Contribution of Glycation and Oxidative Stress to Thyroid Gland Pathology—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (a)
- blood for serum extraction.
- (b)
- tumor sample.
- (c)
- section of tissue surrounding the tumor considered to be a control sample.
- (d)
- sample of adipose tissue surrounding the thyroid gland.
2.2. Immunohistochemistry
2.3. ELISA—Competitive Test
2.4. The Analysis of Fluorescent AGEs and Pentosidine Content Using the Fluorometric Method
2.5. The Assessment of Content of Compounds with Thiol Groups (Glutathione)
2.6. Statistical Analysis
3. Results
3.1. Localization and Semiquantitative Analysis of AGEs, Receptors for Advanced Glycation End-Products (RAGE, SR-A, SR-B) and Nitric Oxide Synthase (NOS-3) in Samples of Thyroid Gland and of Adipose Tissue
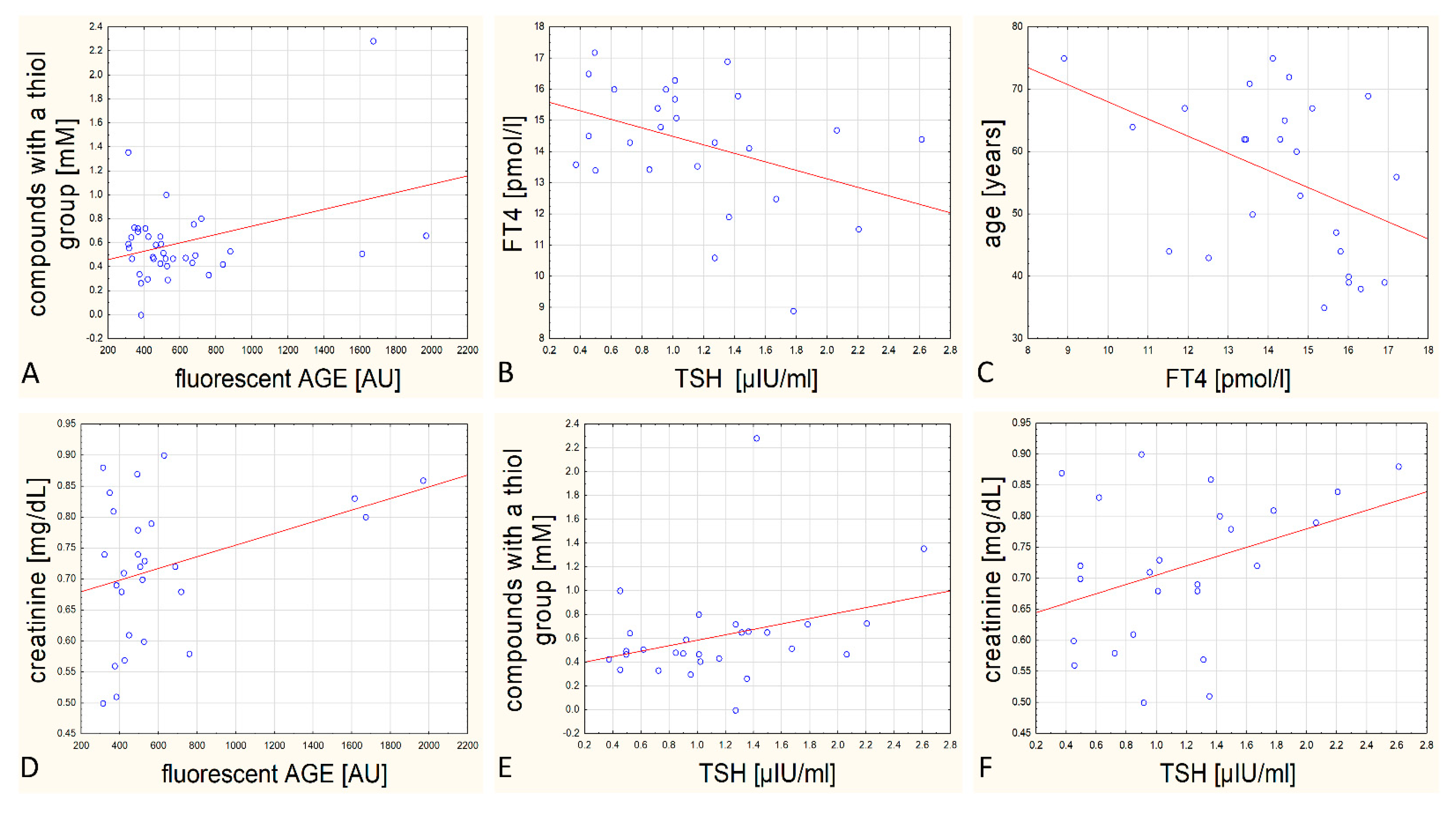
3.2. The Analysis of Correlation between Distinct Biochemical Parameters
- Content of fluorescent AGEs and thiol groups—statistically, the more AGEs in the patient’s blood, the higher the concentration of compounds with thiol group in their blood serum, including glutathione (r = 0.361, p = 0.028; Figure 5, panel A);
- Concentration of TSH and FT4—statistically, the higher the concentration of TSH, the lower level of FT4 hormone in the blood serum (r = −0.395, p = 0.046; Figure 5, panel B);
- Concentration of FT4 and age—statistically, the older the patient, the lower the level of FT4 hormone in the blood serum (r = −0.427, p = 0.03; Figure 5, panel C).
