Stabilization of Dry Sucrose Glasses by Four LEA_4 Proteins from Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LEA Proteins
2.3. Sample Preparation
2.4. FTIR Spectroscopy
3. Results
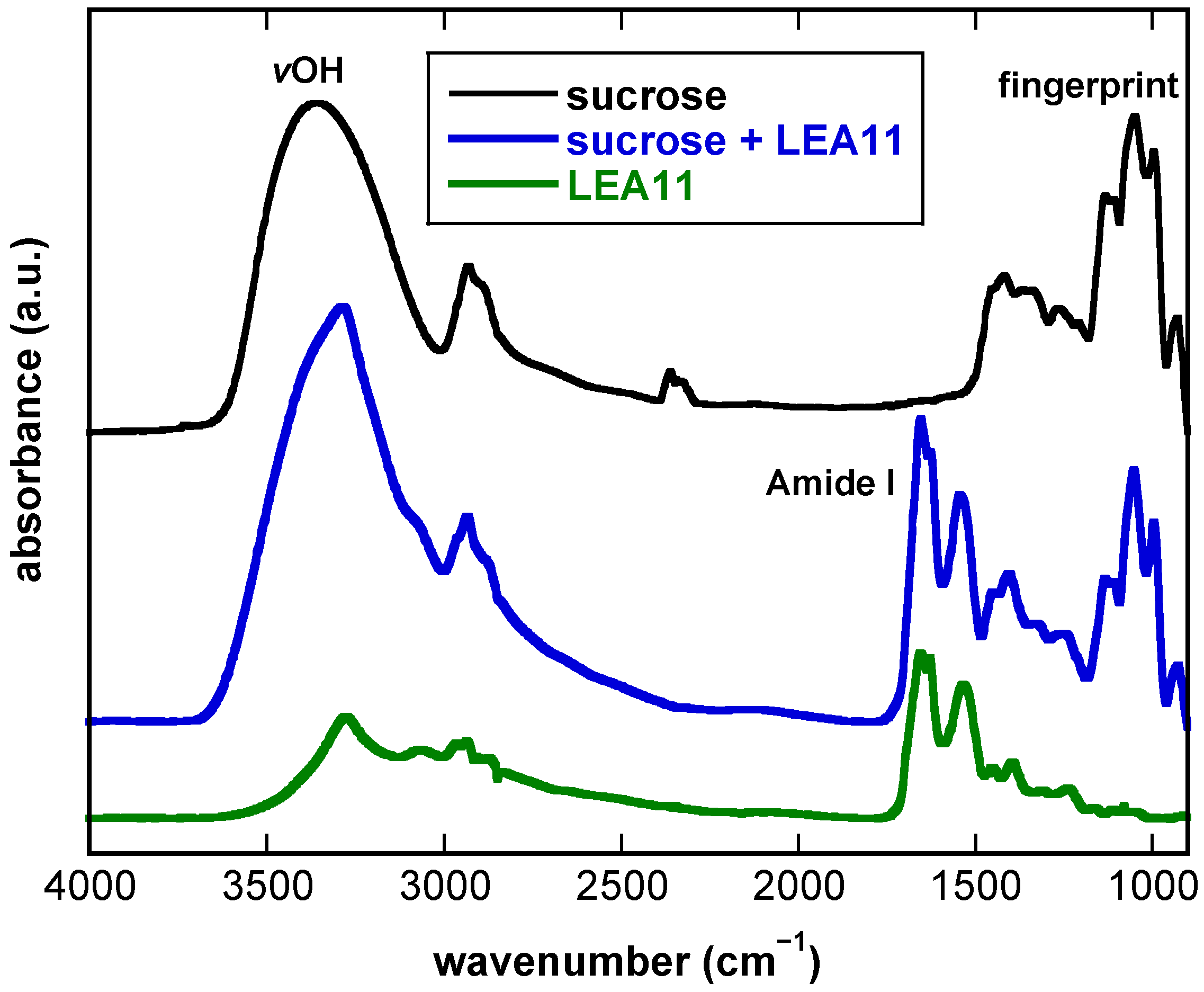
3.1. FTIR Spectra of Dry Suc
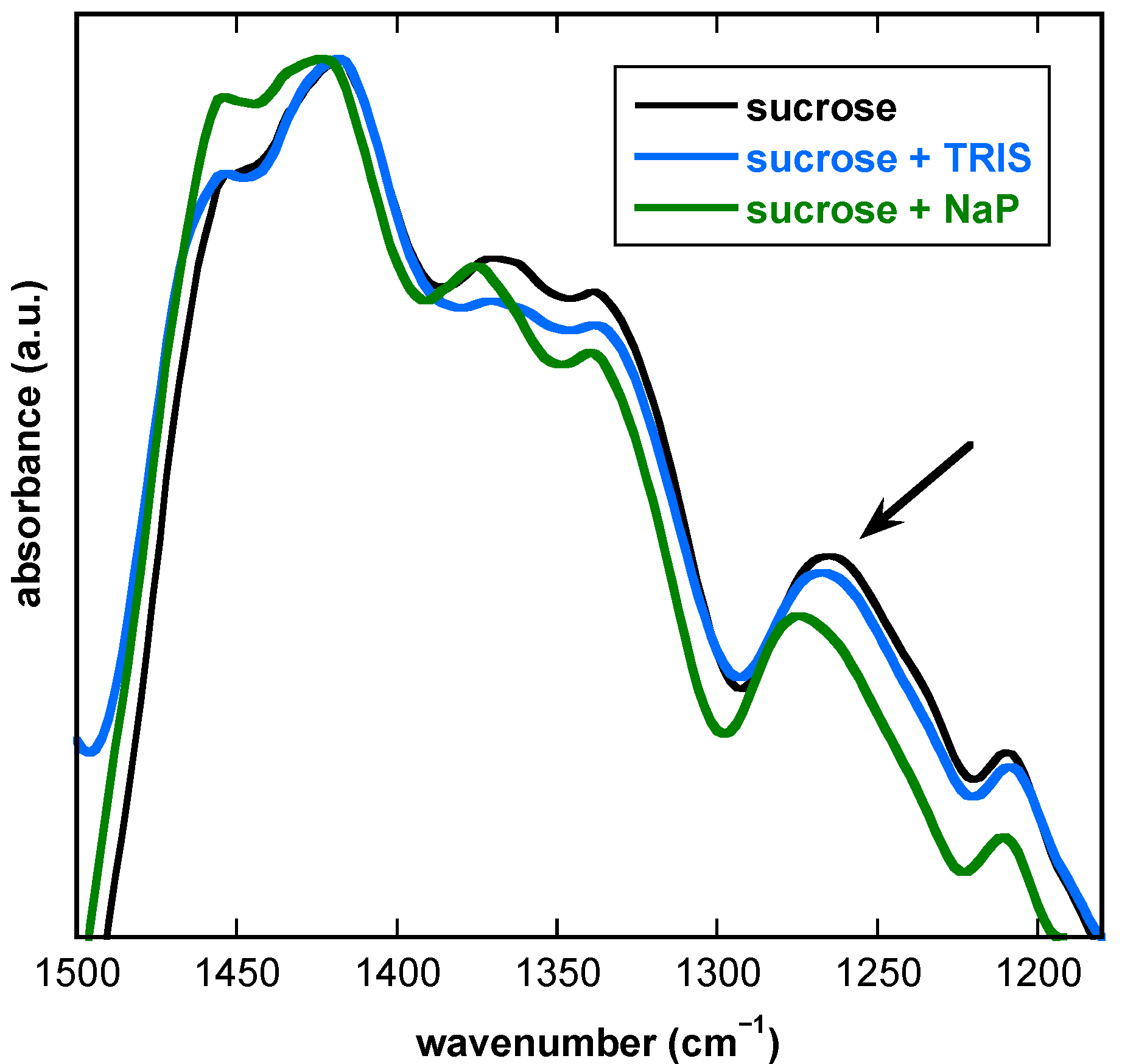
3.2. The Presence of Buffer Components Influences the Properties of Dry Suc Glasses
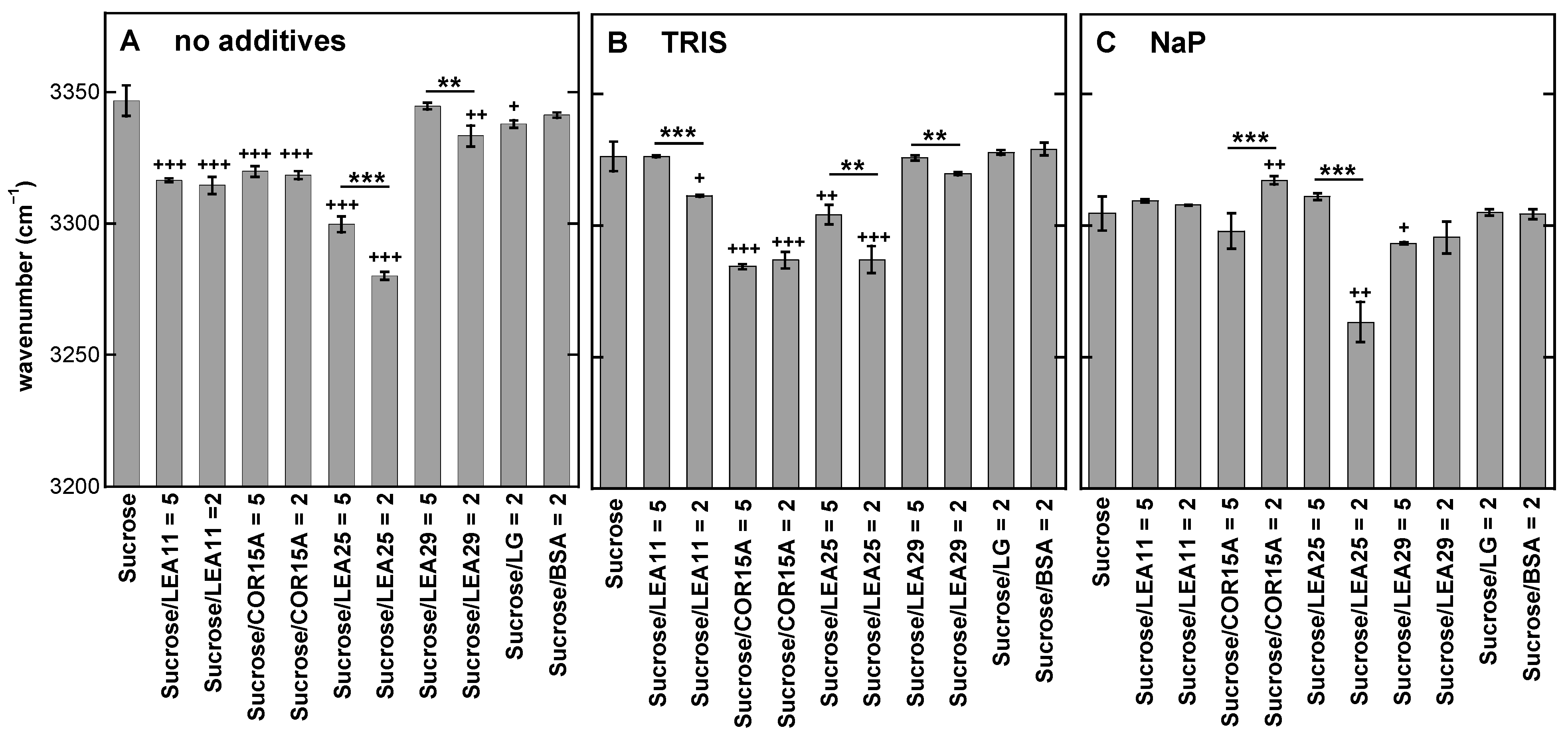
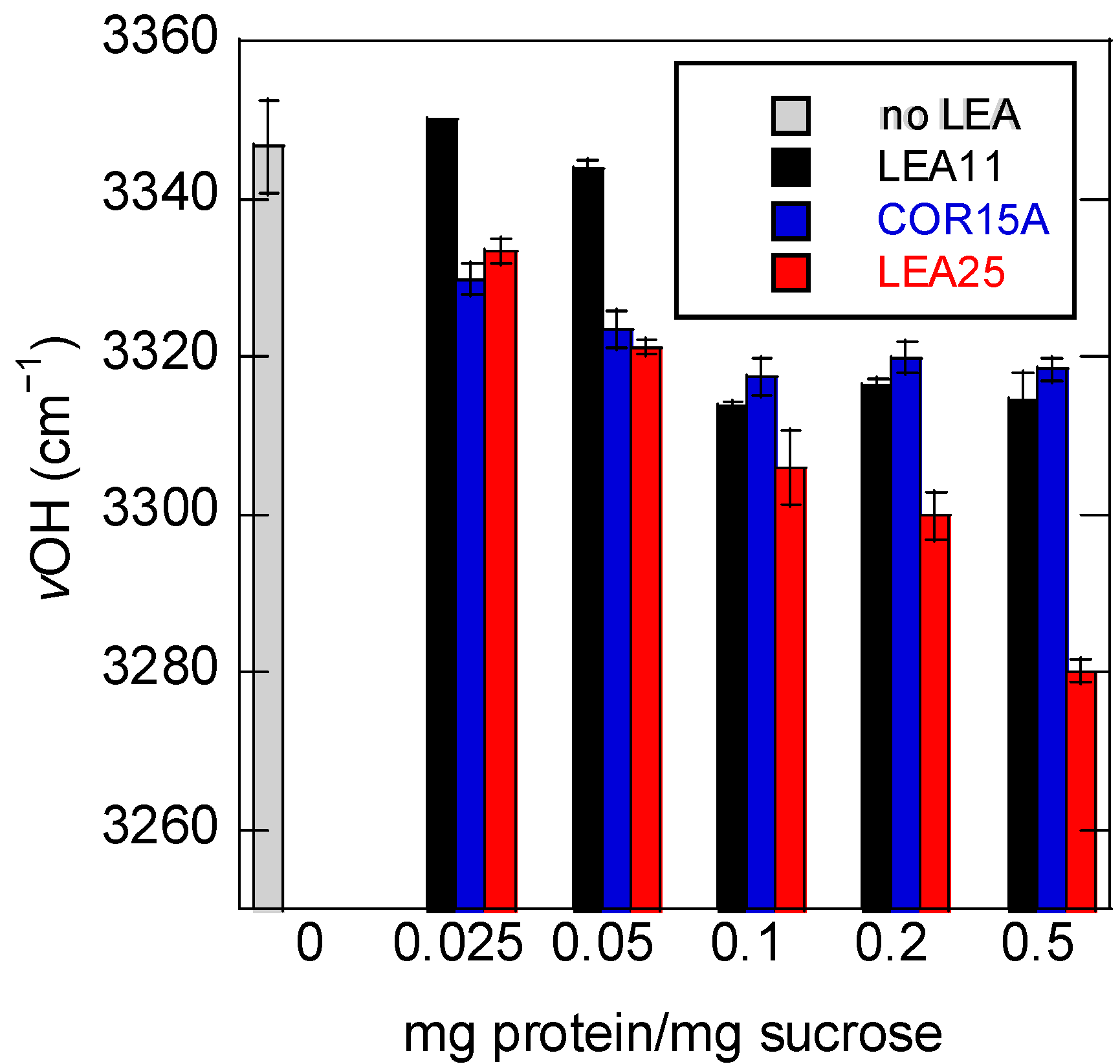
3.3. Addition of Proteins Influences the Position of the νOH Peak of Suc in the Glassy State
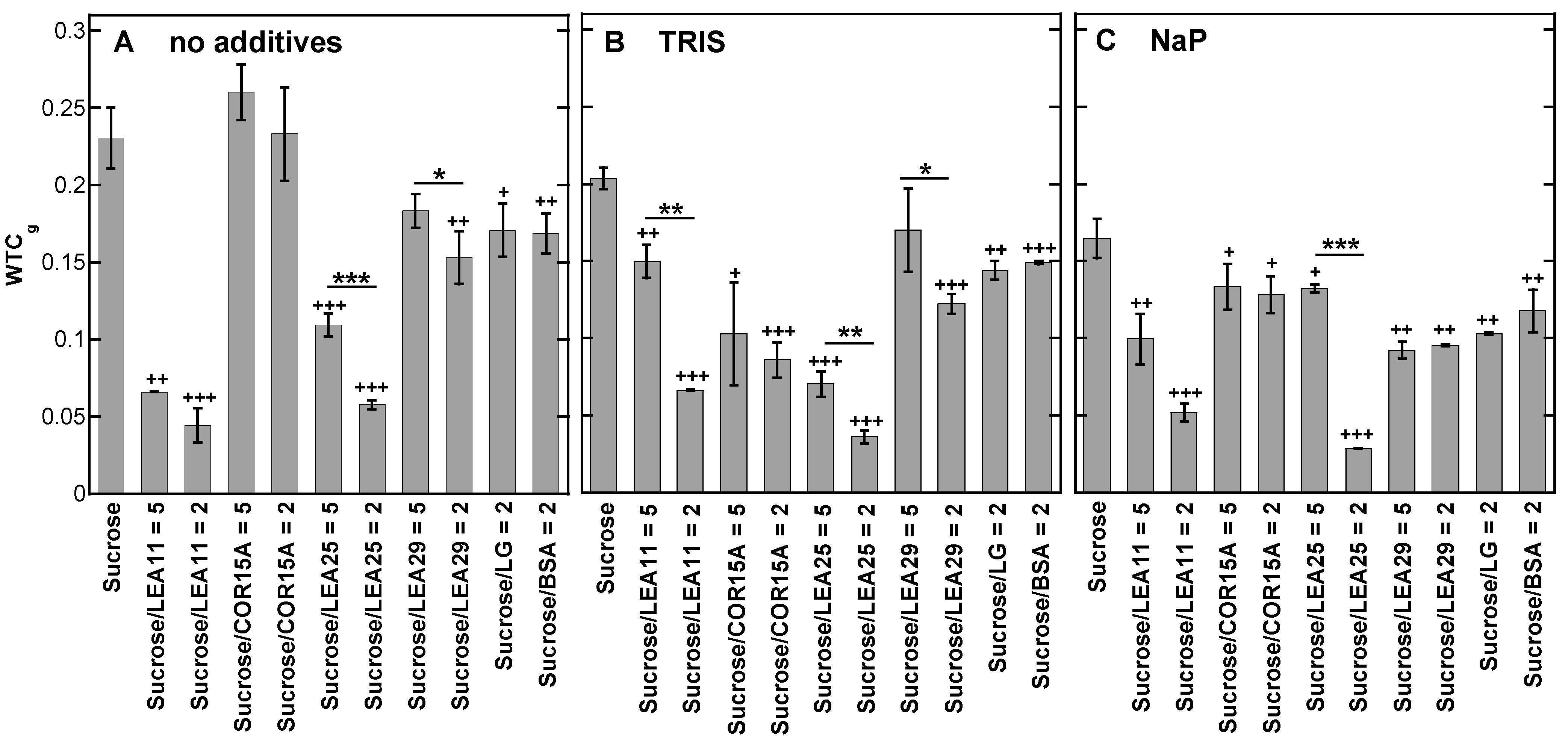
3.4. Strength of H-Bonding Interactions in Dry Suc Glasses
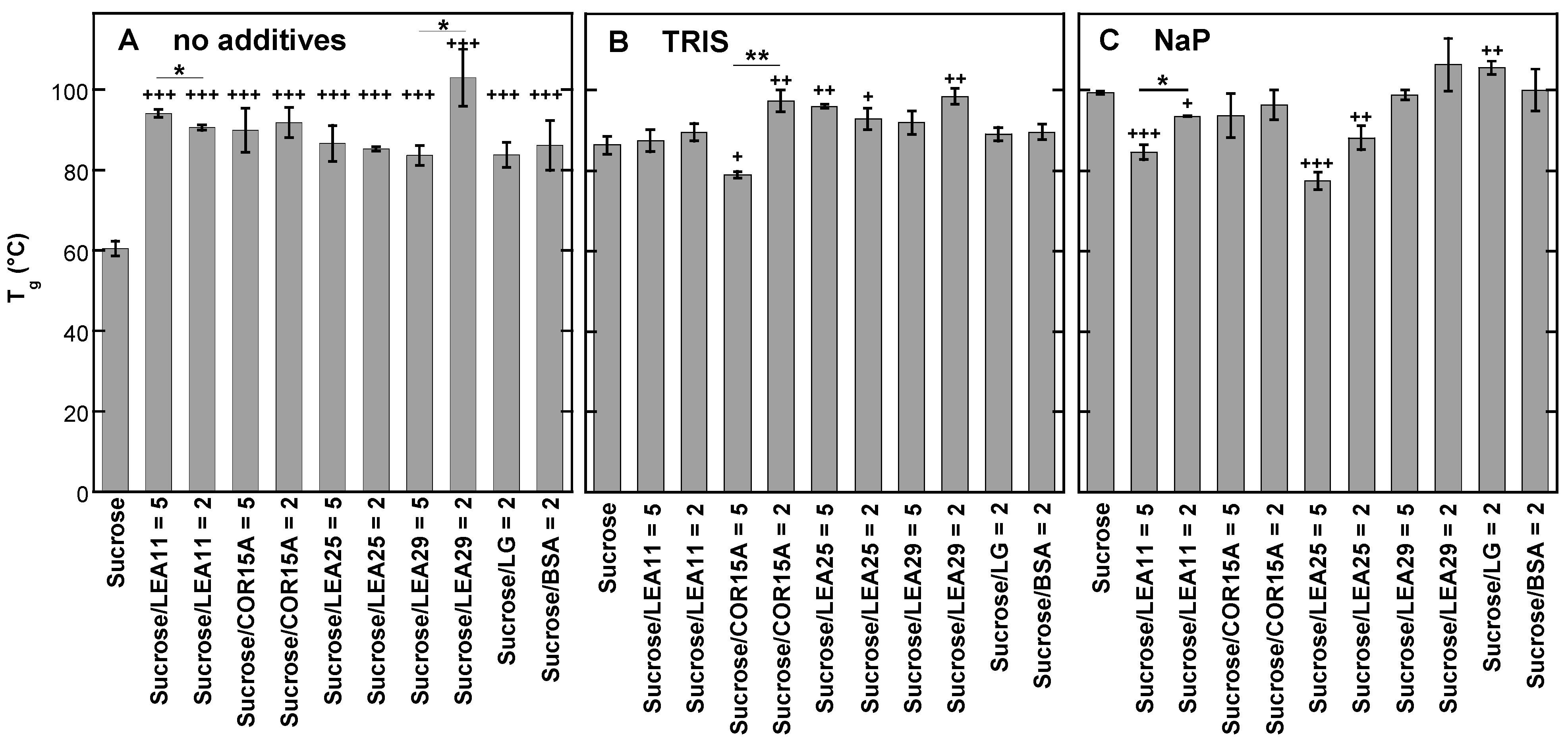
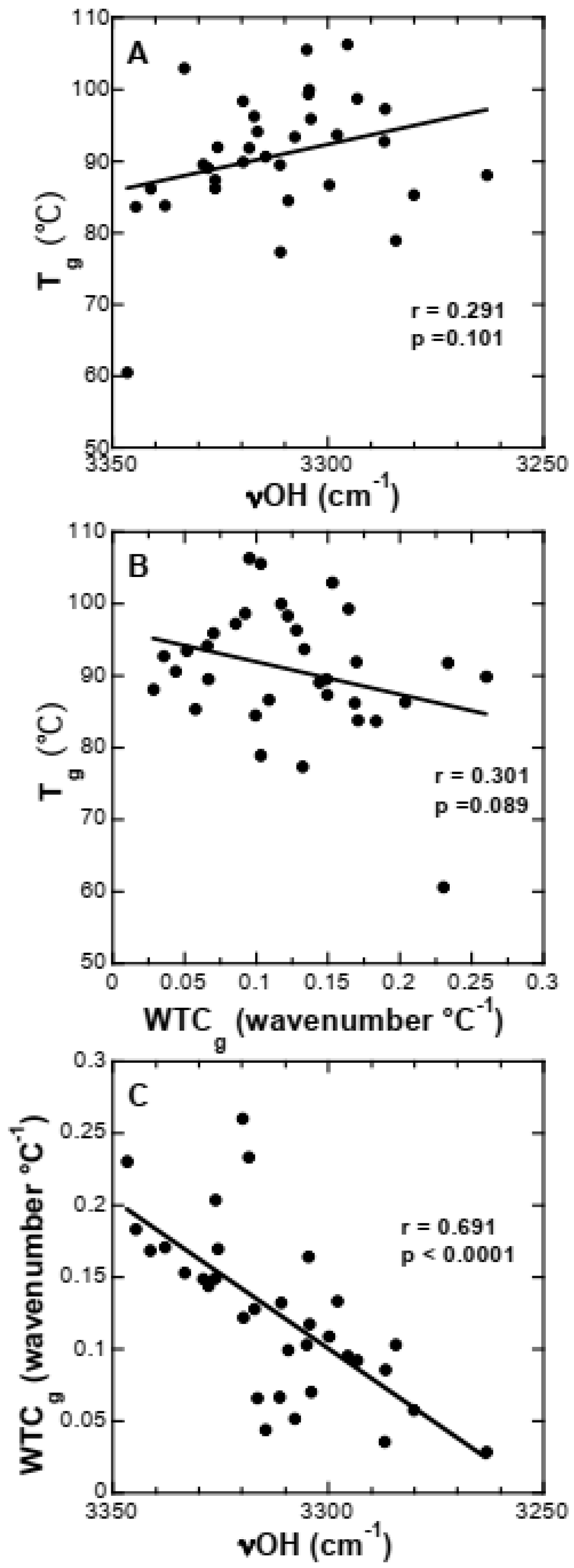
3.5. Effect of Buffer Components and Proteins on Tg
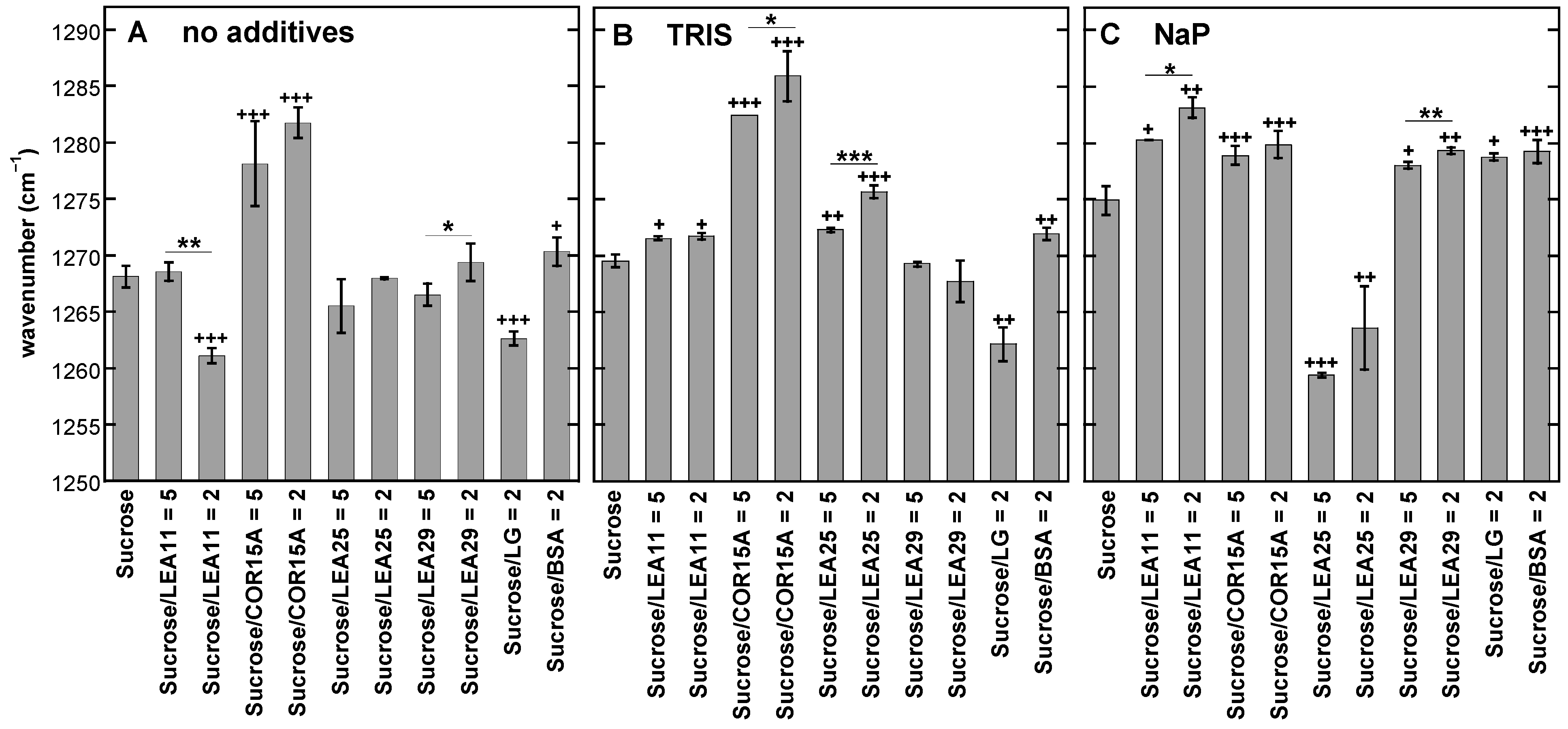
3.6. Influence of Buffer Components and Proteins on the Fingerprint Region of FTIR Spectra from Dry Suc Glasses
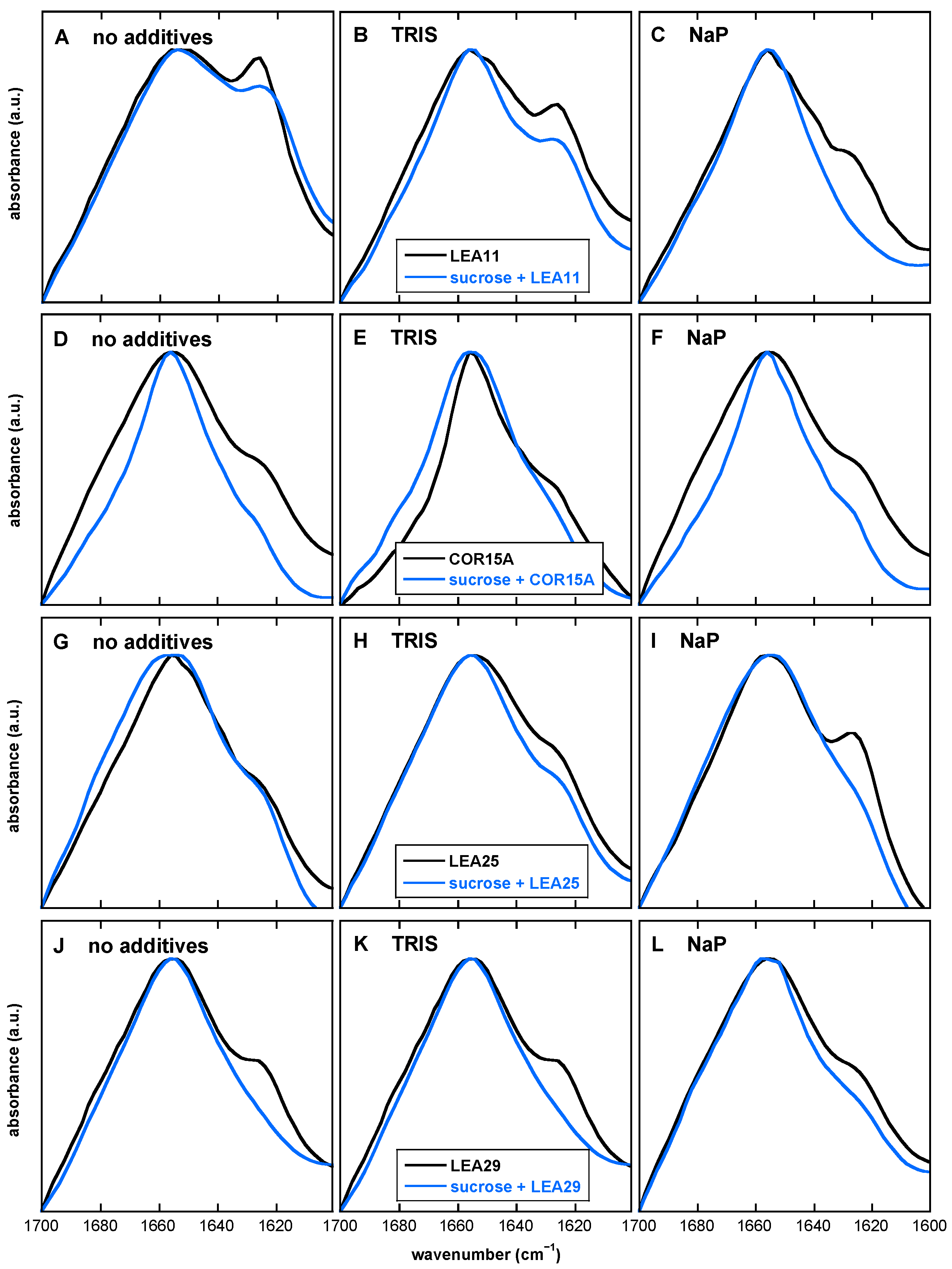
3.7. The Presence of Both Buffer Components and Suc Influences the Extent of LEA Protein Aggregation in the Dry State
4. Discussion
4.1. Tris and Phosphate Buffer Components Increase Number and Strength of H-Bonding Interactions in Suc Glasses and Alter the Molecular Structure of Suc in the Dry State
4.2. LEA Proteins Stabilize Suc Glasses Dehydrated from Water or Tris Buffer and Influence the Molecular Structure of Dry Suc
4.3. The Desiccation-Induced Suc Glassy State Reduced Protein Aggregation of LEA Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| FTIR | Fourier-transform infrared spectroscopy |
| IDP | Intrinsically disordered protein |
| LEA | Late embryogenesis abundant |
| LG | β-lactoglobulin |
| NaP | Sodium phosphate |
| Suc | Sucrose |
| TFE | 2,2,2-trifluoroethanol |
| Tg | Glass transition temperature |
| νOH | Vibration peak of OH groups |
