Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities
Abstract
1. Introduction
2. Nutritional Composition
2.1. Protein
2.2. Minerals and Vitamins
3. Phytochemical Profile
3.1. Essential Oil Profile
3.2. Secondary Metabolite Profile
4. Biological Activities of Annona squamosa L. Leaves
4.1. Anticancer Activity
4.2. Antidiabetic Activity
4.3. Antioxidant Activity
4.4. Antimicrobial Activity
4.5. Hepatoprotective Properties
4.6. Effect of ASLs Extract on the Lipid Profile
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalidindi, N.; Thimmaiah, N.V.; Jagadeesh, N.V.; Nandeep, R.; Swetha, S.; Kalidindi, B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. leaves. J. Food Drug Anal. 2015, 23, 795–802. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; de Lourdes García-Magaña, M.; Abraham Domínguez-Ávila, J.; Yahia, E.M.; Salazar-López, N.J.; González-Aguilar, G.A.; Montalvo-González, E. Annonas: Underutilized species as a potential source of bioactive compounds. Food Res. Int. 2020, 138. [Google Scholar] [CrossRef]
- El-Chaghaby, G.A.; Ahmad, A.F.; Ramis, E.S. Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arab. J. Chem. 2014, 7, 227–233. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.A.; Karthikumar, S.; Shyam Kumar, R.; Samuel, K.J.; Shajahan, S.; Sivasubramanian, V.; Sivashanmugam, P.; Varalakshmi, P.; Syed, A.; Marraiki, N.; et al. An intensified approach for transesterification of biodiesel from Annona squamosa seed oil using ultrasound-assisted homogeneous catalysis reaction and its process optimization. Fuel 2021, 291, 120195. [Google Scholar] [CrossRef]
- Zahid, M.; Mujahid, M.; Singh, P.K.; Farooqui, S.; Singh, K.; Parveen, S.; Arif, M. Annona squamosa linn. (Custard apple): An aromatic medicinal plant fruit with immense nutraceutical and therapeutic potentials (Review). Int. J. Pharm. Sci. Res. 2018, 9, 1745–1759. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Valorisation of black carrot pomace: Microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Evaluation of enzyme and microwave-assisted conditions on extraction of anthocyanins and total phenolics from black soybean (Glycine max L.) seed coat. Int. J. Biol. Macromol. 2019, 135, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Sandhu, K.S.; Grasso, S.; Purewal, S.S.; Kaur, M.; Siroha, A.K.; Kumar, K.; Kumar, V.; Kumar, M. Aspergillus oryzae fermented rice bran: A byproduct with enhanced bioactive compounds and antioxidant potential. Foods 2021, 10, 70. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Amarowicz, R.; Saurabh, V.; Nair, M.S.; Maheshwari, C.; Sasi, M.; Prajapati, U.; Hasan, M.; Singh, S.; et al. Guava (Psidium guajava L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Foods 2021, 10, 752. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Black Carrot (Daucus carota ssp.) and Black Soybean (Glycine max (L.) Merr.) Anthocyanin Extract: A Remedy to Enhance Stability and Functionality of Fruit Juices by Copigmentation. Waste Biomass Valorization 2020, 11, 99–108. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Amarowicz, R.; Kaur, C. Evaluation of cellulolytic enzyme-assisted microwave extraction of Punica granatum peel phenolics and antioxidant Activity. Plant Foods Hum. Nutr. 2020, 75, 614–620. [Google Scholar] [CrossRef]
- Nishad, J.; Dutta, A.; Saha, S.; Rudra, S.G.; Varghese, E.; Sharma, R.R.; Tomar, M.; Kumar, M.; Kaur, C. Ultrasound-assisted development of stable grapefruit peel polyphenolic nano-emulsion: Optimization and application in improving oxidative stability of mustard oil. Food Chem. 2021, 334. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Potkule, J.; Patil, S.; Saxena, S.; Patil, P.G.; Mageshwaran, V.; Punia, S.; Varghese, E.; Mahapatra, A.; Ashtaputre, N.; et al. Extraction of ultra-low gossypol protein from cottonseed: Characterization based on antioxidant activity, structural morphology and functional group analysis. LWT 2021, 140, 110692. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S.; et al. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Tomar, M.; Punia, S.; Singh, S.; Patil, S.; Singh, S.; Ilakiya, T.; Kaur, C.; Kennedy, J.F. Jackfruit seed slimy sheath, a novel source of pectin: Studies on antioxidant activity, functional group, and structural morphology. Carbohydr. Polym. Technol. Appl. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M. Litchi (Litchi chinenis) seed: Nutritional profile, bioactivities, and its industrial applications. Trends Food Sci. Technol. 2021, 108, 58–70. [Google Scholar] [CrossRef]