- Correlation between creatinine concentration and the content of fluorescent AGEs in blood serum, (r = 0.352, p = 0.071; Figure 5, panel D);
- Correlation between the content of compounds with thiol groups and TSH in blood serum (r = 0.335, p = 0.082; Figure 5, panel E);
- Correlation between creatinine concentration and TSH concentration in blood serum (r = 0.369, p = 0.070; Figure 5, panel F).
4. Discussion
4.1. Participation of Glycation in Thyroid Pathology
4.2. RAGE Receptors in Thyroid Pathology
4.3. Scavenger Receptors in Thyroid Pathology
4.4. Discussion of Results Considering Thyroid Hormones, Creatinine and Patients’ Age
4.5. Oxidative Stress in Pathomechanism of Thyroid Diseases
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abs | Absorbance |
| AGEs | Advanced Glycation End-products |
| ATC | Anaplastic Thyroid Carcinoma |
| BSA | Bovine Serum Albumin |
| ATP | Adenosine TriPhosphate |
| CD204 | Cluster of Differentiation 204 |
| CD36 | Cluster of Differentiation 36 |
| CEL | N(ε)-CarboxyEthylLysine |
| CML | CarboxyMethylLysine |
| cNOS | constitutive Nitric Oxide Synthase |
| DAB | 3.3′-DiAminoBenzidine |
| DM | Diabetes Mellitus |
| DNA | DeoxyRibonucleic Acid |
| DPX | Dibutylphthalate Polystyrene Xylene |
| DTNB | 5.5′-DiThiobis-2-NitroBenzoic acid |
| ELISA | Enzyme-Linked Immuno-Sorbent Assay |
| eNOS | endothelial Nitric Oxide Synthase |
| FEEL-I | Link domain-containing scavenger receptor-I |
| FEEL-II | Link domain-containing scavenger receptor-II |
| FT3 | Free Triiodothyronine |
| FT4 | Free Thyroxine |
| FTC | Follicular Thyroid Carcinoma |
| Gal-3 | Galectin-3 |
| GSH | Glutathione |
| GR | Glutathione Reductase |
| GSHPx | Glutathione Peroxidase |
| HbA1C | Glycosylated Hemoglobin type A1C |
| HDL | High-Density Lipoprotein |
| HGB | HaemoGloBin |
| HMW-AGE | High Molecular Weight-Advanced Glycation End products |
| HRP | HorseRadish Peroxidase |
| IgE | Immunoglobulin E |
| IgG | Immonoglobulin G |
| LDL | Low-Density Lipoprotein |
| LMW-AGE | Low Molecular Weight-Advanced Glycation End-products |
| LOX-1 | Lectin-like OXidized low-density lipoprotein receptor-1 |
| MAGE | Melibiose-Derived Advanced-Glycation End-product |
| MDA | MalonDiAldehyde |
| MOLD | MethylglyOxal Lysine Dimer |
| MSR1 | Macrophage Scavenger Receptor 1 |