| WTCg | Wavenumber–temperature coefficient in the glassy state |
References
- Lapinski, J.; Tunnacliffe, A. Anhydrobiosis without trehalose in bdelloid rotifers. FEBS Lett. 2003, 553, 387–390. [Google Scholar] [CrossRef]
- Tunnacliffe, A.; Lapinski, J. Resurrecting Van Leeuwenhoek´s rotifers: A reappraisal of the role of disaccharides in anhydrobiosis. Phil. Trans. R. Soc. Lond. B 2003, 358, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar] [CrossRef]
- Cacela, C.; Hincha, D.K. Low amounts of sucrose are sufficient to depress the phase transition temperature of dry phosphatidylcholine, but not for lyoprotection of liposomes. Biophys. J. 2006, 90, 2831–2842. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Crowe, J.H. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 1989, 28, 3916–3922. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Aurell Wistrom, C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 1987, 242, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Rudolph, A.S.; Aurell Wistrom, C.; Spargo, B.J.; Anchordoguy, T.J. Interaction of sugars with membranes. Biochim. Biophys. Acta 1988, 947, 367–384. [Google Scholar] [CrossRef]
- Ballesteros, D.; Walters, C. Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: Relevance to the physiology of dry biological systems. Plant J. 2011, 68, 607–619. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O. Glass formation in plant anhydrobiotes: Survival in the dry state. Cryobiology 2004, 48, 215–228. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Orford, P.D.; Parker, R.; Ring, S.G. Aspects of the glass transition behaviour of mixtures of carbohydrates of low molecular weight. Carbohydr. Res. 1990, 196, 11–18. [Google Scholar] [CrossRef]