- Al-Nemari, R.; Al-Senaidy, A.; Semlali, A.; Ismael, M.; Badjah-Hadj-Ahmed, A.Y.; Ben Bacha, A. GC-MS profiling and assessment of antioxidant, antibacterial, and anticancer properties of extracts of Annona squamosa L. leaves. BMC Complement. Med. Ther. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical profile and biological activity of cherimoya (Annona cherimola Mill.) and atemoya (Annona atemoya) leaves. Molecules 2020, 25, 2612. [Google Scholar] [CrossRef] [PubMed]
- Hosseinabadi, T. The Medicinal Importance of Annona squamosa fruits. J. Explor. Res. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Lakshmi Kalyani, R.; Chandra, V.S.; Vijaykumar, P.P.N.; Pammi, S.V.N.; Rajkumar, M.; Swamy, P.V.; Murthy, K.V.R. Biosynthesis of silver nanoparticles using Annona squamosa leaf extract with synergistic antibacterial activity. Indian J. Pharm. Sci. 2019, 81, 1036–1044. [Google Scholar]
- Shukry, W.M.; Galilah, D.A.; Elrazek, A.A. Mineral Composition, Nutritional Properties, Vitamins, and Bioactive Compounds in Annona squamosa L. grown at different sites of Egypt. Ser. Bot. Environ. Sci. 2019, 1, 7–22. [Google Scholar]
- Pandey, V.K.; Giri, I.C.; Singh, P.; Srivastava, A. Pharmacognostical and physiochemical study on the leaves of Annona squamosa Linn. Int. J. Res. Pharm. Sci. 2014, 4, 8–12. [Google Scholar]
- Sampathkumar, P. Phytochemical screening and antimicrobial activity of plant extracts for disease management. Int. J. Curr. Sci. 2012, 2012, 209–218. [Google Scholar]
- Akram, M.; Munir, N.; Daniyal, M.; Egbuna, C.; Găman, M.A.; Onyekere, P.F.; Olatunde, A. Vitamins and Minerals: Types, sources and their functions. In Functional Foods and Nutraceuticals; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 149–172. [Google Scholar]
- Varadharaj, V.; Janarthanan, U.D.; Krishnamurthy, V.; Synnah, J. Assessment of Phytonutrients in the Ethanolic Leaf Extract of Annona Squamosa (L.). World J. Pharm. Pharm. Sci. 2014, 3, 725–732. [Google Scholar]
- Himesh, S.; Singhai, A.K.; Sarvesh, S. Quantification of ascorbic acid in leaves of Annona squamosa. Int. J. Pharm. Pharm. Sci. 2012, 4, 144–147. [Google Scholar]
- Meira, C.S.; Guimarães, E.T.; Macedo, T.S.; da Silva, T.B.; Menezes, L.R.A.; Costa, E.V.; Soares, M.B.P. Chemical composition of essential oils from Annona vepretorum Mart. and Annona squamosa L. (Annonaceae) leaves and their antimalarial and trypanocidal activities. J. Essent. Oil Res. 2015, 27, 160–168. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Chauhan, A. Characterization of the Leaf Essential Oil Composition of Annona squamosa L. from Foothills of North India. Med. Aromat. Plants 2016, 5. [Google Scholar] [CrossRef]
- Balbaa, S.I.; Haggag, M.Y.; Taha, K.F. Study of volatile oil content of the leaves of Annona squamosa L. growing in Egypt. Egypt. J. Pharm. Sci. 1977. [Google Scholar]
- Pélissier, Y.; Marion, C.; Kone, D.; Lamaty, G.; Menut, C.; Bessière, J.M. Volatile components of Annona muricata L. J. Essent. Oil Res. 1994, 6, 411–414. [Google Scholar] [CrossRef]
- Thang, T.D.; Dai, D.N.; Hoi, T.M.; Ogunwande, I.A. Study on the volatile oil contents of Annona glabra L., Annona squamosa L., Annona muricata L. and Annona reticulata L., from Vietnam. Nat. Prod. Res. 2013, 27, 1232–1236. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, K.; Kaur, P.; Singh, I. Sitaphal: Unexplored therapeutic potential. Asian J. Res. Chem. Pharm. Sci. 2015, 3, 129–141. [Google Scholar]
- Garg, S.N.; Gupta, D. Composition of the leaf oil of Annona squamosa L. From the north indian plains. J. Essent. Oil Res. 2005, 17, 257–258. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Padhi, L.P.; Panda, S.K.; Satapathy, S.N.; Dutta, S.K. In vitro evaluation of antibacterial potential of Annona squamosa L. and Annona reticulata L. from Similipal Biosphere Reserve. J. Agric. Technol. 2011, 7, 133–142. [Google Scholar]