| MTC | Medullary Thyroid Carcinoma |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate reduced |
| NO | Nitric Oxide |
| NOS-3 | Nitric Oxide Synthase-3 |
| OPD | O-Phenylene Diamine hydrochloride |
| PBS | Phosphate Buffered Saline |
| PBST | Phosphate Buffered Saline with Tween-20 |
| PTC | Papillary Thyroid Carcinoma |
| RAGE | Receptors for Advanced Glycation End-products |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SCTC | Squamous-Cell Thyroid Carcinoma |
| SCARA1 | Scavenger receptor class A member 1 gene |
| SCARB1 | Scavenger receptor class B member 1 gene |
| SDS | Sodium Dodecyl Sulfate |
| SR | Scavenger Receptors |
| SR-A/SRA-I | Scavenger receptor class A member 1 |
| SR-B/SRB-I | Scavenger receptor class B member 1 |
| TBA | ThioBarbituric Acid |
| TCA | TriChloroacetic Acid |
| T3 | Triiodothyronine (L-3,5,3′-Triiodothyronine) |
| T4 | Thyroxine (L-3,5,3′,5′-Tetraiodothyronine) |
| TAMs | Tumor-Associated Macrophages |
| TSH | Thyroid Stimulating Hormone |
| WHO | The World Health Organization |
References
- Köhrle, J. Thyroid hormones and derivatives: Endogenous thyroid hormones and their targets. In Thyroid Hormone Nuclear Receptor. Methods in Molecular Biology, Volume 1801; Plateroti, M.S.J., Ed.; Humana Press: New York, NY, USA, 2018; pp. 85–104. ISBN 978-1-4939-7902-8. [Google Scholar]
- Sherman, S.I.; Perrier, N.; Clayman, G.L. Thyroid cancer. In 60 Years of Survival Outcomes at The University of Texas MD Anderson Cancer Center; Springer: Berlin/Heidelberg, Germany, 2013; pp. 295–310. [Google Scholar]
- Massimino, M.; Evans, D.B.; Podda, M.; Spinelli, C.; Collini, P.; Pizzi, N.; Bleyer, A. Thyroid cancer in adolescents and young adults. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef]
- Popoveniuc, G.; Jonklaas, J. Thyroid nodules. Med. Clin. 2012, 96, 329–349. [Google Scholar] [CrossRef] [Green Version]
- Elgazar, E.H.; Esheba, N.E.; Shalaby, S.A.; Mohamed, W.F. Thyroid dysfunction prevalence and relation to glycemic control in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2513–2517. [Google Scholar] [CrossRef]
- Antognelli, C.; Moretti, S.; Frosini, R.; Puxeddu, E.; Sidoni, A.; Talesa, V.N. Methylglyoxal Acts as a Tumor-Promoting Factor in Anaplastic Thyroid Cancer. Cells 2019, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Gillery, P. Dosage de l’HbA1c et des produits d’Amadori en biologie humaine. Ann. Pharm. Françaises 2014, 72, 330–336. [Google Scholar] [CrossRef]