- Imamura, K.; Fukushima, A.; Sakaura, K.; Sugita, T.; Sakiyama, T.; Nakishi, K. Water sorption and glass transition behaviors of freeze-dried sucrose-dextran mixtures. J. Pharm. Sci. 2002, 91, 2175–2181. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Nguyen, K.H.N.; Crowe, L.M. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta 1996, 1280, 187–196. [Google Scholar] [CrossRef]
- Wolkers, W.F.; McCready, S.; Brandt, W.F.; Lindsey, G.G.; Hoekstra, F.A. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim. Biophys. Acta 2001, 1544, 196–206. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Alberda, M.; Koorneef, M.; Leon-Kloosterziel, K.M.; Hoekstra, F.A. Properties of proteins and the glassy matrix in maturation-defective mutant seeds of Arabidopsis thaliana. Plant J. 1998, 16, 133–143. [Google Scholar] [CrossRef][Green Version]
- Roos, Y.; Karel, M. Plasticizing effect of water on thermal behavior and crystallization of amorphous food models. J. Food Sci. 1991, 56, 38–43. [Google Scholar] [CrossRef]
- Orford, P.D.; Parker, R.; Ring, S.G.; Smith, A.C. Effect of water as a diluent on the glass transition behaviour of malto-oligosaccharides, amylose and amylopectin. Int. J. Biol. Macromol. 1989, 11, 91–96. [Google Scholar] [CrossRef]
- Schaller-Povolny, L.A.; Smith, D.E.; Labuza, T.P. Effect of water content and molecular weight on the moisture isotherms and glass transition properties of inulin. Int. J. Food Properties 2000, 3, 173–192. [Google Scholar] [CrossRef]
- Ohtake, S.; Schebor, C.; Palecek, S.P.; de Pablo, J.J. Effect of pH, counter ion, and phosphate concentration on the glass transition temperature of freeze-dried sugar-phosphate mixtures. Pharm. Res. 2004, 21, 1615–1621. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef]
- Weng, L.; Elliot, G.D. Distinctly different glass transition behavior of trehalose mixed with Na2HPO4 or NaH2PO4: Evidence for its molecular origin. Pharm. Res. 2015, 32, 2217–2228. [Google Scholar] [CrossRef]