- Reza, A.; Mazahery, F.; Dator, R.P.; Concepcion, G.P.; Jacinto, S.D. Murihexocin C from the Leaves of Annona squamosa Linn. Murihexocin C from the Leaves of Annona squamosa Linn. Induces Apoptosis in Human Colon Carcinoma Col 2 Cell Line. Philipp. Agric. Sci. 2009, 92. [Google Scholar]
- Ocker, M.; Höpfner, M. Apoptosis-modulating drugs for improved cancer therapy. Eur. Surg. Res. 2012. [Google Scholar] [CrossRef]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011. [Google Scholar] [CrossRef]
- Al-nemari, R.; Bacha, A.B.; Al-senaidy, A.; Arafah, M.; Al-saran, H. Selective cytotoxic effects of Annona Squamosa Leaves against breast cancer cells via apoptotic signaling proteins. Preprints 2020, 1–14. [Google Scholar] [CrossRef]
- Thakkar, J.H.; Solanki, H.K.; Tripathi, P.; Patel, N.J.; Jani, G.K. Evaluation of antimutagenic potential of Annona squamosa leaf extract. Elixir Hum. Physiol. 2011, 31, 1960–1965. [Google Scholar]
- Nguyen, M.T.; Nguyen, T.; Le, V.M.; Trieu, L.H.; Lam, T.D.; Bui, L.M.; Nhan, L.T.H.; Danh, V.T. Assessment of preliminary phytochemical screening, polyphenol content, flavonoid content, and antioxidant activity of custard apple leaves (Annona squamosa Linn.). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar Chandra, B.A.G.; Gajera, H.H.; Kumar Chandra, A.; Yashwant Kumar, C.; Gajera, H.H. Evaluation of antidiabetic and antioxidant potential of custard apple (Annona squamosa) Leaf extracts: A compositional study. Int. J. Chem. Stud. 2019, 7. [Google Scholar]
- Neethu, S.K.; Santhoshkumar, R.; Kumar, N.S. Phytochemical analysis and antimicrobial activities of Annona squamosa (L) leaf extracts. J. Pharmacogn. Phytochem. 2016, 5, 128–131. [Google Scholar]
- Katole, R.; Gautam, J.; Mokat, D. Phytochemical study of Annona squamosa L. and Annona reticulata L. Int. J. Res. 2018, 5. [Google Scholar]
- Malik, J.; Gandhi, R.; Vishwavidyalaya, P.; Soni, H.; Yadav, A.P.; Yadav, B. Characterization of rutin isolated by leaves Annona squamosa by modern analytical techniques. Eur. J. Biomed. Pharm. Sci. 2018, 5, 484–489. [Google Scholar]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Protective effects of 5,7,4′-trihydroxy-6,3′dimethoxy-flavone 5-O-α-l-rhamnopyranoside, isolated from Annona squamosa leaves in thyrotoxicosis and in hepatic lipid peroxidation in rats. Bioorganic Med. Chem. Lett. 2015, 25, 5726–5728. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. BioFactors 2008, 31, 201–210. [Google Scholar] [CrossRef]
- Nakano, D.; Ishitsuka, K.; Kamikawa, M.; Matsuda, M.; Tsuchihashi, R.; Okawa, M.; Okabe, H.; Tamura, K.; Kinjo, J. Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (III). J. Nat. Med. 2013, 67, 894–903. [Google Scholar] [CrossRef]
- Davis, J.A.; Sharma, S.; Mittra, S.; Sujatha, S.; Kanaujia, A.; Shukla, G.; Katiyar, C.; Lakshmi, B.S.; Bansal, V.S.; Bhatnagar, P.K. Antihyperglycemic effect of Annona squamosa hexane extract in type 2 diabetes animal model: PTP1B inhibition, a possible mechanism of action. Indian J. Pharmacol. 2012, 44, 326–332. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kesari, A.N.; Diwakar, S.; Tyagi, A.; Tandon, V.; Chandra, R.; Watal, G. In vivo evaluation of anti-oxidant and anti-lipidimic potential of Annona squamosa aqueous extract in Type 2 diabetic models. J. Ethnopharmacol. 2008, 118, 21–25. [Google Scholar] [CrossRef]
- Ranjana; Tripathi, Y.B. Insulin secreting and α-glucosidase inhibitory activity of hexane extract of Annona squamosa Linn. in streptozotocin (STZ) induced diabetic rats. Indian J. Exp. Biol. 2014, 52, 623–629. [Google Scholar] [PubMed]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Rizwani, G.H.; Guo, H.; Ahmed, M.; Ahmed, M.; Hassan, S.Z.; Hassan, A.; Chen, Z.S.; Xu, R.H. Annona squamosa Linn: Cytotoxic activity found in leaf extract against human tumor cell lines. Pak. J. Pharm. Sci. 2014, 27, 1559–1563. [Google Scholar]
