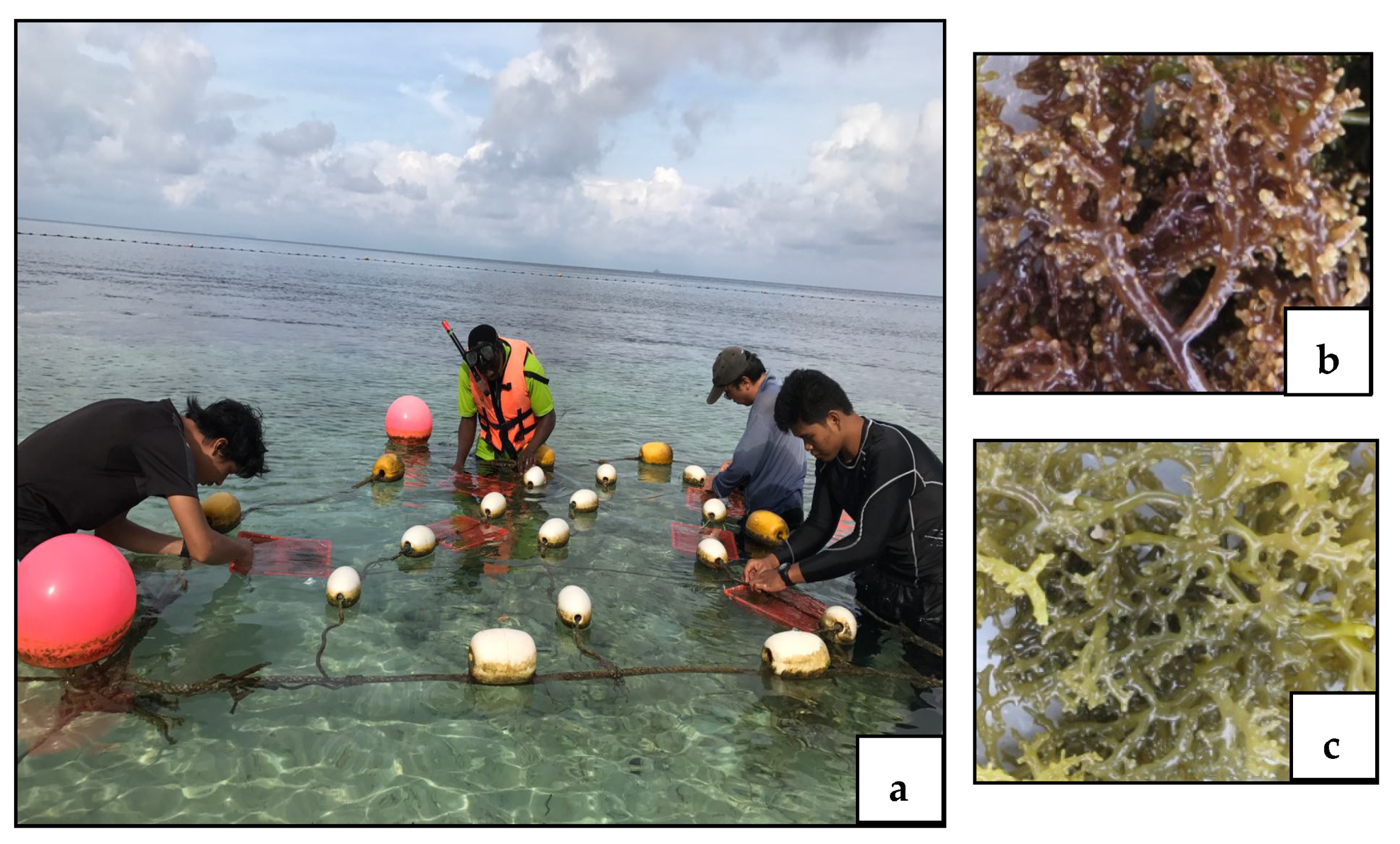
2.1. Biological Material
2.2. Mineral and Heavy Metal Analysis of K. alvarezii and K. striatus
2.3. Extraction and NMR Spectroscopy Analysis of Carrageenan
2.4. Extraction of Soluble and Fat-Soluble Antioxidants and Determination of Antioxidant Capacity
2.5. Quality Assurance
2.6. Statistical Analysis
3. Results
3.1. Mineral and Heavy Metal Composition of K. alvarezii and K. striatus
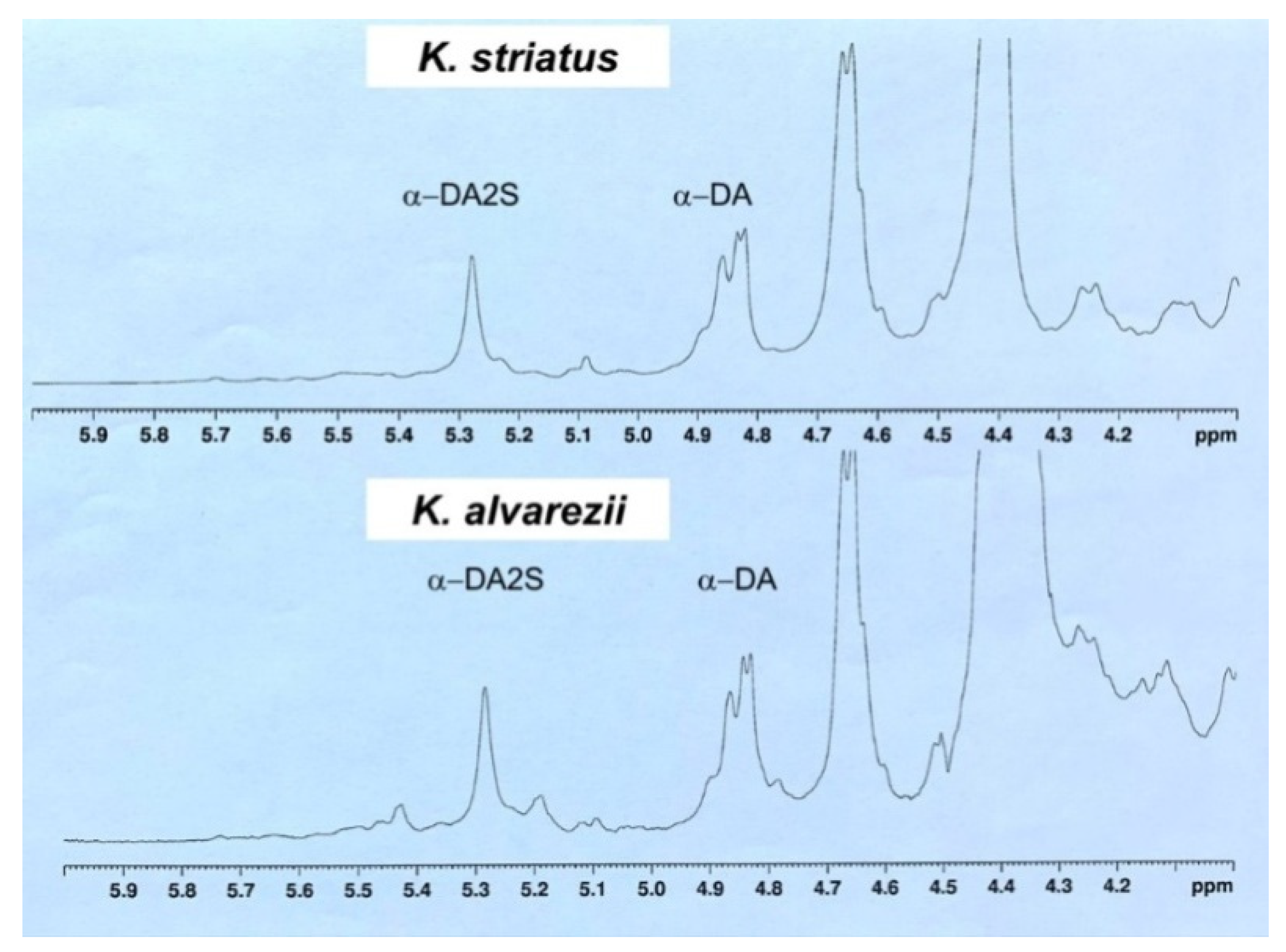
3.2. Characterization of K. alvarezii and K. striatus Carrageenans
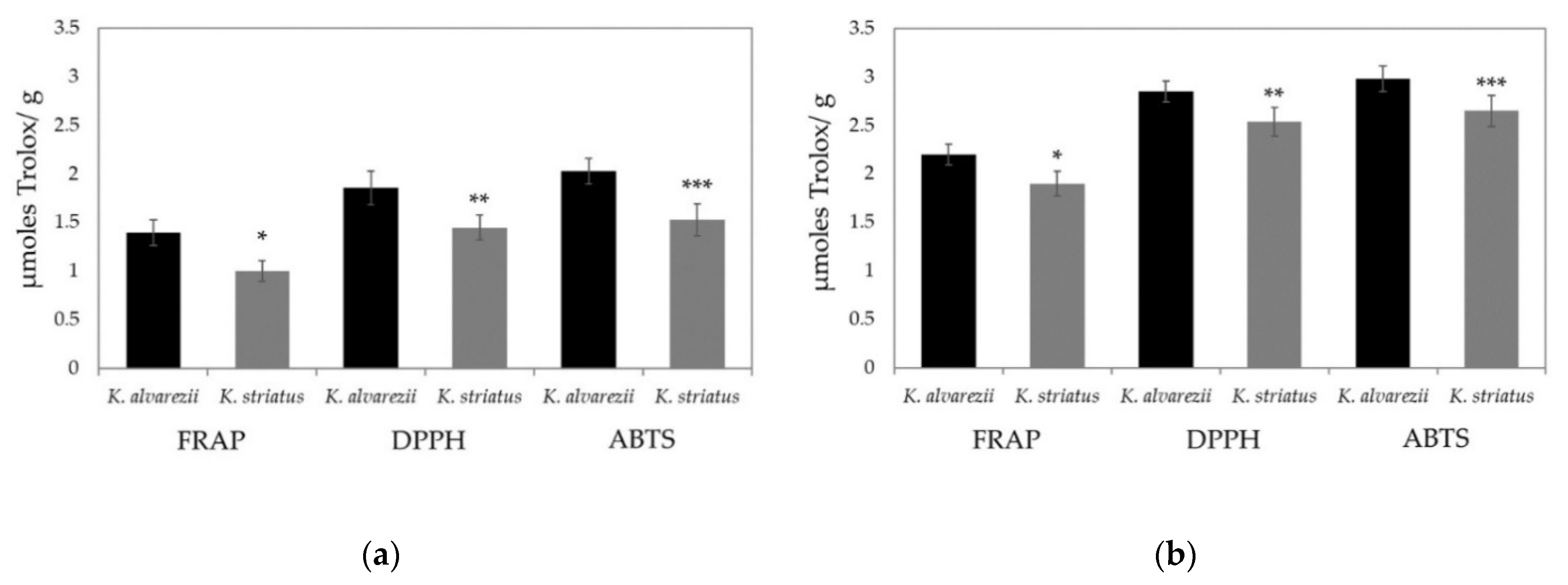
3.3. Soluble and Fat-Soluble Antioxidant Capacity of K. alvarezii and K. striatus
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Evans, F.D.; Critchley, A.T. Seaweeds for animal production use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health Benefits of Algal Polysaccharides in Human Nutrition. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 66, pp. 75–145. [Google Scholar]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. In Inflammation Protocols; Paul, G.W., Derek, A.W., Eds.; Humana Press: Totowa, NJ, USA, 2003; Volume 225, pp. 115–122. [Google Scholar]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Van de Velde, F. Structure and function of hybrid carrageenans. Food Hydrocoll. 2008, 22, 727–734. [Google Scholar] [CrossRef]
- Yong, W.; Chin, J.; Thien, V.; Yasir, S. Heavy metal accumulation in field cultured and tissue cultured Kappaphycus alvarezii and Gracilaria changii. Int. Food Res. J. 2017, 24, 970–975. [Google Scholar]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Farah Diyana, A.; Abdullah, A.; Shahrul Hisham, Z.A.; Chan, K.M. Antioxidant activity of red algae Kappaphycus alvarezii and Kappaphycus striatum. Int. Food Res. J. 2015, 22, 1977–1984. [Google Scholar]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from Macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown Algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, R.; Dubinsky, Z.; Kishimoto, E.; Masaki, K.; Masuda, Y.; Takeuchi, T.; Chihara, M.; Yamamoto, Y.; Niki, E.; Karube, I. A comparison of screening methods for antioxidant activity in seaweeds. J. Appl. Phycol. 1997, 9, 29–35. [Google Scholar] [CrossRef]