- Boothby, T.C.; Tapia, H.; Brozena, A.H.; Piszkiewicz, S.; Smith, A.E.; Giovannini, I.; Rebecchi, L.; Pielak, G.J.; Koshland, D.; Goldstein, B. Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 2017, 65, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.E.; Leprince, O.; Wolkers, W.F.; Hincha, D.K.; Heyer, A.G.; Crowe, J.H. Non-disaccharide-based mechanisms of protection during drying. Cryobiology 2001, 43, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. Lea proteins during water stress: Not just for plants anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef]
- Tunnacliffe, A.; Hincha, D.K.; Leprince, O.; Macherel, D. Lea proteins: Versatility of form and function. In Sleeping Beauties—Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 91–108. [Google Scholar]
- Shih, M.; Hsieh, T.; Lin, T.; Hsing, Y.; Hoekstra, F.A. Characterization of two soybean (Glycine max L.) LEA IV proteins by circular dichroism and Fourier-transform infrared spectroscopy. Plant Cell Physiol. 2010, 51, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kanamori, Y.; Furuki, T.; Kikawada, T.; Okuda, T.; Takahashi, T.; Mihara, H.; Sakurai, M. Desiccation-induced structuralization and glass formation of group 3 late embryogenesis abundant protein model peptides. Biochemistry 2010, 49, 1093–1104. [Google Scholar] [CrossRef]
- Dure, L.I.; Greenway, S.C.; Galau, G.A. Developmental biochemistry of cottonseed embryogenesis and germination: Changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry 1981, 20, 4162–4168. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Knox-Brown, P.; Rindfleisch, T.; Günther, A.; Balow, K.; Bremer, A.; Walther, D.; Miettinen, M.S.; Hincha, D.K.; Thalhammer, A. Similar yet different—Structural and functional diversity among Arabidopsis thaliana LEA_4 proteins. Int. J. Mol. Sci. 2020, 21, 2794. [Google Scholar] [CrossRef]