- Staniszewska, M.; Bronowicka-Szydełko, A.; Gostomska-Pampuch, K.; Szkudlarek, J.; Bartyś, A.; Bieg, T.; Gamian, E.; Kochman, A.; Picur, B.; Pietkiewicz, J.; et al. The melibiose-derived glycation product mimics a unique epitope present in human and animal tissues. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, F. Application of immunohistochemistry in thyroid pathology. Arch. Pathol. Lab. Med. 2015, 139, 67–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspar-Bell, G.; Dhar, I.; Prasad, K. Advanced glycation end products (AGEs) and its receptors in the pathogenesis of hyperthyroidism. Mol. Cell. Biochem. 2016, 414, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Leszek, J.; Ferens-Sieczkowska, M. Fluorescent advanced glycation end products in the sera of Alzheimer’s disease patients. In Perspectives in Neurosciences: In Memory of Professor Agapitos Diacoyannis; Baloyannis, S.J., Ed.; Greece Society for Amelioration of the Quality of Life for Chronic Neurologic Patients: Thessaloniki, Greece, 2005; pp. 373–381. [Google Scholar]
- Diplock, A.T.; Symons, M.C.R.; Rice-Evans, C.A. Techniques in Free Radical Research; Elsevier: New York, NY, USA, 1991; ISBN 0080858910. [Google Scholar]
- Babu, S.; Shetty, J.K.; Mungli, P. Total thiols and MDA levels in patients with acute myocardial infarction before and after reperfusion therapy. Online J. Health Allied Sci. 2010, 9, 6–7. [Google Scholar]
- Chen, X.; Wu, W.; Zhou, Q.; Jie, J.; Chen, X.; Wang, F.; Gong, X. Advanced glycation end-products induce oxidative stress through the Sirt1/Nrf2 axis by interacting with the receptor of AGEs under diabetic conditions. J. Cell. Biochem. 2019, 120, 2159–2170. [Google Scholar] [CrossRef]
- Kawaguchi, Y. Biomarkers of Ossification of the Spinal Ligament. Glob. Spine J. 2019, 9, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Yuan, X.; Gu, J.; Yue, X.; Gu, X.; Nagaraj, R.H.; Crabb, J.W. Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol. Cell. Proteomics 2009, 8, 1921–1933. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.F.; Rodby, R.A.; Justice, A.C.; Hricik, D.E.; Group, C.S. Free pentosidine and neopterin as markers of progression rate in diabetic nephropathy. Kidney Int. 1998, 54, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, T.; Ito, H.; Murata, K.; Hashimoto, M.; Tanaka, M.; Murakami, K.; Nishitani, K.; Azukizawa, M.; Okahata, A.; Doi, K. Distinct biomarkers for different bones in osteoporosis with rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzan, A.; Chwiłkowska, A.; Maksymowicz, K.; Bronowicka-Szydełko, A.; Stach, K.; Pezowicz, C.; Gamian, A. Advanced glycation end products as a source of artifacts in immunoenzymatic methods. Glycoconj. J. 2018, 35, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüel, A.; Oxlund, H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis 1996, 127, 155–165. [Google Scholar] [CrossRef]
- Ling, X.; Sakashita, N.; Takeya, M.; Nagai, R.; Horiuchi, S.; Takahashi, K. Immunohistochemical distribution and subcellular localization of three distinct specific molecular structures of advanced glycation end products in human tissues. Lab. Investig. 1998, 78, 1591–1606. [Google Scholar] [PubMed]
- Leszek, J.; Małyszczak, K.; Bartyś, A.; Staniszewska, M.; Gamian, A. Analysis of Serum of Patients With Alzheimer’s Disease for the Level of Advanced Glycation End Products. Am. J. Alzheimer’s Dis. Other Dementiasr 2006, 21, 360–365. [Google Scholar] [CrossRef]
- Prasad, K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol. Cell. Biochem. 2019, 451, 139–144. [Google Scholar] [CrossRef]
- Pasupulati, A.K.; Chitra, P.S.; Reddy, G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol. Concepts 2016, 7, 293–309. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Guo, C.; Fisher, P.B.; Subjeck, J.R.; Wang, X.-Y. Scavenger receptors: Emerging roles in cancer biology and immunology. In Advances in Cancer Research; Elsevier: New York, NY, USA, 2015; Volume 128, pp. 309–364. ISBN 0065-230X. [Google Scholar]
- Du, F.-M.; Kuang, H.-Y.; Duan, B.-H.; Liu, D.-N.; Yu, X.-Y. Effects of thyroid hormone and depression on common components of central obesity. J. Int. Med. Res. 2019, 47, 3040–3049. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, H.I.; Oh, H.K.; Kim, T.H.; Jang, H.W.; Chung, J.H.; Shin, M.H.; Kim, S.W. Age- and gender-specific reference intervals of TSH and free T4 in an iodine-replete area: Data from Korean National Health and Nutrition Examination Survey IV (2013–2015). PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, A.; D’Orta, A.; Licito, A.; Coppola, D.; De Monaco, A. Age Related Ft3/Ft4 Ratio As Possible Indicator of Chronic Disease and Cancer Development: A Pilot Study. World Cancer Res. J. 2017, 4, 1–4. [Google Scholar]
- Sawant, S.U.; Chandran, S.; Almeida, A.F.; Rajan, M.G.R. Correlation between Oxidative Stress and Thyroid Function in Patients with Nephrotic Syndrome. Int. J. Nephrol. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Akinci, M.; Kosova, F.; Çetin, B.; Sepici, A.; Altan, N.; Aslan, S.; Çetin, A. Oxidant/antioxidant balance in patients with thyroid cancer. Acta Cir. Bras. 2008, 23, 551–554. [Google Scholar] [CrossRef] [Green Version]
- Erdamar, H.; Çimen, B.; Gülcemal, H.; Saraymen, R.; Yerer, B.; Demirci, H. Increased lipid peroxidation and impaired enzymatic antioxidant defense mechanism in thyroid tissue with multinodular goiter and papillary carcinoma. Clin. Biochem. 2010, 43, 650–654. [Google Scholar] [CrossRef]
- Senthil, N.; Manoharan, S. Lipid peroxidation and antioxidants status in patients with papillary thyroid carcinoma in India. Asia Pac. J. Clin. Nutr. 2004, 13, 391–395. [Google Scholar] [PubMed]
- Rovcanin, B.R.; Gopcevic, K.R.; Kekic, D.L.; Zivaljevic, V.R.; Diklic, A.D.; Paunovic, I.R. Papillary thyroid carcinoma: A malignant tumor with increased antioxidant defense capacity. Tohoku J. Exp. Med. 2016, 240, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Feng, J.-F.; Zeng, P.; Yang, Y.-H.; Luo, J.; Yang, Y.-W. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr. Relat. Cancer 2011, 18, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donckier, J.E.; Michel, L.; Delos, M.; Havaux, X.; Van Beneden, R. Interrelated overexpression of endothelial and inducible nitric oxide synthases, endothelin-1 and angiogenic factors in human papillary thyroid carcinoma. Clin. Endocrinol. 2006, 64, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Fenton, C.; Terrell, R.; Powers, P.A.; Dinauer, C.; Tuttle, R.M.; Francis, G.L. Nitrotyrosine, inducible nitric oxide synthase (iNOS), and endothelial nitric oxide synthase (eNOS) are increased in thyroid tumors from children and adolescents. J. Endocrinol. Investig. 2002, 25, 675–683. [Google Scholar] [CrossRef]
