Chemical Constituents of the Egg Cases of Tenodera angustipennis (Mantidis ootheca) with Intracellular Reactive Oxygen Species Scavenging Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Insect Material
2.3. Extraction and Isolation
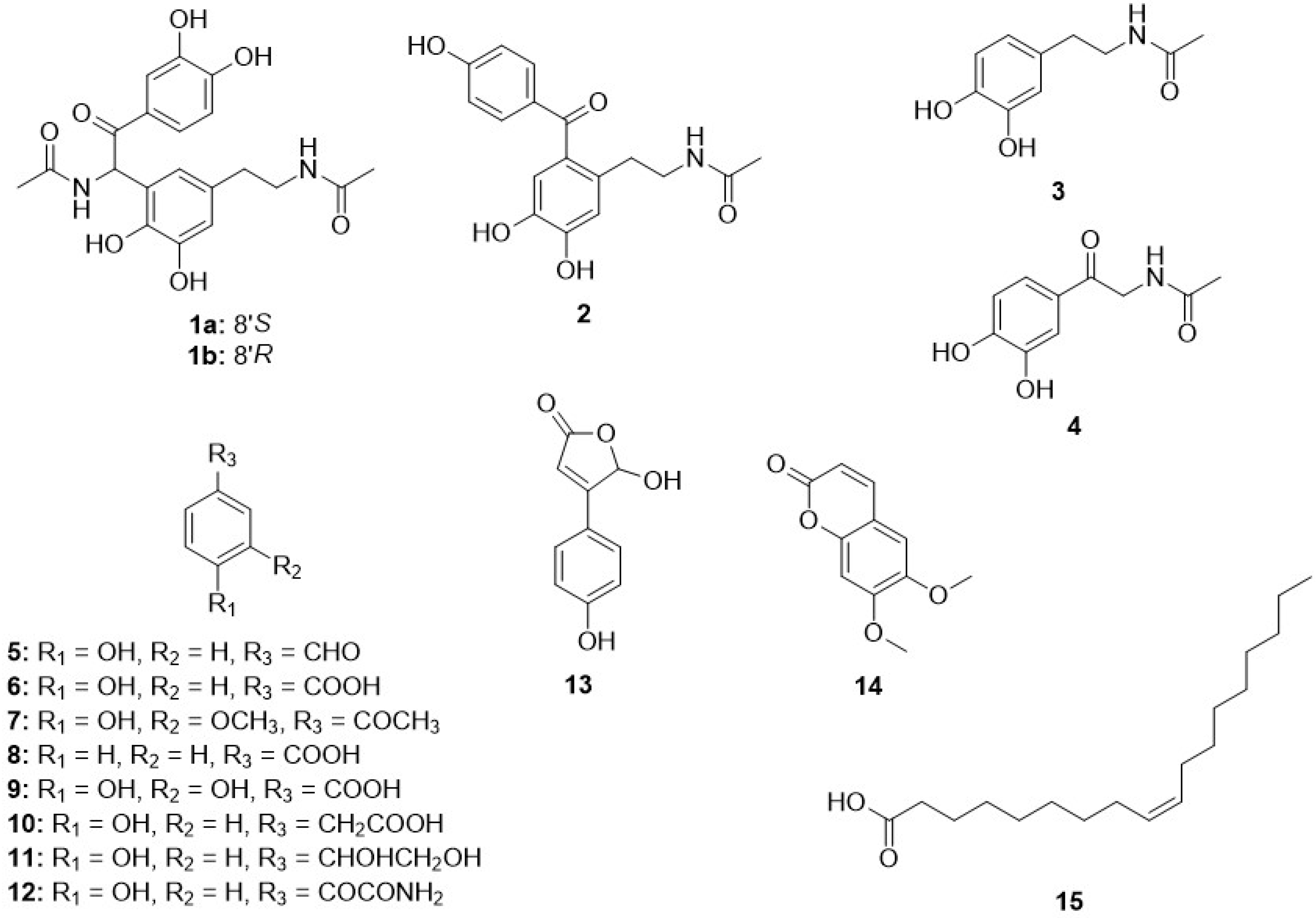
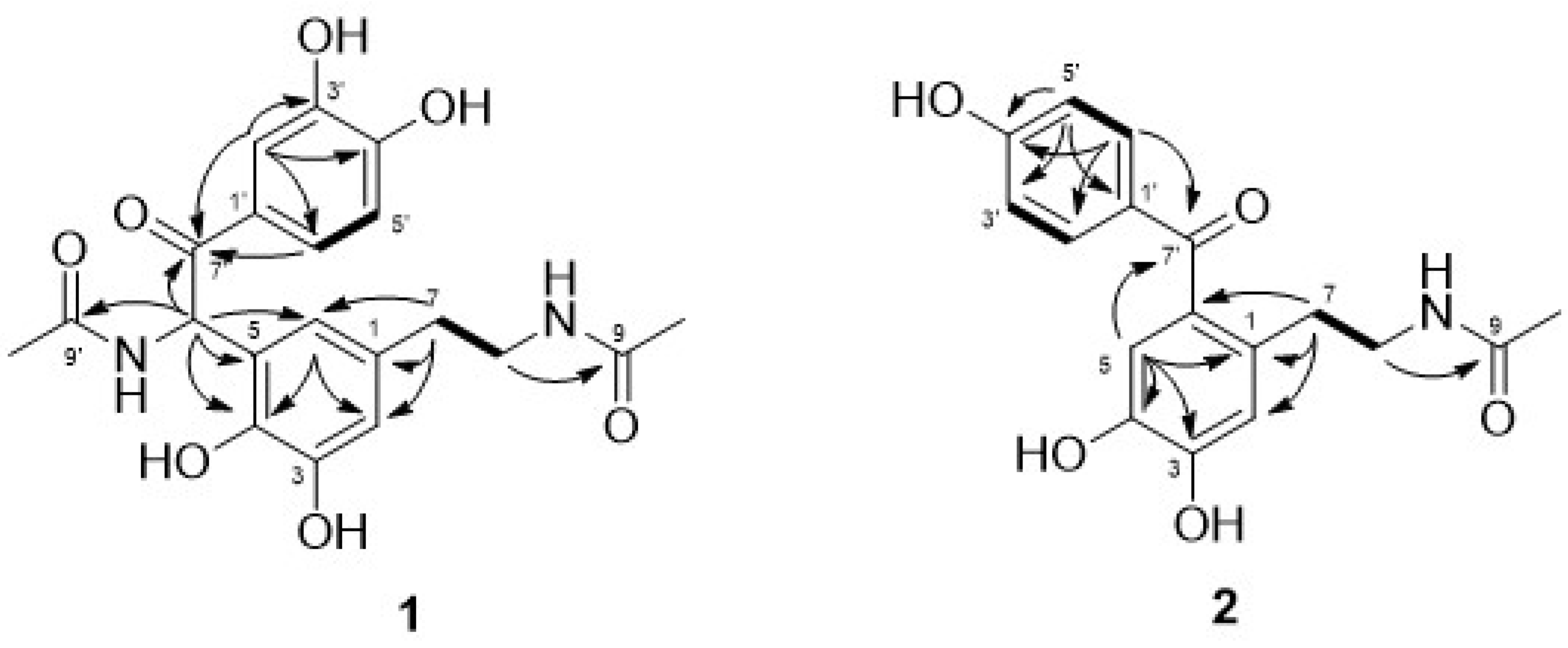
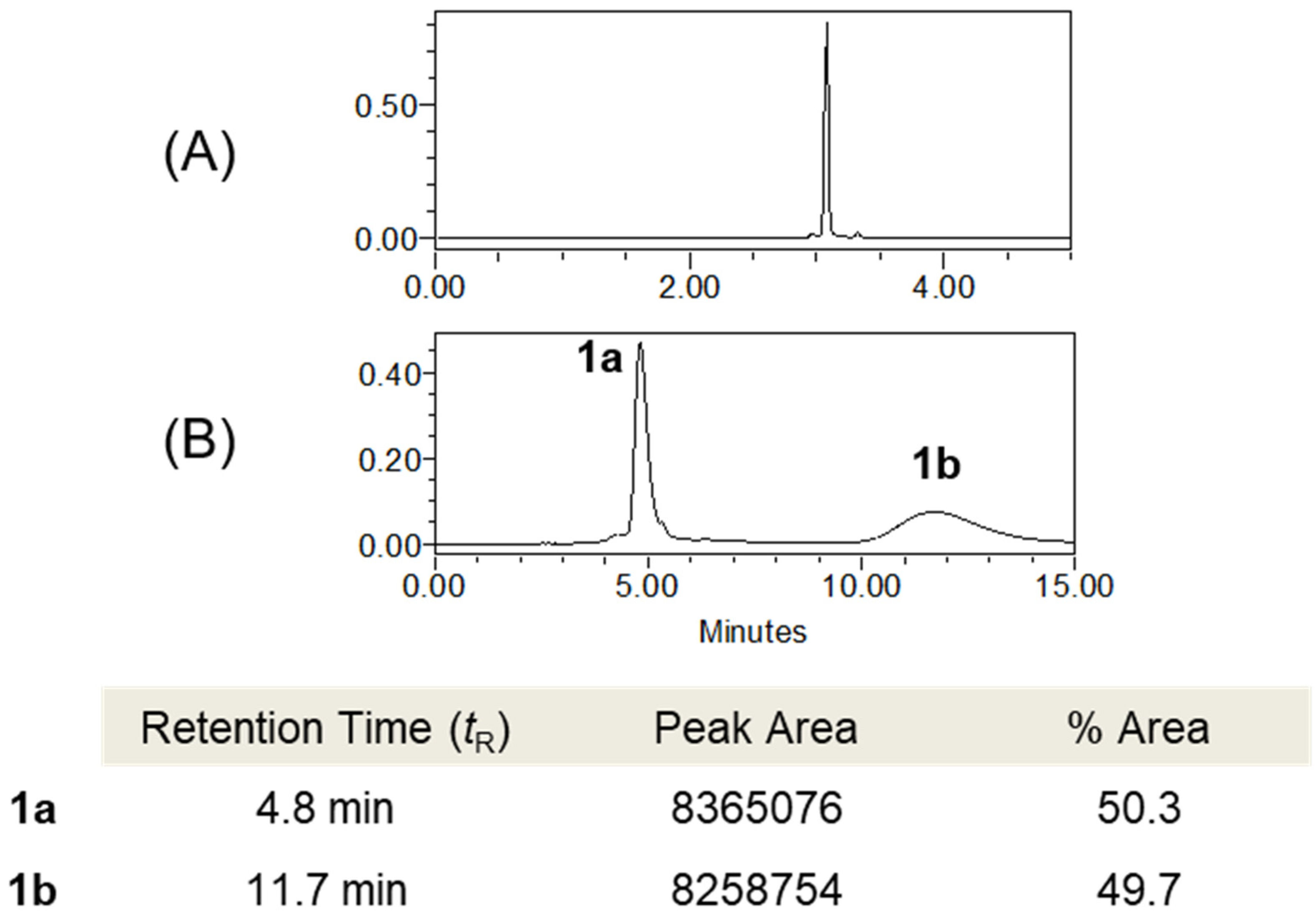