- Bremer, A.; Wolff, M.; Thalhammer, A.; Hincha, D.K. Folding of intrinsically disordered plant LEA proteins is driven by glycerol-induced crowding and the presence of membranes. FEBS J. 2017, 284, 919–936. [Google Scholar] [CrossRef]
- Thalhammer, A.; Hundertmark, M.; Popova, A.V.; Seckler, R.; Hincha, D.K. Interaction of two intrinsically disordered plant stress proteins (COR15a and COR15b) with lipid membranes in the dry state. Biochim. Biophys. Acta 2010, 1798, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Thomashow, M.F. A cold-regulated Arabidopsis gene encodes a polypeptide having potent cryoprotective activity. Biochem. Biophys. Res. Comm. 1992, 183, 1103–1108. [Google Scholar] [CrossRef]
- Nakayama, K.; Okawa, K.; Kakizaki, T.; Honma, T.; Itoh, H.; Inaba, T. Arabidopsis COR15am is a chloroplast stromal protein that has cryoprotective activity and forms oligomers. Plant Physiol. 2007, 144, 513–523. [Google Scholar] [CrossRef]
- Pouchkina-Stantcheva, N.N.; McGee, B.M.; Boschetti, C.; Tolleter, D.; Chakrabortee, S.; Popova, A.V.; Meersman, F.; Macherel, D.; Hincha, D.K.; Tunnacliffe, A. Functional divergence of former alleles in an ancient asexual invertebrate. Science 2007, 318, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, A.; Bryant, G.; Sulpice, R.; Hincha, D.K. Disordered cold regulated15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 2014, 166, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Artus, N.N.; Uemura, M.; Steponkus, P.L.; Gilmour, S.J.; Lin, C.; Thomashow, M.F. Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proc. Natl. Acad. Sci. USA 1996, 93, 13404–13409. [Google Scholar] [CrossRef]
- Moore, D.S.; Hansen, R.; Hand, S.C. Liposomes with diverse compositions are protected during desiccation by LEA proteins from Artemia franciscana and trehalose. Biochim. Biophys. Acta 2016, 1858, 104–115. [Google Scholar] [CrossRef]