- Fadholly, A.; Proboningrat, A.; Dewi Iskandar, R.; Rantam, F.; Sudjarwo, S. In vitro anticancer activity Annona squamosa extract nanoparticle on WiDr cells. J. Adv. Pharm. Technol. Res. 2019, 10, 149–154. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; El Rabey, H.A. The Antidiabetic Effect of Low Doses of Moringa oleifera Lam. Seeds on Streptozotocin Induced Diabetes and Diabetic Nephropathy in Male Rats. Biomed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pallela, P.N.V.K.; Pammi, S.V.N.; Padavala, V.S.; Kolapalli, V.R.M. Synergetic antibacterial and anticarcinogenic effects of Annona squamosa leaf extract mediated silver nano particles. Mater. Sci. Semicond. Process. 2019, 100, 301–309. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013. [Google Scholar] [CrossRef]
- Kulkarni, V.R.; Chandrashekar, C. Isolation, Characterizations and Free radical scavenging activity of Annona squamosa leaf. J. Pharm. Res. 2011, 4, 610–611. [Google Scholar]
- Shenoy, C.; Patil, M.B.; Kumar, R. Antibacterial and wound healing activity of the leaves of Annona squamosa Linn. (Annonaceae). Res. J. Pharmacogn. Phytochem. 2009, 1, 44–50. [Google Scholar]
- Gowdhami, M.; Sarkar, B.L.; Ayyasamy, P.M. Screening of Phytochemicals and Antibacterial Activity of Annona Squamosa Extracts. Int. J. Pharm. Sci. Invent. 2014, 3, 30–39. [Google Scholar]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kesari, A.N.; Murthy, P.S.; Chandra, R.; Tandon, V.; Watal, G.; Gupta, R.K.; Kesari, A.N.; Murthy, P.S.; Chandra, R.; et al. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J. Ethnopharmacol. 2005, 99, 75–81. [Google Scholar] [CrossRef]
- Kaleem, M.; Asif, M.; Ahmed, Q.U.; Bano, B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med. J. 2006, 47, 670–675. [Google Scholar] [PubMed]
- Shirwaikar, A.; Rajendran, K.; Kumar, C.D.; Bodla, R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin-nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 2004, 91, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, N.; Yadav, A.K.; Mishra, P.K.; Jain, P.K.; Rao, C.V. Evaluation of hepatoprotective activity of Annona squamosa leaves and bark extract against carbon tetrachloride liver damage in wistar rats. World J. Pharm. Pharm. Sci. 2016, 5, 1353–1360. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Rajendran, K.; Kumar, C.D. Oral antidiabetic activity of Annona squamosa leaf alcohol extract in NIDDM rats. Pharm. Biol. 2004, 42, 30–35. [Google Scholar] [CrossRef]
- Sobiya Raj, D.; Vennila, J.J.; Aiyavu, C.; Panneerselvam, K. The hepatoprotective effect of alcoholic extract of Annona squamosa leaves on experimentally induced liver injury in swiss albino mice. Int. J. Integr. Biol. 2009, 5, 182–186. [Google Scholar]
- El-baz, D.M.; Hssan, A.K. Effects of Egyptian Annona squamosa leaves extracts against alloxan induced hyperglycemia in rats. World J. Pharm. Pharm. Sci. 2019, 8, 145–163. [Google Scholar] [CrossRef]
- Rajeshkumar, A.; Tamilarasan, B.; Sivakumar, V. Phytochemical screening and hepatoprotective efficacy of leaves extracts of Annona squamosa against paracetamol induced liver Toxicity in rats. Int. J. Pharmacogn. 2015, 22, 178–185. [Google Scholar] [CrossRef]
- Ibrahim, F.; Jaber, A.; Ibrahim, G.; Cheble, E. Antioxidant activity and total phenol content of different plant parts of Lebanese Annona Squamosa Linn. Int. J. Pharm. Pharm. Sci. 2020, 100–105. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Vijayalakshmi, S.; Devi Rajeswari, V. Phytochemical and pharmacological properties of Annona muricata: A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 3–6. [Google Scholar]
- Sharma, S.K.; Gupta, M.L.; Kumar, B. Hypocholesterolemic Efficacy of Annona Squamosa (L.) Extract In Mice Diabetic Models. J. Biotechnol. Biochem. 2019, 5, 41–45. [Google Scholar] [CrossRef]
- Tomar, R.S.; Sisodia, S.S. Antidiabetic activity of Annona squamosa Linn. in alloxan-induced diabetic rats. Int. J. Green Pharm. 2014, 8, 237–241. [Google Scholar] [CrossRef]
- Pandey, N.; Barve, D. Phytochemical and pharmacological review on Annona squamosa Linn. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1404–1412. [Google Scholar]