- D’Errico, G.; Vitiello, G.; De Tommaso, G.; Abdel-Gawad, F.K.; Brundo, M.V.; Ferrante, M.; De Maio, A.; Trocchia, S.; Bianchi, A.R.; Ciarcia, G.; et al. Electron Spin Resonance (ESR) for the study of Reactive Oxygen Species (ROS) on the isolated frog skin (Pelophylax bergeri): A non-invasive method for environmental monitoring. Environ. Res. 2018, 165, 11–18. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The duckweed, lemna minor modulates heavy metal-induced oxidative stress in the Nile Tilapia, Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vivo studies and clinical trials. Mar. Drugs 2019, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Casas-Valdez, M.; Hernández-Contreras, H.; Marín-Alvarez, A.; Aguila-Ramírez, R.; Hernández-Guerrero, C.; Sánchez-Rodríguez, I.; Carrillo-Domínguez, S. The seaweed Sargassum (Sargassaceae) as tropical alternative for goats’ feeding. Rev. Biol. Trop. 2006, 54, 83–92. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; El-Deek, A.A. Effect of different dietary concentrations of brown marine algae (Sargassum dentifebium) prepared by different methods on plasma and yolk lipid profiles, yolk total carotene and lutein plus zeaxanthin of laying hens. Ital. J. Anim. Sci. 2012, 11, e64. [Google Scholar] [CrossRef]
- Mizpal, A.; Rosazman, H.; Suhaimi, M.; Ahmad Tarmizi, A. Projek estet mini rumpai laut dan penglibatan komuniti nelayan di daerah Semporna, Sabah. J. Borneo Transform. Stud. 2015, 1, 122–138. [Google Scholar]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of nutritional and antinutritional traits among different species ( Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and varieties of lupin seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Mahrose, K. Seaweeds, intact and processed, as a valuable component of poultry feeds. J. Mar. Sci. Eng. 2020, 8, 620. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in european poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Doty, M. Taxonomy of economic seaweeds: With reference to some Pacific and Caribbean Species. In Prodomus Ad Systematica Eucheumatoideorum: A Tribe of Commercial Seaweeds Related to Eucheuma (Solieriaceae, Gigartinales); Abott, I., Ed.; 1985; Available online: https://escholarship.org/content/qt6xm1n104/qt6xm1n104.pdf (accessed on 27 March 2021).
- Li, H.; Liu, J.; Zhang, L.; Pang, T. Antioxidant responses and photosynthetic behaviors of Kappaphycus alvarezii and Kappaphycus striatum (Rhodophyta, Solieriaceae) during low temperature stress. Bot. Stud. 2016, 57, 21. [Google Scholar] [CrossRef]
- Olanrewaju Oladokun, S.; Abdelaziz, A.; Guerriero, G. Risk Analysis of Aquaculture Farming System; Olanrewaju Oladokun, S., Abdelaziz, A., Guerriero, G., Eds.; LAP LAMBERT Academic Publishing: Sunnyvale, CA, USA, 2016. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 20th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2015. [Google Scholar]
- Ariano, A.; Marrone, R.; Andreini, R.; Smaldone, G.; Velotto, S.; Montagnaro, S.; Anastasio, A.; Severino, L. Metal concentration in muscle and digestive gland of common octopus (Octopus vulgaris) from two coastal site in Southern Tyrrhenian Sea (Italy). Molecules 2019, 24, 2401. [Google Scholar] [CrossRef]
- Barnard, T.W.; Crockett, M.I.; Ivaldi, J.C.; Lundberg, P.L. Design and evaluation of an echelle grating optical system for ICP-OES. Anal. Chem. 1993, 65, 1225–1230. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; Bianchi, A.R.; Mistretta, C.; Vitale, E.; Parisi, C.; Guerriero, G.; Magliulo, V.; De Maio, A. The ageing process affects the antioxidant defences and the poly (ADPribosyl)ation activity in cistus incanus l. leaves. Antioxidants 2019, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Vitale, L.; Vitale, E.; Costanzo, G.; De Maio, A.; Arena, C. Photo-protective mechanisms and the role of poly (Adp-ribose) polymerase activity in a facultative cam plant exposed to long-term water deprivation. Plants 2020, 9, 1192. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Kausar, H.; Jaferi, S.S.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. An insight into the algal evolution and genomics. Biomolecules 2020, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D. Nutraceuticals and functional foods regulations in the United States and around the world. Toxicology 2006, 221, 1–3. [Google Scholar] [CrossRef]
- Hafting, J.T.; Critchley, A.T.; Cornish, M.L.; Hubley, S.A.; Archibald, A.F. On-land cultivation of functional seaweed products for human usage. J. Appl. Phycol. 2012, 24, 385–392. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Ganesan, K.; Subba Rao, P.V. Phycoremediation of heavy metals by the three-color forms of Kappaphycus alvarezii. J. Hazard. Mater. 2007, 143, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.S.; Yong, W.T.L.; Ng, S.E.; Anton, A.; Yassir, S. Chemical composition of farmed and micropropagated Kappaphycus alvarezii (Rhodophyta, Gigartinales), a commercially important seaweed in Malaysia. J. Appl. Phycol. 2015, 27, 1271–1275. [Google Scholar] [CrossRef]
- Tropin, I.V. Distribution of metals in thalluses of red alga with special reference to their taxonomy and ecology. Oceanol. Russ. Acad. Sci. 2013, 35, 92–98. [Google Scholar]
- Srinivasan, V.S. Bioavailability of nutrients: A practical approach to in vitro demonstration of the availability of nutrients in multivitamin-mineral combination products. J. Nutr. 2001, 131, 1349S–1350S. [Google Scholar] [CrossRef]
- Chuah, X.Q.; Teo, S.S. Evaluation of sub-chronic toxicity and heavy metal toxicity of Kappaphycus alvarezii in-vivo. Int. J. Pharm. Sci. Res. 2016, 7, 573–578. [Google Scholar] [CrossRef]
- Andrieu, S. Is there a role for organic trace element supplements in transition cow health? Vet. J. 2008, 176, 77–83. [Google Scholar] [CrossRef]