2.3.1. Tenoderin A (1)
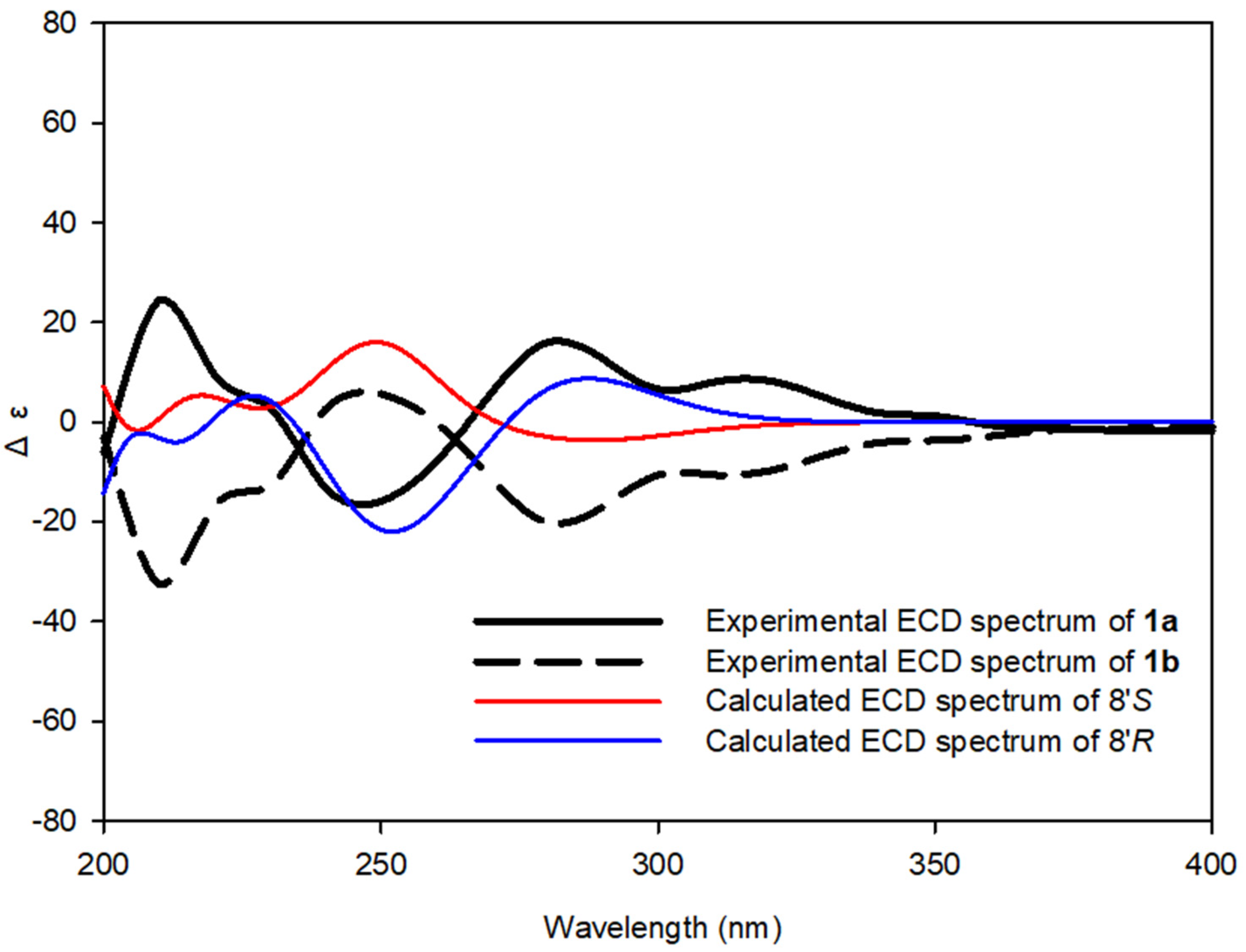
(+)-tenoderin A (1a)
(−)-tenoderin A (1b)
2.3.2. Tenoderin B (2)
2.4. Computational Methods