- Wu, Y.C.; Hung, Y.C.; Chang, F.R.; Cosentino, M.; Wang, H.K.; Lee, K.H. Identification of ent-16β,17-dihydroxykauran-19-oic acid as an anti- HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J. Nat. Prod. 1996, 59, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Prakash Mishra, A.; Nautiyal, A.R.; Mishra, A.P.; Nautiyal, A.R.; Prakash Mishra, A.; Nautiyal, A.R. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—A review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Nandita, H.; Manohar, M.; Gowda, D.V. Recent review on oxidative stress, cellular senescence and age-associated diseases. Int. J. Res. Pharm. Sci. 2020, 11, 1331–1342. [Google Scholar] [CrossRef]
- Ola, A.R.B.; Sugi, Y.; Lay, C.S. Isolation, identification and antimicrobial activity of secondary metabolites of endophytic fungi from annona leaves (Annona squamosa L.) growing in dry land. IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012039. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Fang-nan, K.O.; Wen-yan, L.I.; You, W.E.; Jing, Z.H.; Jing, Z.H.; Zhi-qiang, Y.A.; Xiu-fen, Z. Optimization of Extraction Process of Polyphenols from Annona squamosa Leaves and Its Antioxidant Activity in Vitro. Sci. Technol. Food Ind. 2020, 41, 162–168. [Google Scholar]
- Babu Marahatta, A.; Aryal, A.; Chandra Basnyat, R.; Anant Babu Marahatta, C. The phytochemical and nutritional analysis and biological activity of Annona squamosa Linn. Int. J. Herb. Med. 2019, 7, 19–28. [Google Scholar]
- Cosentino, S.; Tuberoso, C.I.G.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Shanker, K.S.; Kanjilal, S.; Rao, B.V.S.K.; Kishore, K.H.; Misra, S.; Prasad, R.B.N. Isolation and antimicrobial evaluation of isomeric hydroxy ketones in leaf cuticular waxes of Annona squamosa. Phytochem. Anal. 2007, 18, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Dholvitayakhun, A.; Trachoo, N.; Sakee, U.; Cushnie, T.P.T.T. Potential applications for Annona squamosa leaf extract in the treatment and prevention of foodborne bacterial disease. Nat. Prod. Commun. 2013, 8, 1934–1948. [Google Scholar] [CrossRef]
- Uduman, M.S.T.S.; Sundarapandian, R.; Muthumanikkam, A.; Kalimuthu, G.; Parameswari, S.A.; Srinivas, T.R.V.; Karunakaran, G. Protective effect of methanolic extract of Annona squamosa linn in isoniazid-rifampicin induced hepatotoxicity in rats. Pak. J. Pharm. Sci. 2011, 24, 129–134. [Google Scholar]
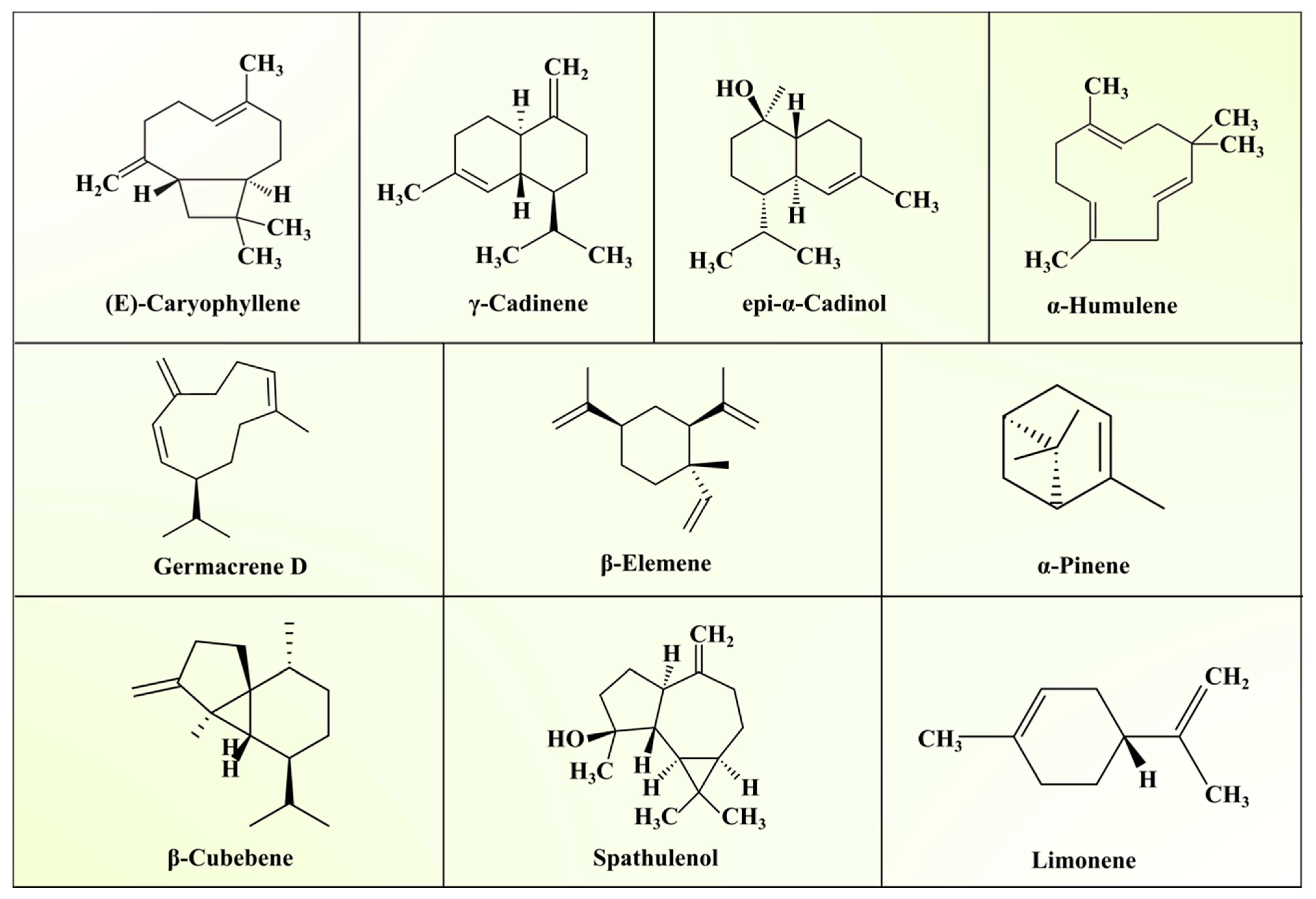
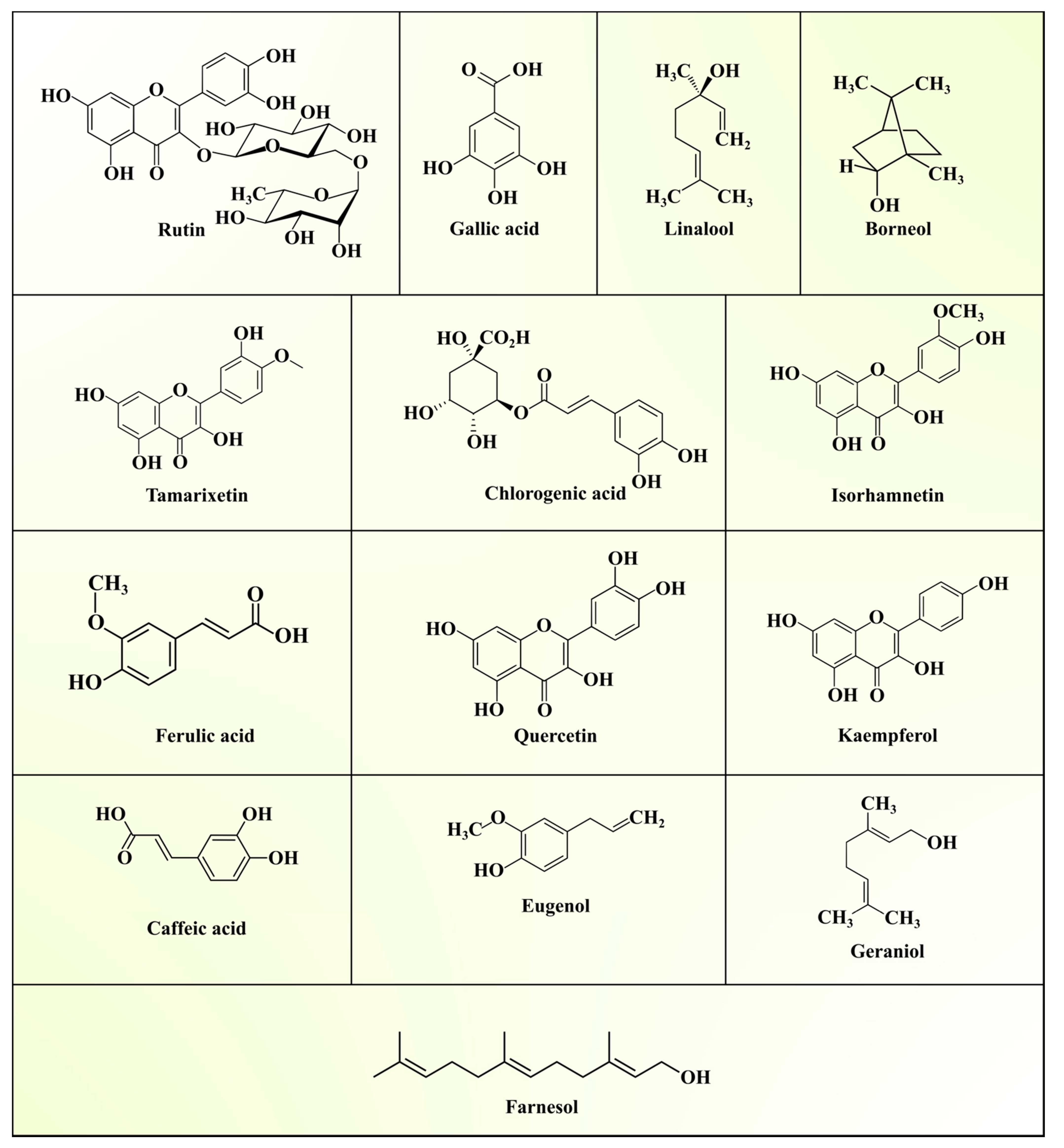
| Group | Composition | References |
|---|---|---|
| Protein | mg/g of FW (fresh weight) | [27] |
| Egyptian sites | ||
| Menofia | 13.47 ± 0.11 | |
| Giza | 6.80 ± 0.11 | |
| Alexandria | 3.52 ± 0.10 | |
| Mansoura | 17.26 ± 0.02 | |
| Essential oil profile | ||
| North Indian (Foothills region) ASLEO | Composition (%) | [35] |
| (E)-Caryophyllene | 15.9 | |
| γ-Cadinene | 11.2 | |
| epi-α-Cadinol | 9.4 | |
| (Z)-Caryophyllene | 7.3 | |
| γ-Muurolene | 5.4 | |
| α-Humulene | 5.2 | |
| North Indian (Plains) ASLEO | [36] | |
| β-Caryophyllene | 22.9 | |
| Germacrene D | 21.3 | |
| Bicyclogermacrene | 8.5 | |
| β-Elemene | 7.8 | |
| γ-Cadinene | 6.7 | |
| α-Muurolol | 5.7 | |
| Vietnamese ASLEO | [37] | |
| α-Pinene | 1.0–11.9 | |
| Limonene | 0.8–11.7 | |
| β-Cubebene | 0.5–13.0 | |
| β-Caryophyllene | 11.6–24.5 | |
| Spathulenol | 0.8–9.0 | |
| Caryophyllene oxide | 1.0–10.6 | |
| α-Cadinol | 3.3–7.8 | |
| Brazilian ALEO | [38] | |
| (E)-Caryophyllene | 27.4 | |
| Germacrene D | 17.1 | |
| Bicyclogermacrene | 10.8 | |
| (Z)-Caryophyllene | 7.3 | |
| β-Elemene | 6.2 | |
| δ-Elemene | 4.1 |
| Variety | Type of Extract | Essential Oil Components Identified | References |
|---|---|---|---|
| ASLs, Lucknow, India | Hydro-distilled essential oil | Limonene, terpinolene, bicyclogermacrene, γ-cadinene, α-copaene, α-muurolol, β-bourbonene, δ-cadinene, (Z)-nerolidol, β-elemene, β-caryophyllene, (E)-nerolidol, caryophyllene oxide, γ-elemene, aromadendrene, γ-eudesmol, germacrene D, α-humulene | [36] |
| ASLs | Methanol, petroleum ether, chloroform, and water extracts | Linalool, borneol, eugenol, farnesol, geraniol | [39] |