- Arthington, J.D. Trace Mineral Nutrition and Immune Competence in Cattle. In Proceedings of the California Animal Nutrition Conference, Minneapolis, MN, USA, 2005; p. 106. Available online: https://docs.ufpr.br/~freitasjaf/artigos/simposioflorida/Arthington1.pdf (accessed on 27 March 2021).
- Hesari, B.A.; Mohri, M.; Seifi, H.A. Effect of copper edetate injection in dry pregnant cows on hematology, blood metabolites, weight gain and health of calves. Trop. Anim. Health Prod. 2012, 44, 1041–1047. [Google Scholar] [CrossRef]
- Gressley, T.F. Zinc, copper, manganese, and selenium in dairy cattle rations. In Proceedings of the 7th Annual mid-Atlantic Nutrition Conference; Zimmermann, N., Ed.; Agris: College Park, MD, USA, 2009; pp. 56–71. [Google Scholar]
- Siciliano-Jones, J.L.; Socha, M.T.; Tomlinson, D.J.; DeFrain, J.M. Effect of trace mineral source on lactation performance, claw integrity, and fertility of dairy cattle. J. Dairy Sci. 2008, 91, 1985–1995. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef]
- Boland, M.P.; Lonergan, P. Trace minerals in production and reproduction in dairy cows. Adv. Dairy Technol. 2003, 15, 330. [Google Scholar]
- Arcella, D.; Gergelova, P.; Innocenti, M.L.; López-Gálvez, G.; Steinkellner, H. Occurrence data of nickel in feed and animal exposure assessment. EFSA J. 2019, 17, 5754. [Google Scholar] [CrossRef]
- Drugă, M.; Trif, A.; Drugă, M.; Ştef, D.; Driha, A. Aluminium intake impact on broiler chickens during a production cycle on some bioproductive parameters. Sci. Pap. Anim. Sci. Biotechnol. 2010, 43, 47–50. [Google Scholar]
- European Food Safety Authority (EFSA). Advises on the Safety of Aluminium in Food. Available online: https://www.efsa.europa.eu/en/news/efsa-advises-safety-aluminium-food (accessed on 13 March 2021).
- Directive (EC). No 2002/32 of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; 2002; p. 10. Available online: http://data.europa.eu/eli/dir/2002/32/oj (accessed on 27 March 2021).