- Bremer, A.; Kent, B.; Hauß, T.; Thalhammer, A.; Yepuri, N.R.; Darwish, T.A.; Garvey, C.J.; Bryant, G.; Hincha, D.K. Intrinsically disordered stress protein COR15a resides at the membrane surface during dehydration. Biophys. J. 2017, 113, 572–579. [Google Scholar] [CrossRef]
- Sakurai, T.; Satou, M.; Akiyama, K.; Iida, K.; Seki, M.; Kuromori, T.; Itoh, M.; Konagaya, A.; Toyoda, T.; Shinozaki, K. Rarge: A large-scale database of RIKEN Arabidopsis resources ranging from transcriptome to phenome. Nucl. Acids Res. 2005, 33, D647–D650. [Google Scholar] [CrossRef]
- Seki, M.; Narusaka, M.; Kamiya, A.; Ishida, J.; Satou, M.; Sakurai, T.; Nakajima, M.; Enju, A.; Akiyama, K.; Oono, Y.; et al. Functional annotation of a full-length Arabidopsis cDNA collection. Science 2002, 296, 141–145. [Google Scholar] [CrossRef]
- Sowemimo, O.T.; Knox-Brown, P.; Borcherds, W.; Rindfleisch, T.; Thalhammer, A.; Daughdrill, G.W. Conserved glycines control disorder and function in the cold-regulated protein, COR15a. Biomolecules 2019, 9, 84. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDA. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E.; Hellwege, E.M.; Heyer, A.G. Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology 2002, 12, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.V.; Hincha, D.K. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: A Fourier-transform infrared spectroscopy study. Biophys. J. 2003, 85, 1682–1690. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oldenhof, H.; Alberda, M.; Hoekstra, F.A. A Fourier-transform infrared microspectroscopy study of sugar glasses: Application to anhydrobiotic higher plant cells. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 1998, 1379, 83–96. [Google Scholar] [CrossRef]
- Kacurakova, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef]
- Brizuela, A.B.; Bichara, L.C.; Romano, E.; Yurquina, A.; Locatelli, S.; Brandan, S.A. A complete characterization of the vibrational spectra of sucrose. Carbohydr. Res. 2012, 361, 212–218. [Google Scholar] [CrossRef]