| ASLs, city of Sao Cristovao, Sergipe, Brazil | Hydro-distilled essential oil using a Clevenger-type apparatus | α-Pinene, camphene, limonene, δ-elemene, α-copaene, β-bourbonene, β-elemene, (Z)-caryophyllene, (E)-caryophyllene, α-humulene, germacrene D, viridiflorene, bicyclogermacrene, germacrene A, γ-cadinene, δ- cadinene, germacrene B, spathulenol, caryophyllene oxide, epi-α-cadinol, α-cadinol | [40] |
| Variety | Type of Extract | Bioactive Compounds Identified | References |
|---|---|---|---|
| ASLs, Diliman, Quezon City, Philippines | 95% ethanol extract | Acetogenin murihexocin C | [48] |
| ASLs | Ethanol extract | Stigmasterol acetate, 4,4-tert-butylcalix(4)areve, sodium benzoate, 4,4-dimethylcholertrol, isoamylacetyate, butyloctylpthalate | [49] |
| ASLs, Mayurbhanj, Orissa, India | Water, petroleum ether, and methanol extracts | Linalool, flavonoids, eugenol, borneol, geraniol, farnesol | [50] |
| ASLs, Fortaleza, State of Ceara, Brazil | 80% methanol extract | O-methylarmepavine, C37 trihydroxy adjacent bistetrahydrofuran acetogenins | [51] |
| ASLs, Chennai, Tamilnadu, India | Ethanol extract | Rutin, kamepherol, quercetin, isorhamnetin, farmarixetin | [52] |
| ASLs, Fukuoka, Japan | Methanol extract | Lanuginosine, liriodenine, lysicamine | [53] |
| ASLs | Methanol extract | 5,7,4′-trihydroxy-6,3′dimethoxy-flavone 5-O-α-L-rhamnopyranoside | [54] |
| ASLs, Dharwad, Karnataka, India | Water, methanol, and chloroform extracts | Phenols, glycosides, flavonoids, saponins, tannins, alkaloids, steroids, and carbohydrates | [1] |
| ASLs, Kattakada, Thiruvananthapuram, Kerala, India | Water, acetone, and chloroform extracts | Alkaloids, glycosides, saponins, oils, and flavanoids | [55] |
| ASLs, Pune, India | Water and methanol extracts | Saponins, tannins, anthroquinones, phenols, flavonoids, and glycosides | [56] |
| ASLs, Bhopal, Madhya Pradesh, India | 80% ethanol extract | Rutin (quercetin-3-rhamnosyl glucoside) | [57] |
| ASLs, Junagadh, Gujarat, India | Methanol extract | Gallic acid, quercetin, chlorogenic, cinnamic acid, ferullic acid, caffeic acid, and salicylic acid | [58] |
| ASLs, Duyen Hai, Tra Vinh, Vietnam | Water and ethanol extracts | Alkaloids, saponins, coumarins, flavonoids, cardiac glycosides, phenols, and tannins | [59] |
| Variety | Type of Extract | Bioactive Compounds Identified | Type of Cell Lines/Type of Study | Major Findings and Molecular Mechanisms of Action | References |
|---|---|---|---|---|---|
| Anti-cancer activities | |||||
| Leaves were obtained from a local plant nursery in Ta’if City, Saudi Arabia | Methanolic and acetonic extracts | Phenolics, annonaceous acetogenins, saponins, flavonoids, alkaloids, glycosides, alkaloids, steroids, and terpenoids | MCF-7 and MDA-MB-231 breast cancer cell lines | 100 µg/mL extract decreases cell viability and reduced their proliferation to ~60%. | [62] |
| Leaves were obtained from botanical garden in Narsapur, W.G.Dt, South India | Extracts prepared using double distilled water | NA | HeLa (cervical) cancer cell line | IC50 value against HeLa cells was estimated to be 25 μg/mL. | [67] |
| Leaves were obtained from Lumajang Regency, East Java, Indonesia. | Ethanolic extract | 12,15-cis-squamostatin-A, bullatacin | Human colon cancer cell lines (WiDr) | IC50 value against WiDr cells was estimated to be 292.39 µg/mL. | [68] |
| Leaves were obtained from department of Pharmacognosy, Faculty of Pharmacy, University of Karachi, Pakistan | Ethanolic extract | Annoreticuin and Isoannoreticuin | Colon cancer cell line (HCT-116), breast carcinoma cell line (MDA-MB-435), prostatic cancer cell line (DU145), human epidermoid carcinoma cell line (KB-3-1), lung cancer line (H460), and hepatocellular carcinoma cell line (BEL7404) | IC50 values of 13.66 µg/mL for KB 3-1 cells, 1.37 µg/mL for HCT-116 cells, 74.51 µg/mL for HEK293 cells, 53.86 µg/mL for KB-3-1 cells, and 15.06 µg/mL for HCT-116 cells. | [55] |
| Anti-diabetic activity | |||||
| ASLs were collected from IARI, New Delhi, India | Ethanolic extract | In vivo (Wistar strain of rats with alloxan (80 mg/kg) and STZ (50 mg/kg i.p.) induced diabetes) | Administration of ASLs extract (350 mg/kg), significantly reduced the FBG by 6%, 26.8%, and 13% in normal, alloxan-induced, and STZ induces diabetic rats, respectively, and improved the glucose tolerance in diabetic rats. ASL also reduced TC (by 49.3%), LDL (by 71.9%), and TG (by 28.7%) and increased HDL (by 30.3%) in severely diabetic mouse. | [69] | |