2.5. DPPH and ABTS Radical Scavenging Activities
2.6. Detection of Intracellular ROS
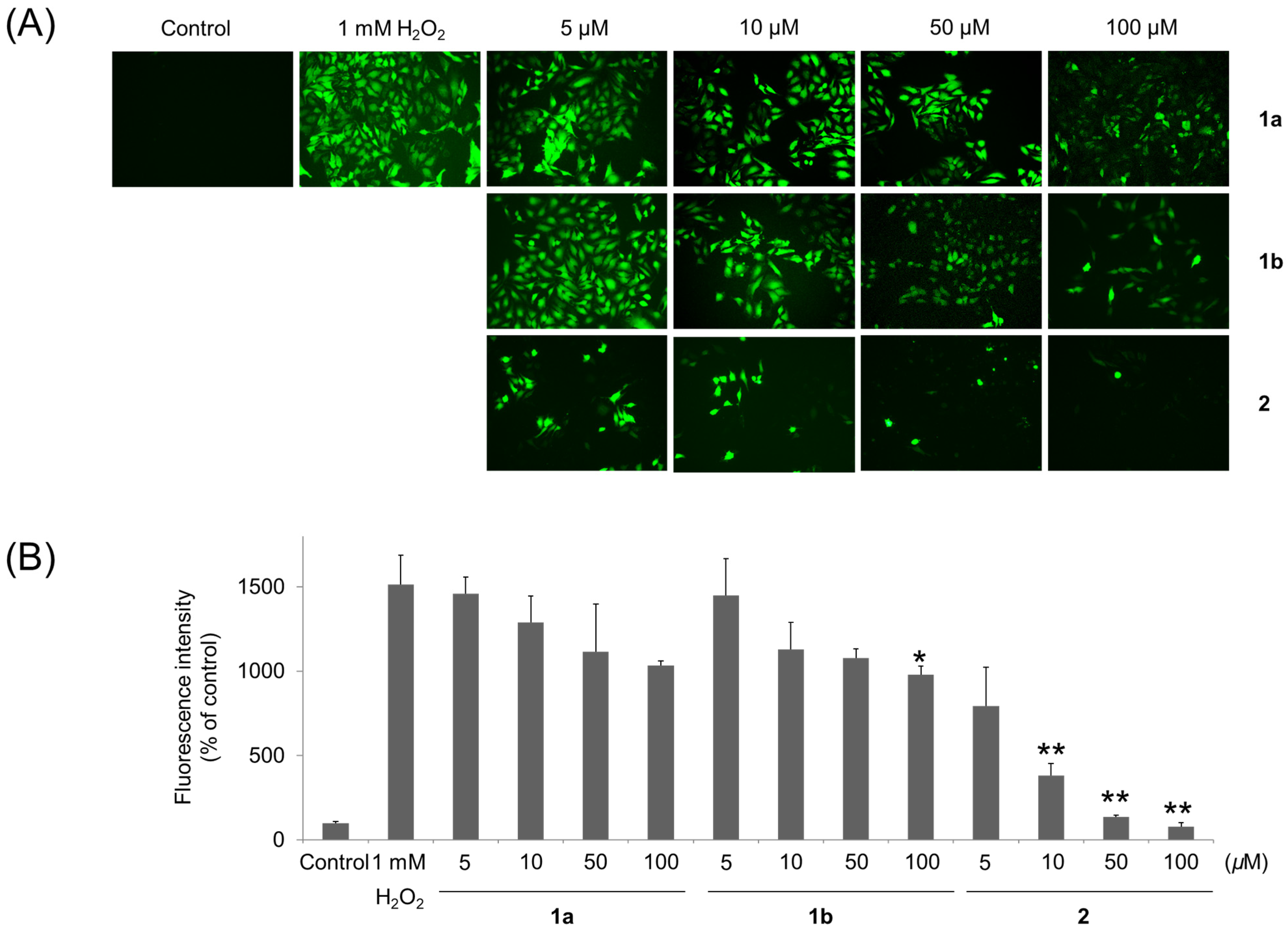
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defining Dictionary for Medicinal Herbs. Korea Institute of Oriental Medicine. Available online: http://boncho.kiom.re.kr/codex (accessed on 4 April 2019).