- Ribero, G.C.; Costa, T.M.H.; Pereira, A.S.; Cassol, L.A.; Perottoni, C.A.; Balzaretti, N.M. In situ infrared spectroscopy study of sucrose up to 14 GPa. Vibr. Spectrosc. 2011, 57, 152–156. [Google Scholar]
- Hundertmark, M.; Popova, A.V.; Rausch, S.; Seckler, R.; Hincha, D.K. Influence of drying on the secondary structure of intrinsically disordered and globular proteins. Biochem. Biophys. Res. Commun. 2012, 417, 122–128. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Surewicz, W.K.; Mantsch, H.H. New insight into protein structure from resolution-enhanced infrared spectra. Biochim. Biophys. Acta 1988, 952, 115–130. [Google Scholar] [CrossRef]
- Arrondo, J.L.R.; Goni, F.M. Structure and dynamics of membrane proteins as studied by infrared spectroscopy. Prog. Biophys. Mol. Biol. 1999, 72, 367–405. [Google Scholar] [CrossRef]
- Dong, A.; Matsuura, J.; Manning, M.C.; Carpenter, J.F. Intermolecular β-sheet results from trifluoroethanol-induced nonnative α-helical structure in β-sheet predominant proteins: Infrared and circular dichroism spectroscopic study. Arch. Biochem. Biophys. 1998, 355, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Susi, H.; Byler, M. Resolution-enhanced Fourier-transform infrared spectroscopy of enzymes. Meth. Enzymol. 1986, 130, 290–311. [Google Scholar]
- Popova, A.V.; Hundertmark, M.; Seckler, R.; Hincha, D.K. Structural transitions in the intrinsically disordered plant dehydration stress protein LEA7 upon drying are modulated by the presence of membranes. Biochim. Biophys. Acta 2011, 1808, 1879–1887. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Hand, S.C.; Crowe, L.M.; Crowe, J.H. Cryoprotection of phosphofructokinase with organic solutes: Characterization of enhanced protection in the presence of divalent cations. Arch. Biochem. Biophys. 1986, 250, 505–512. [Google Scholar] [CrossRef]