- Roleda, M.Y.; Lage, S.; Aluwini, D.F.; Rebours, C.; Brurberg, M.B.; Nitschke, U.; Gentili, F.G. Chemical profiling of the Arctic sea lettuce Ulva lactuca (Chlorophyta) mass-cultivated on land under controlled conditions for food applications. Food Chem. 2021, 341, 127999. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control. 2019, 95, 121–134. [Google Scholar] [CrossRef]
- De la Moneda, A.; Carro, M.D.; Weisbjerg, M.R.; Roleda, M.Y.; Lind, V.; Novoa-Garrido, M.; Molina-Alcaide, E. Variability and potential of seaweeds as ingredients of ruminant diets: An in vitro study. Animals 2019, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.M.P.; Gouveia, A.; Rema, P.; Matos, J.; Gomes, E.F.; Pinto, I.S. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Kalla, A.; Yoshimatsu, T.; Araki, T.; Zhang, D.-M.; Yamamoto, T.; Sakamoto, S. Use of Porphyra spheroplasts as feed additive for red sea bream. Fish. Sci. 2008, 74, 104–108. [Google Scholar] [CrossRef]
- Ergün, S.; Soyutürk, M.; Güroy, B.; Güroy, D.; Merrifield, D. Influence of Ulva meal on growth, feed utilization, and body composition of juvenile Nile tilapia (Oreochromis niloticus) at two levels of dietary lipid. Aquac. Int. 2009, 17, 355–361. [Google Scholar] [CrossRef]
- Kamunde, C.; Sappal, R.; Melegy, T.M. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PLoS ONE 2019, 14, e0219792. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, V.; Ceci, E.; Tufarelli, V. Productive traits and meat fatty acid profile of broiler chickens fed diets containing micronized fava beans (Vicia faba L. var. minor) as the main protein source. J. Appl. Poult. Res. 2011, 20, 12–20. [Google Scholar] [CrossRef]
- Ali, A.; Memon, M.S. Green seaweed as component of poultry feed. Int. J. Biol. Biotechnol. 2014, 5, 211–214. [Google Scholar]
- Abudabos, A.M.; Okab, A.B.; Aljumaah, R.S.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A. Nutritional Value of green seaweed (Ulva Lactuca) for broiler chickens. Ital. J. Anim. Sci. 2013, 12, e28. [Google Scholar] [CrossRef]
- Zahid, P.B.; Aisha, K.; Ali, A. Green seaweed as component of poultry feed. Bangladesh J. Bot. 1995, 24, 146–153. [Google Scholar]
- Wang, S.; Shi, X.; Zhou, C.; Lin, Y. Entermorpha prolifera: Effects on performance, carcass quality and small intestinal digestive enzyme activities of broilers. Chin. J. Anim. Nutr. 2013, 25, 1332–1337. [Google Scholar]
- Kulshreshtha, G.; Rathgeber, B.; Stratton, G.; Thomas, N.; Evans, F.; Critchley, A.; Hafting, J.; Prithiviraj, B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. 2014, 93, 2991–3001. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. Electr. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- El-Deek, A.A.; Brikaa, M.A. Nutritional and biological evaluation of marine seaweed as a feedstuff and as a pellet binder in poultry diet. Int. J. Poult. Sci. 2009, 8, 875–881. [Google Scholar] [CrossRef]
- El-Deek, A.A.; Brikaa, M.A. Effect of different levels of seaweed in starter and finisher diets in pellet and mash form on performance and carcass quality of ducks. Int. J. Poult. Sci. 2009, 8, 1014–1021. [Google Scholar] [CrossRef]
- Ventura, M.R.; Castañon, J.I.R.; McNab, J.M. Nutritional value of seaweed (Ulva rigida) for poultry. Anim. Feed Sci. Technol. 1994, 49, 87–92. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Tommonaro, G.; Guerriero, G.; Fogliano, C.; Iodice, C.; Velotto, G.; Tramice, A. New Insight into Marine Biotechnology: Carrageenans Chemical Features and Acetylcholinesterase (AChE) Inhibition Activity of Two Edible Seaweeds of the Genus Kappaphycus. In Proceedings of the In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions (2nd Edition): Proceedings of Euro-Mediterranean Conference for Environmental Integration, Tunisia, 2019; Ksibi, M., Ghorbal, A., Chakraborty, S., Chaminé, H.I., Barbieri, M., Guerriero, G., Hentati, O., Negm, A., Lehmann, A., Römbke, J., et al., Eds.; Springer International Publishing: New York, NY, USA, 2021; pp. 2203–2207. [Google Scholar]