- Kim, C.M.; Shin, M.K.; Ahn, D.K.; Lee, K.S. Chinese Medicine Dictionary; Jungdam: Seoul, Korea, 2006; Volume 5, pp. 2184–2186. [Google Scholar]
- Tan, Z.; Lei, Y.; Zhang, B.; Huang, L. Comparison of Pharmacological Studies on Ootheca Mantidis. China J. Chin. Mater. Med. 1997, 22, 496–499. [Google Scholar]
- Kim, H.Y.; Lee, Y.J.; Han, B.H.; Yoon, J.J.; Ahn, Y.M.; Hong, M.H.; Tan, R.; Kang, D.G.; Lee, H.S. Mantidis Ootheca Induces Vascular Relaxation through Pi3k/Akt-Mediated Nitric Oxid-Cyclic Gmp-Protein Kinase G Signaling in Endothelial Cells. J. Physiol. Pharmacol. 2017, 68, 215–221. [Google Scholar] [PubMed]
- Ahn, M.Y.; Ryu, K.S.; Lee, Y.W.; Kim, Y.S. Cytotoxicity and L-Amino Acid Oxidase Activity of Crude Insect Drugs. Arch. Pharmacal Res. 2000, 23, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Moon, B.C.; Lim, H. Effects of 14 Chung-Bu Medicinal Materials Described in the Dongui Bogam on Inflammatory Cytokines Production in Hacat Keratinocytes. J. Soc. Cosmet. Sci. Korea 2020, 46, 195–204. [Google Scholar]
- Wang, W.; Zhang, N.; Chanda, W.; Liu, M.; UD Din, S.R.; Diao, Y.; Liu, L.; Cao, J.; Wang, X.; Li, X. Antibacterial and Anti-Biofilm Activity of the Lipid Extract from Mantidis Ootheca on Pseudomonas aeruginosa. J. Zhejiang Univ. Sci. B 2018, 19, 364–371. [Google Scholar] [CrossRef]
- Xu, M. Study of the Anti-Dpph Free Radical Component from Mantidis Ootheca Extracts. J. Anhui Agric. Univ. 2014, 2014, 11619–11620. [Google Scholar]
- Xu, M. Anti-Atherosclerotic Activities of Two Compounds from Mantidis Ootheca. J. Anhui Agric. Univ. 2012, 2012, 15722–15723. [Google Scholar]
- Song, J.; Cha, J.; Moon, B.C.; Kim, W.J.; Yang, S.; Choi, G. Mantidis Oötheca (Mantis Egg Case) Original Species Identification Via Morphological Analysis and DNA Barcoding. J. Ethnopharmacol. 2020, 252, 112574–112584. [Google Scholar] [CrossRef]
- Lim, H.; Seo, Y.S.; Ryu, S.M.; Moon, B.C.; Choi, G.; Kim, J. Two-Week Repeated Oral Dose Toxicity Study of Mantidis Ootheca Water Extract in C57bl/6 Mice. J. Evid. Based Complementary Altern. Med. 2019, 2019, 6180236. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of Dpph Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of Phytochemical Content and Antioxidant Activity of Some Selected Traditional Indian Medicinal Plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, L.; Jiang, L.; Di, L.; Yan, Y.; Tu, Z.; Yang, C.; Zuo, Z.; Hou, B.; Xia, H.; et al. Dopamine Derivatives from the Insect Polyrhachis Dives as Inhibitors of Rock1/2 and Stimulators of Neural Stem Cell Proliferation. Tetrahedron 2014, 70, 8852–8857. [Google Scholar] [CrossRef]
- Bai, H.; Li, Y.; Qin, F.; Yan, Y.; Wang, S.; Zhang, H.; Cheng, Y. Periplanetols A–F, Phenolic Compounds from Periplaneta Americana with Potent Cox-2 Inhibitory Activity. Fitoterapia 2020, 143, 104589. [Google Scholar] [CrossRef]