- Miller, D.P.; de Pablo, J.J.; Corti, H. Thermophysical properties of trehalose and its concentrated aqueous solutions. Pharm. Res. 1997, 14, 578–590. [Google Scholar] [CrossRef]
- Miller, D.P.; Anderson, R.E.; de Pablo, J.J. Stabilization of lactate dehydrogenase following freeze-thawing and vacuum-drying in the presence of trehalose and borate. Pharm. Res. 1998, 15, 1215–1221. [Google Scholar] [CrossRef]
- Sydykov, B.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Hydrogen bonding interactions and enthalpy relaxation in sugar/protein glasses. J. Pharm. Sci. 2017, 106, 761–769. [Google Scholar] [CrossRef]
- Boudet, J.; Buitink, J.; Hoekstra, F.A.; Rogniaux, H.; Larre, C.; Satour, P.; Leprince, O. Comparative analysis of the heat stable proteome of radicles of Medicago truncatula seeds during germination identifies late embryogenesis abundant proteins associated with desiccation tolerance. Plant Physiol. 2006, 140, 1418–1436. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hincha, D.K.; Zuther, E.; Popova, A.V. Stabilization of Dry Sucrose Glasses by Four LEA_4 Proteins from Arabidopsis thaliana. Biomolecules 2021, 11, 615. https://doi.org/10.3390/biom11050615
Hincha DK, Zuther E, Popova AV. Stabilization of Dry Sucrose Glasses by Four LEA_4 Proteins from Arabidopsis thaliana. Biomolecules. 2021; 11(5):615. https://doi.org/10.3390/biom11050615
Chicago/Turabian StyleHincha, Dirk K., Ellen Zuther, and Antoaneta V. Popova. 2021. "Stabilization of Dry Sucrose Glasses by Four LEA_4 Proteins from Arabidopsis thaliana" Biomolecules 11, no. 5: 615. https://doi.org/10.3390/biom11050615
APA StyleHincha, D. K., Zuther, E., & Popova, A. V. (2021). Stabilization of Dry Sucrose Glasses by Four LEA_4 Proteins from Arabidopsis thaliana. Biomolecules, 11(5), 615. https://doi.org/10.3390/biom11050615