| Young ASLs were collected from Painkulam village, Tamil Nadu, India. | 95% Ethanol extract | In vivo (Wister male albino rats with STZ (65 mg/kg i.p.) induced diabetes) | Administration of ASLs extract (250 mg/kg and 500 mg/kg) exhibited a significant reduction of FBG and increased the insulin level. | [70] | |
| ASLs | Hexane extract | In vitro (L6 Myotubes) and in vivo (Ob/ob mice modal) | ASLs hexane extract (500 mg/kg b.i.d. p.o.) improved the glucose uptake, stimulated the IR-β and IRS-1 phosphorylation, and promoted the upregulation of mRNA (GLUT4 and PI3 kinase) in L6 myotubes. ASLs hexane extract inhibited the PTP1B with an IC50 17.4 µg/mL Oral administration of ASL hexane extract significantly declined random glucose (27.7%) and TG (30.5%). | [51] | |
| ASLs | Water extract, Hexane extract, and methanol extract | In vivo (CF strain rats) | Hexane extract inhibited the α-glucosidase (75.69 ± 1.7%) in comparison to acarbose (53.60 ± 1.45%) Hexane extract (100 mg/kg and 400 mg/kg) improved insulin level (11.58 ± 1.8 µU/mL and 16.28 ± 1.2 µU/mL) and reduced BGL (41.18 ± 2.46% and 78.10 ± 1.57%), respectively. | [52] | |
| Young ASLs were collected from the Regional Research Institute of Unani Medicine, Aligarh, India. | Aqueous extract | In vivo (male albino Wister rats with STZ (55 mg/kg i.p.) induced diabetes) | Oral administration of ASLs extract (300 mg/kg) significantly reduced the BGL, lipid peroxidation and also increased the activity of the antioxidant enzymes. | [71] | |
| ASLs collected from Kolli Hills, Tamil Nadu, India | 95% ethanol extract | In vivo (male albino Wister rats with STZ (50 mg/kg i.p.) induced diabetes) | ASLs extract (100 mg/kg) significantly reduced BGL, glycosylated hemoglobin, creatinine, and urea. | [72] | |
| ASLs were collected from IARI, New Delhi, India | Water extract | In vivo (Albino Wister rats with STZ (50 mg/kg i.p.) induced diabetes) | ASLs extract (350 mg/kg) significantly improved the lipid profile and increased the activities of antioxidant enzymes. | [66] | |
| ASLs were collected locally, Indore, India. | 80% Methanol | Quercetin-3-O-glucoside | In vivo (Wistar male rats with alloxan monohydrate (120 mg/kg i.p.) induced diabetes) | Quercetin-3-O-glucoside (15 mg/kg p.o.) significantly increased serum insulin, decreased glucose, reduced oxidation of lipid (p < 0.001), and increased antioxidant enzyme activity (p < 0.001). | [49] |
| Antioxidant activities | |||||
| ASLs, Rajshahi, Bangladesh | Methanol extracts | Phenols and flavonoids | In vitro | IC50 of 7.81 ± 0.1 μg·mL−1 for DPPH, IC50 of 29.60 ± 0.17 (μM of Trolox for oxygen radical absorbance capacity (ORAC). | [73] |
| ASLs, Tamil Nadu, India | Ethanol extracts | Flavonoids | In vitro | IC50 of DPPH, ABTS, superoxide dismutase, nitric oxide, and lipid peroxidation were found to be 110, 40, 115, 60, and 955 μg·mL−1, respectively. | [74] |
| ASLs, Andhra Pradesh, India | Ethanol extracts | Flavones | In vitro | % DPPH radical scavenging was 45.62 at 100 (μg·mL−1) concentration. | [60] |
| ASLs, Egypt | Methanol 80%, acetone 50%, ethanol 50%, and boiling water. | Phenols | In vitro | Total antioxidant activity was reported highest in acetone extract i.e., 1625.38 ± 68.55 ascorbic acid/g of extract, and lowest in case of water 639.65 ± 22.17 ascorbic acid/g of extract. | [3] |
| Anti-microbial activities | |||||
| Leaf Extract (India) | Control (1 mL of 2% Gum acacia) | Steroids Alkaloids Glycosides Saponin Flavonoid Tannin Triterpenoid | Antibacterial activity and measurement of wound healing activity of ASLs extract in Albino wistar rats. | Period of epithelisation—25 days | [61] |
| Petroleum ether | Zone of inhibition—19–22 mm at MIC-200 mg/ 0.1 mL. On addition of 300 mg/ kg (ED50 value) petroleum ether extract, wound healing induced within 16 days. | ||||