- Noda, N.; Kubota, S.; Miyata, Y.; Miyahara, K. Optically Active N-Acetyldopamine Dimer of the Crude Drug “Zentai,” the Cast-Off Shell of the Cicada Cryptotympana sp. Chem. Pharm. Bull. 2000, 48, 1749–1752. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J.; Lu, F.; Ralph, S.A.; Boudet, A.M.; MacKay, J.J.; Sederoff, R.R.; Ito, T.; Kawai, S.; Ohashi, H. Nmr Analysis of Lignins in Cad-Deficient Plants. Part 1. Incorporation of Hydroxycinnamaldehydes and Hydroxybenzaldehydes into Lignins. Org. Biomol. Chem. 2003, 1, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Jiang, C.; Zhang, X.; Jiang, Y.; Ma, D. Cui-Catalyzed Hydroxylation of Aryl Bromides under the Assistance of 5-Bromo-2-(1h-Imidazol-2-Yl) Pyridine and Related Ligands. Tetrahedron Lett. 2011, 52, 5593–5595. [Google Scholar] [CrossRef]
- Luo, J.; Ma, Q.; Zhao, Y.; Yi, T.; Li, C.; Zhou, J. Palaeophytochemical Components from the Miocene-Fossil Wood of Pinus Griffithii. J. Chin. Chem. Soc. 2009, 56, 600–605. [Google Scholar] [CrossRef]
- Bonaparte, A.C.; Betush, M.P.; Panseri, B.M.; Mastarone, D.J.; Murphy, R.K.; Murphree, S.S. Novel Aerobic Oxidation of Primary Sulfones to Carboxylic Acids. Org. Lett. 2011, 13, 1447–1449. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Kudo, A.; Tokuda, S.; Matsumoto, K.; Hosoyama, H. Identification of a New Natural Vasorelaxatant Compound,(+)-Osbeckic Acid, from Rutin-Free Tartary Buckwheat Extract. J. Agric. Food Chem. 2010, 58, 10876–10879. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.E.; Storz, T.; Colyer, J.T.; Thiel, O.R.; Dilmeghani Seran, M.; Larsen, R.D.; Murry, J.A. Iodide-Catalyzed Reductions: Development of a Synthesis of Phenylacetic Acids. J. Org. Chem. 2011, 76, 9519–9524. [Google Scholar] [CrossRef]
- Chang, L.; Ouyang, L.M.; Xu, Y.; Pan, J.; Xu, J.H. Highly Enantioselective Hydrolysis of Phenyl-1, 2-Ethanediol Cyclic Carbonates by Newly Isolated Bacillus Sp. Ecu0015. J. Mol. Catal. B Enzym. 2010, 66, 95–100. [Google Scholar] [CrossRef]
- Matsuki, Y.; Ito, T.; Komatsu, S.; Nambara, T. Studies on the Metabolism of Atenolol. Characterization and Determination of a New Urinary Metabolite in the Rat. Chem. Pharm. Bull. 1982, 30, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Wolff, C.; Rudolph, H. Compartmentalization of Phenolic Constituents in Sphagnum. Phytochemistry 1995, 38, 35–39. [Google Scholar] [CrossRef]
- Fillion, E.; Dumas, A.M.; Kuropatwa, B.A.; Malhotra, N.R.; Sitler, T.C. Yb (Otf) 3-Catalyzed Reactions of 5-Alkylidene Meldrum’s Acids with Phenols: One-Pot Assembly of 3, 4-Dihydrocoumarins, 4-Chromanones, Coumarins, and Chromones. J. Org. Chem. 2006, 71, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.H.; Martinez, L.J.; Coleman, M.C.; Spitz, D.R.; Weintraub, N.L.; Kader, K.N. Mechanisms of H2o2-Induced Oxidative Stress in Endothelial Cells. Free Radic. Biol. Med. 2006, 40, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| δC, Type 2 | δH, Multi (J in Hz) 1 | COSY | HMBC | δC, Type | δH, Multi (J in Hz) | COSY | HMBC | |