- Lobo Júnior, J.P.; Brescansin, C.P.; Santos-Weiss, I.C.R.; Welter, M.; de Souza, E.M.; Rego, F.G.d.M.; Picheth, G.; Alberton, D. Serum Fluorescent Advanced Glycation End (F-AGE) products in gestational diabetes patients. Arch. Endocrinol. Metab. 2017, 61, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, F.; Omrani, G.R. Effects of selenium and vitamin C on the serum level of antithyroid peroxidase antibody in patients with autoimmune thyroiditis. J. Endocrinol. Investig. 2019, 42, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
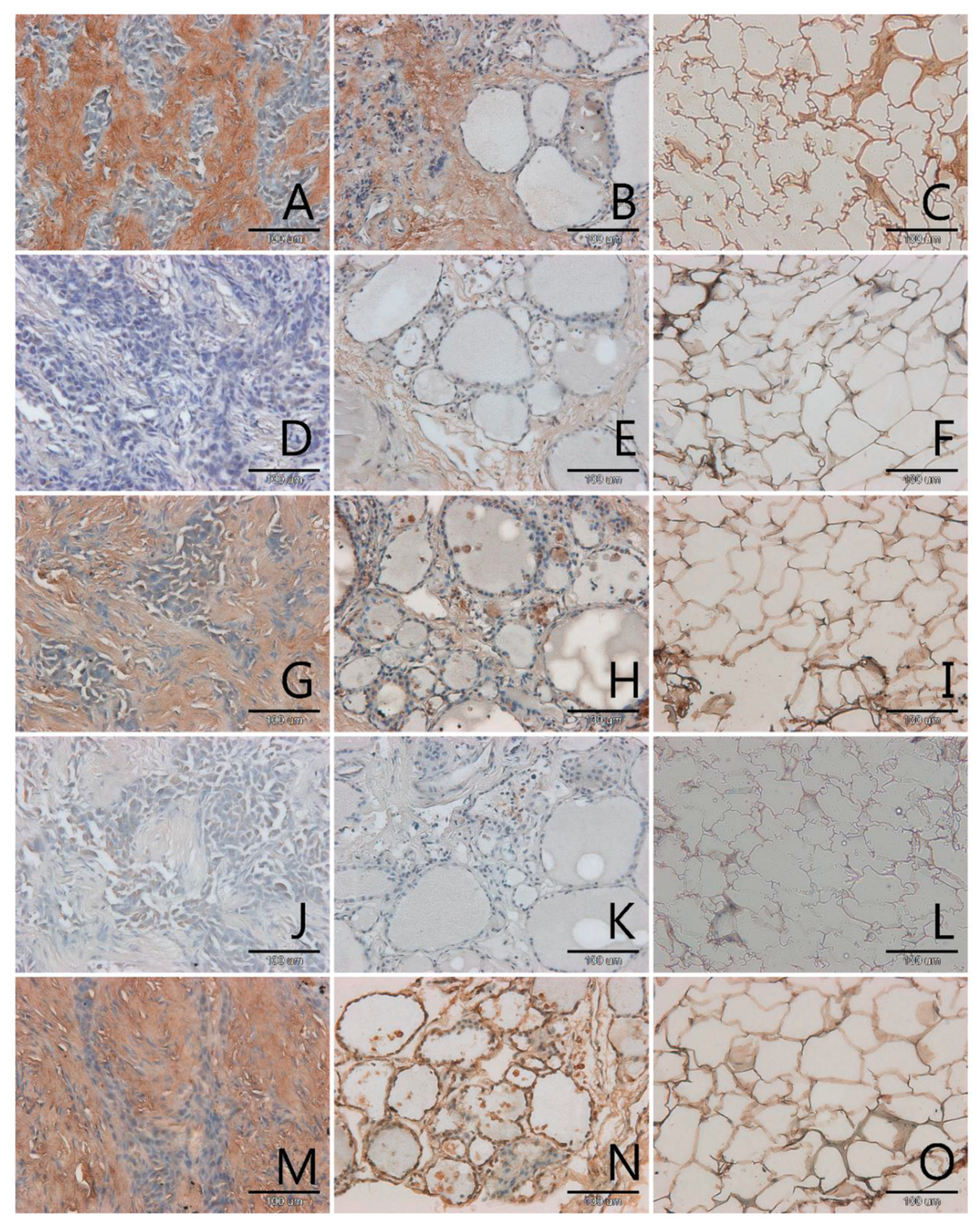
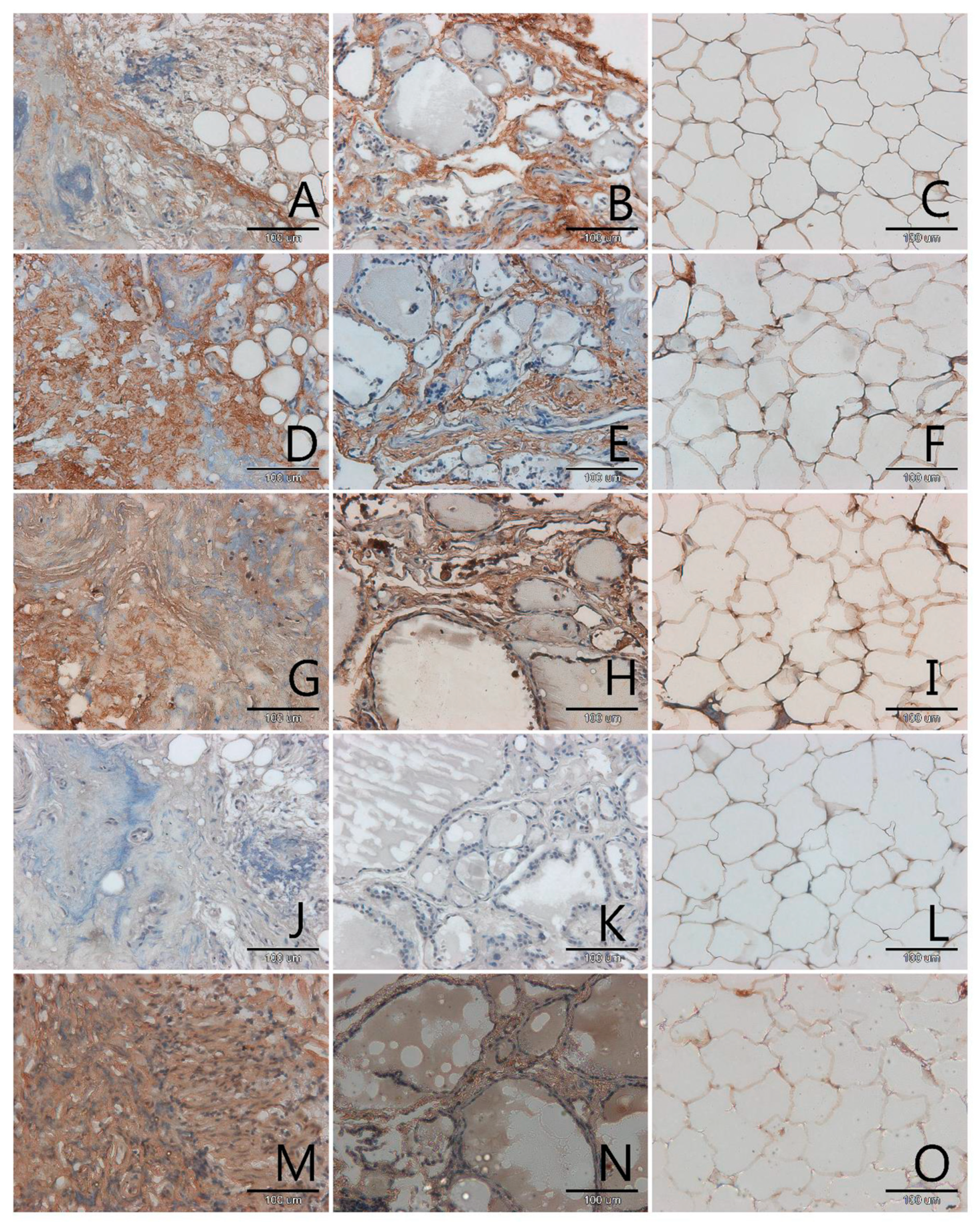
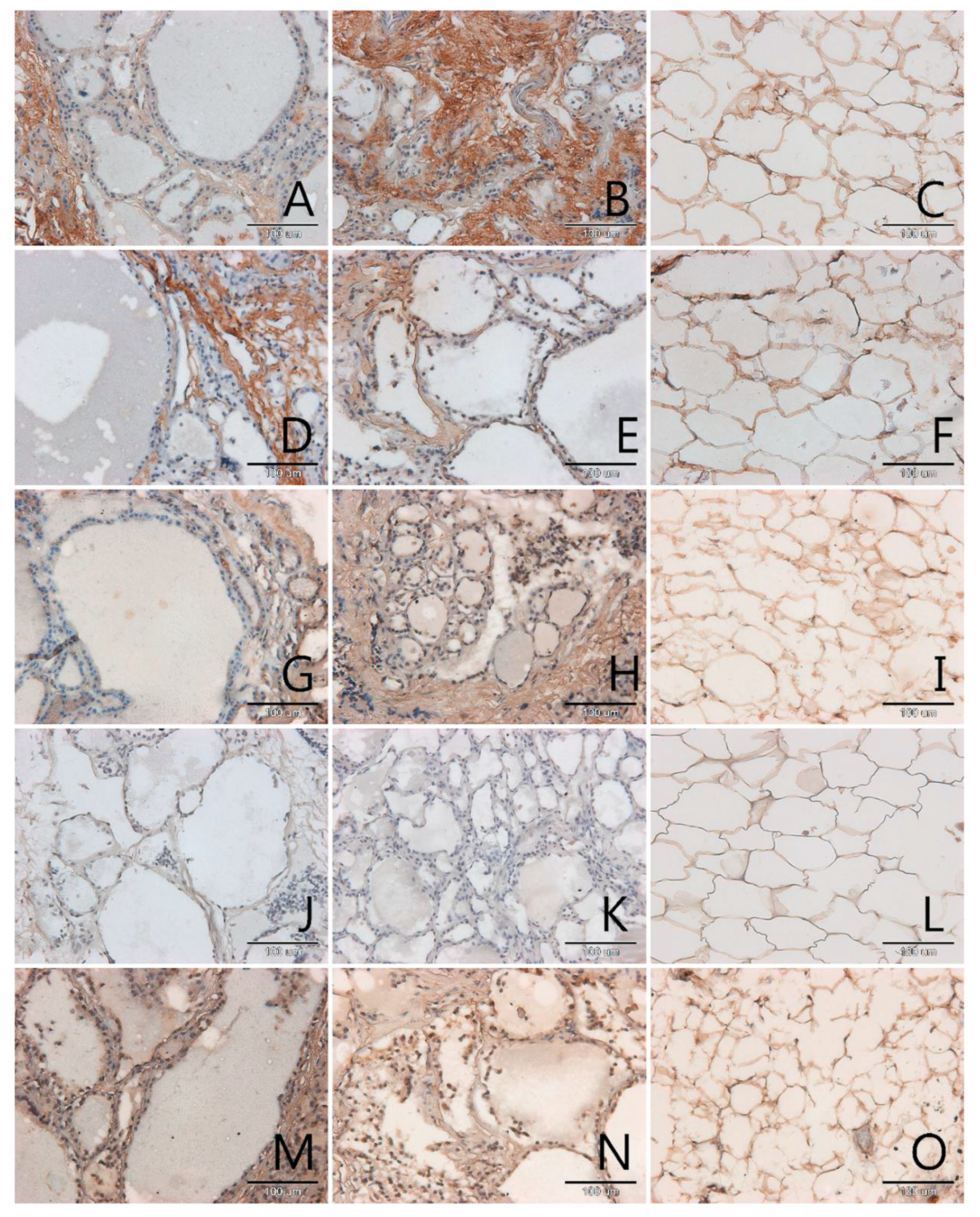
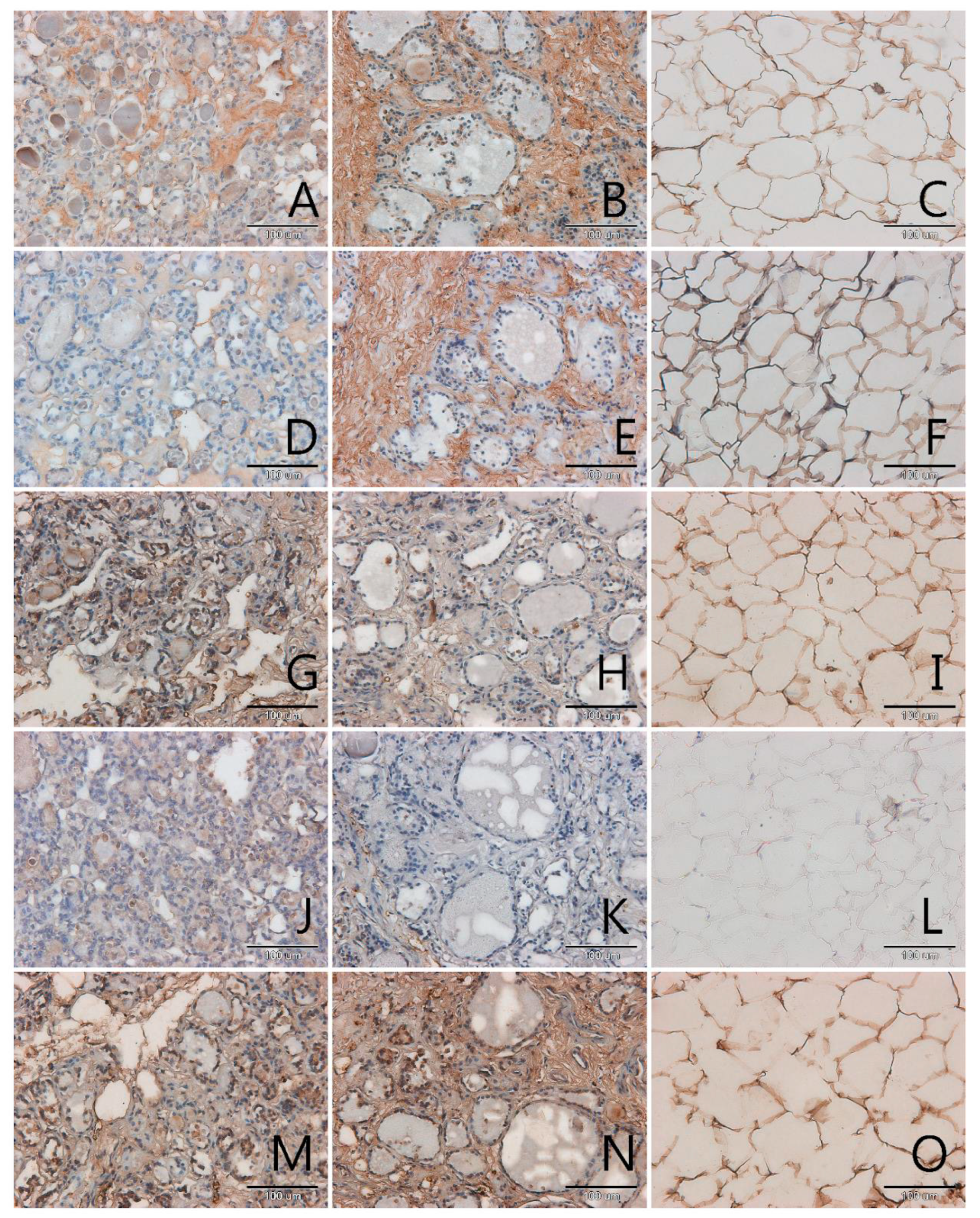

| Papillary Cancer n = 4 | Squamous Cell Carcinoma n = 1 | Follicular Adenoma n = 3 | Nodular Goitre n = 29 | |
|---|---|---|---|---|
| Age (years) (Mean +/− SD) | 65.8 (±5.4) | 65 | 61.3 (±14.0) | 53.0 (±14.0) |
| Gender (% of women) | 100 | 100 | 66.7 | 82.76 |
| TSH (µIU/mL) (Mean +/− SD) | 1.09 (±0.52) | 2.61 | 1.4 (±0.54) | 1.05 (±0.5) |
| FT4 (pmol/L) (Mean +/− SD) | 13.94 (±0.47) | 14.4 | 12.30 (±4.82) | 14.53 (±1.86) |
| FT3 (pmol/L) (Mean +/− SD) | 4.51 (±0.93) | 4.59 | 5.61 | 6.36 (±0.75) |
| Haemoglobin (g/dL) (Mean +/− SD) | 13.93 (±0.61) | 13.3 | 13.97 (±0.91) | 13.65 (±1.13) |
| Fasting glucose (mg/dL) (Mean +/− SD) | 107.5 (±20.5) | 112 | 97 | 94.4 (±11.06) |
| Creatinine (mg/dL) (Mean +/− SD) | 0.72 (±0.05) | 0.88 | 0.77 (±0.05) | 0.70 (±0.12) |
| MAGE (µg/mL) (Mean +/− SD) | 1069.0 (±628.2) | 1704.5 | 1000.9 (±275.7) | 1017.6 (±462.7) |
| Fluorescent AGEs (AU) (Mean +/− SD) | 439.6 (±75.9) | 311.2 | 339.0 (±25.2) | 658.8 (±408.6) |
| Pentosidine (AU) (Mean +/− SD) | 413.0 (±91.3) | 550.3 | 559.1 (±371.2) | 612.5 (±232.9) |
| Compounds with thiol groups (mM) (Mean +/− SD) | 0.45 (±0.32) | 1.36 | 0.59 (±0.37) | 0.59 (±0.37) |
| MDA (nM) (Mean +/− SD) | 0.85 (±0.09) | 1.35 | 0.96 (±0.24) | 0.94 (±0.27) |
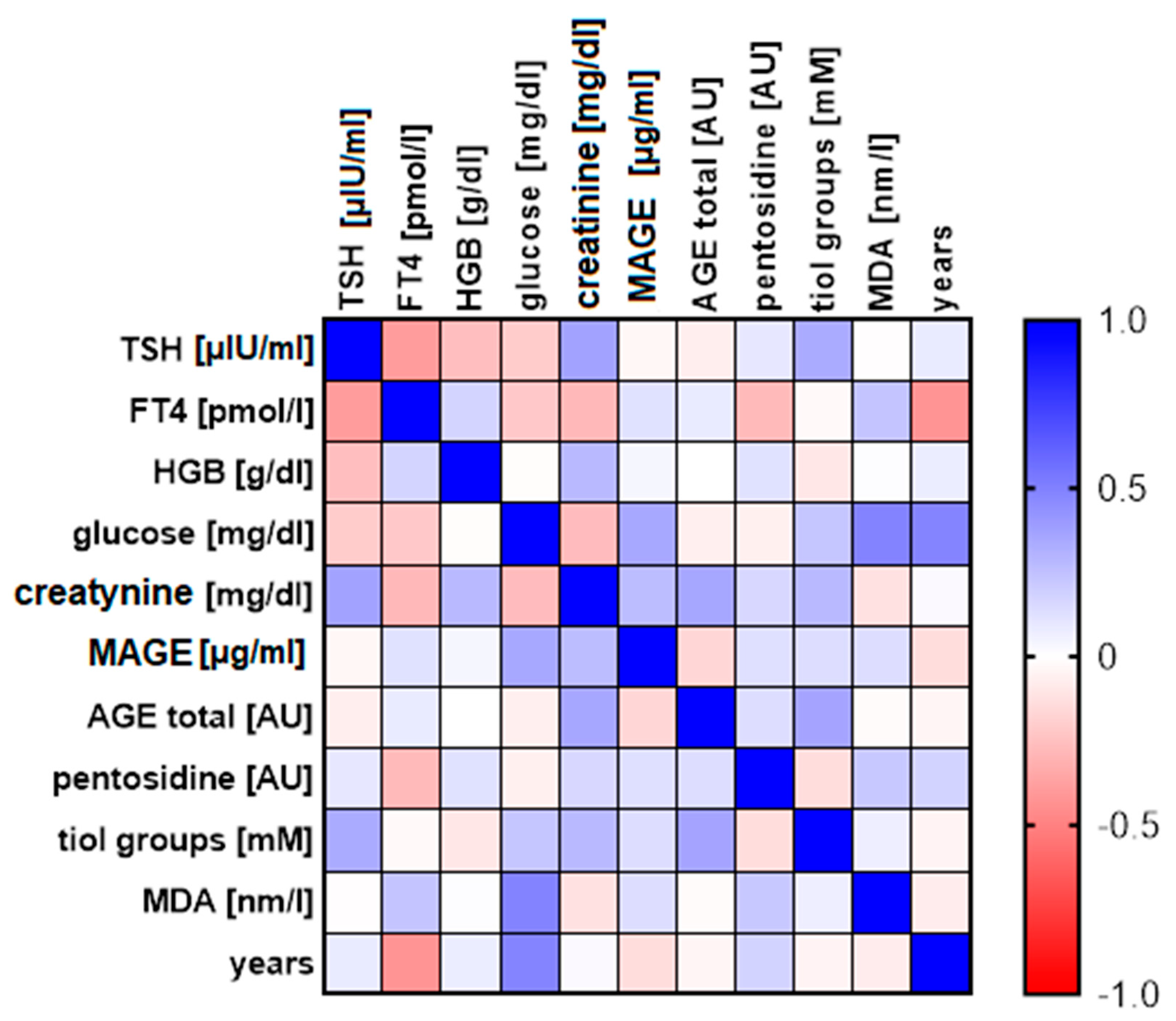
| r | TSH (µIU/mL) | FT4 (pmol/L) | HGB (g/dL) | Creatinine (mg/dL) | MAGE (µg/mL) | Fluorescent AGE (AU) | Pentosidine (AU) | Tiol Groups (mM) | MDA (nm/L) | Age (Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| TSH [µIU/mL] | 1.000 | |||||||||