| Chloroform-water | Zone of inhibition- 19–21 mm at MIC-200 mg/ 0.1 mL. On addition of 300 mg/kg (ED50 value) chloroform-water extract, wound healing induced within 19 days. | ||||
| Alcohol | Zone of inhibition- 18–20 mm at MIC-200 mg/ 0.1 mL. On addition of 300 mg/ kg alcoholic extract, wound healing induced within 18 days. | ||||
| ASLs extract (Egypt) | Methanol 80% | Carbohydrates, tannins, phenolic compounds, polyphenols, and flavonoids | Antibacterial activity of ASLs extracts using disc-diffusion method against six bacterial species. | Zone of inhibition diameter: 12–13 mm with 38–43% inhibition. | [3] |
| Acetone 50% | Zone of inhibition diameter: 14–16 mm with 41–51% inhibition. | ||||
| Ethanol 50% | Zone of inhibition diameter: 12–14 mm with 35–48% inhibition. | ||||
| Boiling water | Zone of inhibition diameter: 9–11 mm with 28–36% inhibition. | ||||
| ASLs extract (India) | Ethanol extract 25% 50% 75% 100% | Polyphenols tannins, and terpenoids | Estimation of the effect of ASLs extract on inhibition of six bacterial species | Zone of inhibition (in mm) 8–15 mm 10–17 mm 13–19 mm 15–22 mm | [74] |
| Hepato-protective and lipid lowering effect | |||||
| Leaves obtained near to NBRI, Lucknow, India | Ethanolic extract | carbon tetrachloride induced Wistar rats | Significant hepatoprotective effect was reported at oral dose of 450 mg/kg. Effects were comparable to silymarin. | [75] | |
| ASLs extract | Ethanolic extract | Diethylnitrosamine induced Swiss albino mice | Dose of 5 g/Kg exhibited hepatoprotective effects. | [76] | |
| ASLs extract | Aqueous extracts | Albino rats | ASLs extract protect the hepatic cells, paracetamol-induced increased level of bilirubin, cholesterol, and triglycerides level, which get normalised after treatment. | [77] | |
| ASLs extract | Methanolic extract | 5,7,40-trihydroxy- 6,30dimethoxy flavone 3-O-a-L-rhamnopyranoside | Wistar albino rats | Significantly reduced hepatic lipid peroxidation and improved serum lipid profile. | [78] |
| ASLs collected from Madurai, TN, India | Methanolic extract | - | Isoniazid-rifampicin induced rats | ALs extract protects against rifampicin-induced oxidative liver injury. | [79] |
| Leafy twigs of A. squamosa | Methanolic extract | - | Streptozotocin induced mice diabetic models | Significantly lower levels of TC and TGs was reported compared to diabetic control mice. | [74] |
| Fresh ASLs collected from Al-Nobaria, Egypt | Aqueous and ethanolic extract | - | Alloxan-induced hyperglycemic rats | Significantly reduced CL, TGs, and LDL-cholesterol and increased HDL-cholesterol compared to diabetic rats. | [80] |
| ASLs collected near Udaipur, India | 70% alcohol | - | Streptozotocin induced Albino rats | Maintained the lipid profile (reduced CL, TGs, and LDL-cholesterol and increased HDL). | [81] |
| ASLs were collected near IARI, New Delhi, India | Ethanolic extract | - | Alloxan and streptozotocin induced Wistar rats and albino rabbits | After 15 days of treatment, lipid profile maintains nearly normal level and increased the HDL cholesterol. | [69] |
| ASLs extract | Water extract | - | Streptozotocin induced Albino Wistar rats | Treatment enhanced the activity of antioxidant enzyme and reduces MDA, CL, and TGs. | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. https://doi.org/10.3390/biom11050614
Kumar M, Changan S, Tomar M, Prajapati U, Saurabh V, Hasan M, Sasi M, Maheshwari C, Singh S, Dhumal S, et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules. 2021; 11(5):614. https://doi.org/10.3390/biom11050614
Chicago/Turabian StyleKumar, Manoj, Sushil Changan, Maharishi Tomar, Uma Prajapati, Vivek Saurabh, Muzaffar Hasan, Minnu Sasi, Chirag Maheshwari, Surinder Singh, Sangram Dhumal, and et al. 2021. "Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities" Biomolecules 11, no. 5: 614. https://doi.org/10.3390/biom11050614
APA StyleKumar, M., Changan, S., Tomar, M., Prajapati, U., Saurabh, V., Hasan, M., Sasi, M., Maheshwari, C., Singh, S., Dhumal, S., Radha, Thakur, M., Punia, S., Satankar, V., Amarowicz, R., & Mekhemar, M. (2021). Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules, 11(5), 614. https://doi.org/10.3390/biom11050614