| 1 | 132.4, C | 132.5, C | ||||||
| 2 | 116.8, CH | 6.61, d (1.8) | 3, 4, 6, 7 | 118.7, CH | 6.78, s | 3, 4, 6, 7 | ||
| 3 | 147.4, C | 149.1, C | ||||||
| 4 | 142.8, C | 144.1, C | ||||||
| 5 | 125.3, C | 118.1, CH | 6.75, s | 1, 3, 4, 7′ | ||||
| 6 | 120.3, CH | 6.39, d (1.9) | 2, 4, 7, 8′ | 131.7, C | ||||
| 7 | 36.0, CH2 | 2.55, t (7.2) | 8 | 1, 2, 6, 8 | 33.1, CH2 | 2.73, t (7.0) | 8 | 1, 2, 6, 8 |
| 8 | 42.2, CH2 | 3.24, t (7.2) | 7 | 1, 7, 9 | 42.8, CH2 | 3.30, overlap 3 | 7 | 1, 7, 9 |
| 9 | 173.4, CO | 173.4, CO | ||||||
| 10 | 22.6, CH3 | 1.80, s | 9 | 22.7, CH3 | 1.83, s | 9 | ||
| 1′ | 128.5, C | 131.3, C | ||||||
| 2′ | 116.7, CH | 7.44, d (2.0) | 3′, 4′, 6′, 7′ | 134.3, CH | 7.68, d (8.2) | 3′ | 3′, 4′, 6′, 7′ | |
| 3′ | 146.5, C | 116.2, CH | 6.84, d (8.3) | 2′ | 1′, 4′, 5′ | |||
| 4′ | 152.6, C | 164.0, C | ||||||
| 5′ | 115.9, CH | 6.72, d (8.4) | 6′ | 1′, 3′, 4′ | 116.2, CH | 6.84, d (8.3) | 6′ | 1′, 3′, 4′ |
| 6′ | 123.9, CH | 7.47, dd (8.4, 2.1) | 5′ | 2′, 4′, 7′ | 134.3, CH | 7.68, d (8.2) | 5′ | 2′, 4′, 5′, 7′ |
| 7′ | 196.3, CO | 199.1, CO | ||||||
| 8′ | 54.4, CH | 6.66, s | 4, 5, 6, 7′, 9′ | |||||
| 9′ | 173.2, CO | |||||||
| 10′ | 22.4, CH3 | 2.02, s | 9′ | |||||
| Samples | IC50 (μM) | |
|---|---|---|
| DPPH | ABTS | |
| 1a | 81.50 ± 0.77 1 | 81.98 ± 0.48 |
| 1b | 46.54 ± 0.56 | 62.74 ± 0.69 |
| 2 | 19.45 ± 0.42 | 37.23 ± 0.26 |
| Gallic acid | 8.95 ± 0.20 | 10.82 ± 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, S.M.; Nam, H.-h.; Kim, J.S.; Song, J.-h.; Seo, Y.H.; Kim, H.S.; Lee, A.Y.; Kim, W.J.; Lee, D.; Moon, B.C.; et al. Chemical Constituents of the Egg Cases of Tenodera angustipennis (Mantidis ootheca) with Intracellular Reactive Oxygen Species Scavenging Activity. Biomolecules 2021, 11, 556. https://doi.org/10.3390/biom11040556
Ryu SM, Nam H-h, Kim JS, Song J-h, Seo YH, Kim HS, Lee AY, Kim WJ, Lee D, Moon BC, et al. Chemical Constituents of the Egg Cases of Tenodera angustipennis (Mantidis ootheca) with Intracellular Reactive Oxygen Species Scavenging Activity. Biomolecules. 2021; 11(4):556. https://doi.org/10.3390/biom11040556
Chicago/Turabian StyleRyu, Seung Mok, Hyeon-hwa Nam, Joong Sun Kim, Jun-ho Song, Young Hye Seo, Hyo Seon Kim, A Yeong Lee, Wook Jin Kim, Dongho Lee, Byeong Cheol Moon, and et al. 2021. "Chemical Constituents of the Egg Cases of Tenodera angustipennis (Mantidis ootheca) with Intracellular Reactive Oxygen Species Scavenging Activity" Biomolecules 11, no. 4: 556. https://doi.org/10.3390/biom11040556
APA StyleRyu, S. M., Nam, H.-h., Kim, J. S., Song, J.-h., Seo, Y. H., Kim, H. S., Lee, A. Y., Kim, W. J., Lee, D., Moon, B. C., & Lee, J. (2021). Chemical Constituents of the Egg Cases of Tenodera angustipennis (Mantidis ootheca) with Intracellular Reactive Oxygen Species Scavenging Activity. Biomolecules, 11(4), 556. https://doi.org/10.3390/biom11040556