| FT4 [pmol/L] | −0.395 (0.046) * | 1.000 | ||||||||
| HGB [g/dL] | −0.262 (0.178) | 0.172 (0.402) | 1.000 | |||||||
| creatinine [mg/dL] | 0.369 (0.070) | −0.286 (0.176) | 0.276 (0.163) | 1.000 | ||||||
| MAGE [µg/mL] | −0.037 (0.852) | 0.121 (0.555) | 0.035 (0.856) | 0.266 (0.181) | 1.000 | |||||
| Fluorescent AGE [AU] | −0.071 (0.718) | 0.079 (0.702) | −0.003 (0.988) | 0.352 (0.071) | −0.168 (0.320) | 1.000 | ||||
| pentosidine [AU] | 0.096 (0.626) | −0.275 (0.174) | 0.118 (0.534) | 0.158 (0.432) | 0.129 (0.448) | 0.137 (0.420) | 1.000 | |||
| tiol groups [mM] | 0.335 (0.082) | −0.025 (0.904) | −0.099 (0.602) | 0.278 (0.160) | 0.134 (0.429) | 0.361 (0.028) * | −0.139 (0.411) | 1.000 | ||
| MDA [nm/L] | −0.011 (0.956) | 0.234 (0.251) | 0.005 (0.980) | −0.122 (0.544) | 0.137 (0.425) | −0.017 (0.921) | 0.216 (0.205) | 0.068 (0.694) | 1.000 | |
| Age [years] | 0.086 (0.664) | −0.427 (0.030) * | 0.071 (0.709) | 0.020 (0.922) | −0.141 (0.419) | −0.040 (0.819) | 0.174 (0.317) | −0.049 (0.780) | −0.081 (0.649) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzan, A.; Królewicz, E.; Nowakowska, K.; Stach, K.; Kaliszewski, K.; Domosławski, P.; Kotyra, Ł.; Gamian, A.; Kustrzeba-Wójcicka, I. Contribution of Glycation and Oxidative Stress to Thyroid Gland Pathology—A Pilot Study. Biomolecules 2021, 11, 557. https://doi.org/10.3390/biom11040557
Kuzan A, Królewicz E, Nowakowska K, Stach K, Kaliszewski K, Domosławski P, Kotyra Ł, Gamian A, Kustrzeba-Wójcicka I. Contribution of Glycation and Oxidative Stress to Thyroid Gland Pathology—A Pilot Study. Biomolecules. 2021; 11(4):557. https://doi.org/10.3390/biom11040557
Chicago/Turabian StyleKuzan, Aleksandra, Emilia Królewicz, Karolina Nowakowska, Kamilla Stach, Krzysztof Kaliszewski, Paweł Domosławski, Łukasz Kotyra, Andrzej Gamian, and Irena Kustrzeba-Wójcicka. 2021. "Contribution of Glycation and Oxidative Stress to Thyroid Gland Pathology—A Pilot Study" Biomolecules 11, no. 4: 557. https://doi.org/10.3390/biom11040557
APA StyleKuzan, A., Królewicz, E., Nowakowska, K., Stach, K., Kaliszewski, K., Domosławski, P., Kotyra, Ł., Gamian, A., & Kustrzeba-Wójcicka, I. (2021). Contribution of Glycation and Oxidative Stress to Thyroid Gland Pathology—A Pilot Study. Biomolecules, 11(4), 557. https://doi.org/10.3390/biom11040557