- Guibet, M.; Kervarec, N.; Génicot, S.; Chevolot, Y.; Helbert, W. Complete assignment of 1H and 13C NMR spectra of Gigartina skottsbergii λ-carrageenan using carrabiose oligosaccharides prepared by enzymatic hydrolysis. Carbohydr. Res. 2006, 341, 1859–1869. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Rapid assays to evaluate the antioxidant capacity of phenols in virgin olive oil. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 625–635. [Google Scholar]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Škrovánková, S. Seaweed Vitamins as Nutraceuticals. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 64, pp. 357–369. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; De Maio, A.; Lionetti, E.; Bianchi, A.R.; Rabbito, D.; Ariano, A.; Majdoubi, F.-Z.; Guerriero, G. Sea farms as a safe and sustainable food source: An investigation on use of seaweeds for liver detoxification and reduced DNA damage in lates calcarifer (Bloch, 1790). In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions (2nd Edition): Proceedings of Euro-Mediterranean Conference for Environmental Integration, Tunisia (2019); Ksibi, M., Ghorbal, A., Chakraborty, S., Chaminé, H.I., Barbieri, M., Guerriero, G., Hentati, O., Negm, A., Lehmann, A., Römbke, J., et al., Eds.; Springer International Publishing: New York, NY, USA, 2021; pp. 671–675. [Google Scholar]

| Chemical Composition (% Dry Matter) | Kappaphycusalvarezii | Kappaphycus striatus | RMSE | p-Value |
|---|---|---|---|---|
| Dry matter | 88.93 | 83.88 | 1.87 | 0.0031 |
| Ash | 45.37 | 35.88 | 2.29 | 0.0088 |
| Crude Protein | 6.81 | 6.95 | 0.12 | 0.5216 |
| Ether extract | 0.38 | 0.47 | 0.084 | 0.1539 |
| Crude fiber | 4.36 | 5.67 | 0.28 | 0.0560 |
| NFE | 43.08 | 51.03 | 0.95 | 0.0029 |
| Mineral composition (mg kg−1 dry matter) | ||||
| Al | 0.915 | 0.307 | 0.262 | 0.0024 |
| Cu | 0.035 | 0.070 | 0.036 | 0.1177 |
| Mn | 0.091 | 0.070 | 0.013 | 0.0224 |
| Fe | 40.009 | 21.889 | 6.515 | 0.0070 |
| Ni | 0.033 | 0.033 | 0.0145 | 0.9991 |
| Se | 0.153 | 0.156 | 0.039 | 0.9076 |
| Zn | 0.265 | 0.216 | 0.084 | 0.3413 |
| Heavy metal composition (mg kg−1 dry matter) | ||||
| As | 0.097 | 0.082 | 0.010 | 0.0343 |
| Cd | 0.009 | 0.010 | 0.004 | 0.7319 |
| Pb | 0.004 | 0.004 | 0.002 | 0.9213 |
| Hg | 0.006 | 0.005 | 0.001 | 0.1992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariano, A.; Musco, N.; Severino, L.; De Maio, A.; Tramice, A.; Tommonaro, G.; Damiano, S.; Genovese, A.; Olanrewaju, O.S.; Bovera, F.; et al. Chemistry of Tropical Eucheumatoids: Potential for Food and Feed Applications. Biomolecules 2021, 11, 804. https://doi.org/10.3390/biom11060804
Ariano A, Musco N, Severino L, De Maio A, Tramice A, Tommonaro G, Damiano S, Genovese A, Olanrewaju OS, Bovera F, et al. Chemistry of Tropical Eucheumatoids: Potential for Food and Feed Applications. Biomolecules. 2021; 11(6):804. https://doi.org/10.3390/biom11060804
Chicago/Turabian StyleAriano, Andrea, Nadia Musco, Lorella Severino, Anna De Maio, Annabella Tramice, Giuseppina Tommonaro, Sara Damiano, Angelo Genovese, Oladokun Sulaiman Olanrewaju, Fulvia Bovera, and et al. 2021. "Chemistry of Tropical Eucheumatoids: Potential for Food and Feed Applications" Biomolecules 11, no. 6: 804. https://doi.org/10.3390/biom11060804
APA StyleAriano, A., Musco, N., Severino, L., De Maio, A., Tramice, A., Tommonaro, G., Damiano, S., Genovese, A., Olanrewaju, O. S., Bovera, F., & Guerriero, G. (2021). Chemistry of Tropical Eucheumatoids: Potential for Food and Feed Applications. Biomolecules, 11(6), 804. https://doi.org/10.3390/biom11060804









