Eat, Train, Sleep—Retreat? Hormonal Interactions of Intermittent Fasting, Exercise and Circadian Rhythm
Abstract
1. Introduction
2. Materials and Methods
3. Results
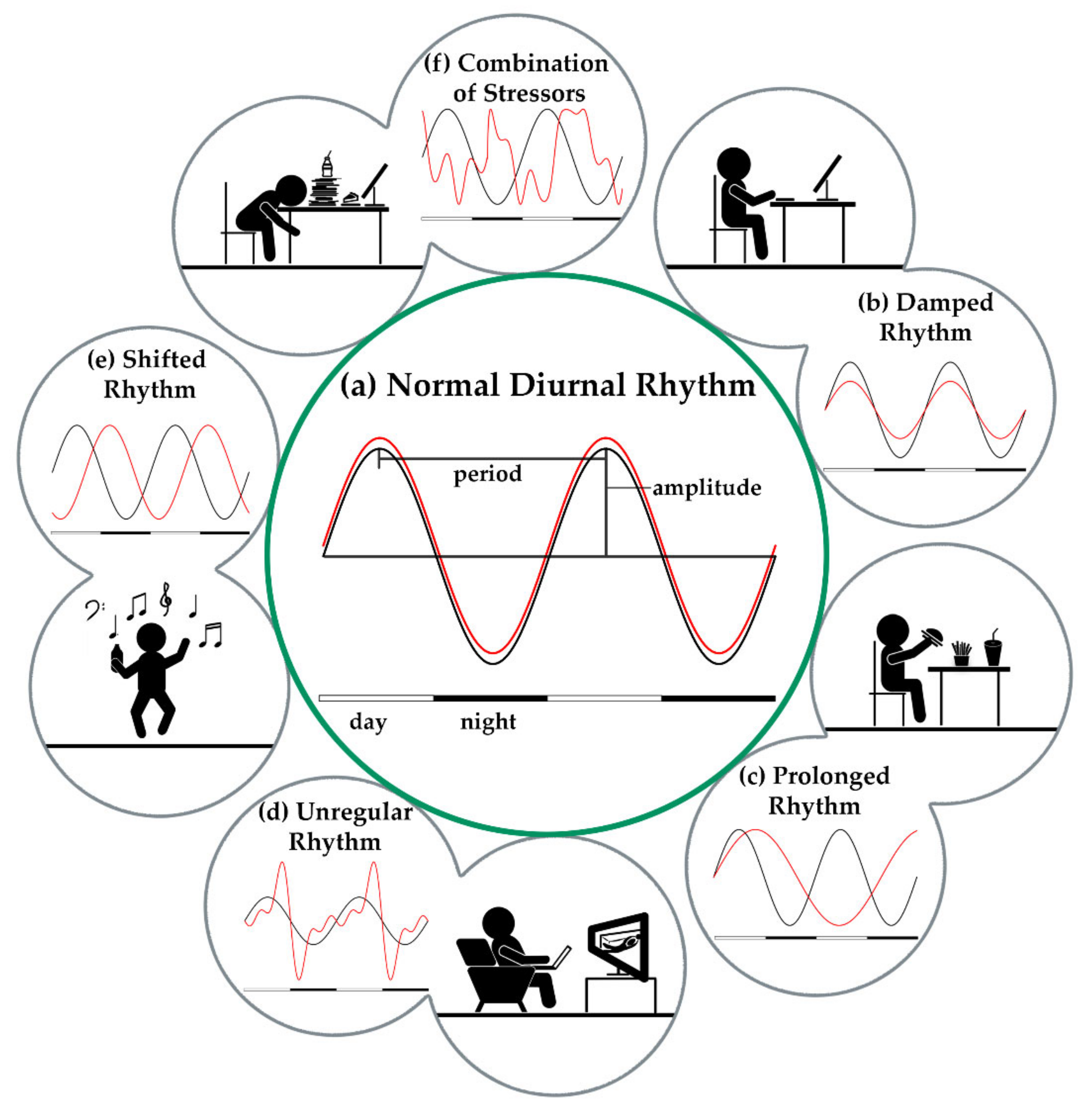
3.1. Circadian Rhythm
3.1.1. Hormonal Pathways and Its Effects on the Circadian Rhythm
3.1.2. External Stressors of the Circadian Rhythm
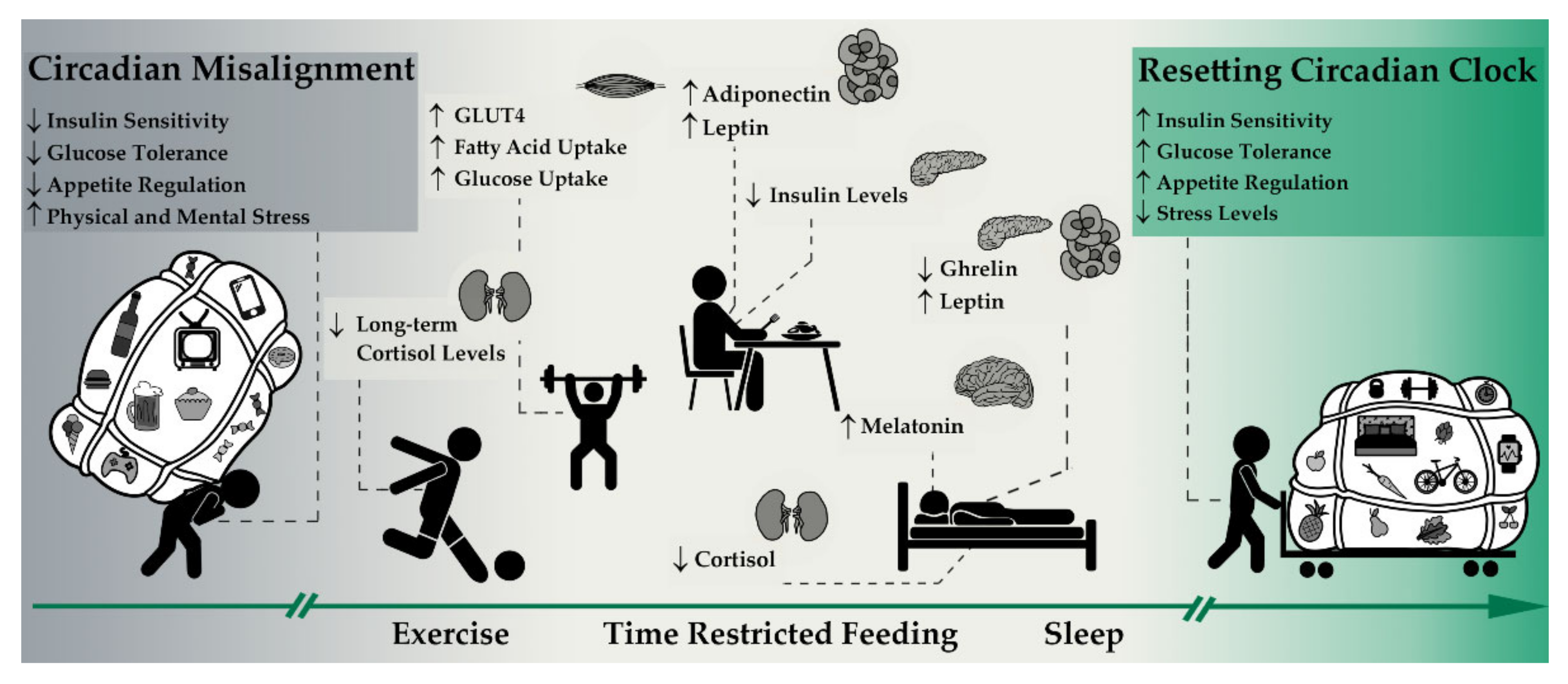
3.2. Intermittent Fasting as a Lifestyle
3.2.1. Intermittent Fasting and Its Influence on Central Clock Genes/Hormones
3.2.2. Intermittent Fasting and Hormonal Pathways
3.3. Exercise as a Lifestyle
3.3.1. Macronutrient Timing
3.3.2. Exercise Timing
3.3.3. Exercise and Other Hormonal Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schott, M.; Bornstein, S.R.; Stratakis, C.A. Hormone and Metabolic Research: 50 Years of Research. Horm. Metab. Res. 2019, 51, 8–10. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef]
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Booth, F.W.; Chakravarthy, M.V.; Gordon, S.E.; Spangenburg, E.E. Waging war on physical inactivity: Using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 2002, 93, 3–30. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Booth, F.W. Eating, exercise, and “thrifty” genotypes: Connecting the dots toward an evolutionary understanding of modern chronic diseases. J. Appl. Physiol. 2004, 96, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Danguir, J.; Nicolaidis, S. Dependence of sleep on nutrients’ availability. Physiol. Behav. 1979, 22, 735–740. [Google Scholar] [CrossRef]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef]
- Dobbs, R.; Sawers, C.; Thompson, F.; Manyika, J.; Woetzel, J.; Child, P.; McKenna, S.; Spatharou, A. Overcoming Obesity: An Initial Economic Analysis; The McKinsey Global Institute: New York, NY, USA, 2014. [Google Scholar]
- Bergouignan, A.; Rudwill, F.; Simon, C.; Blanc, S. Physical inactivity as the culprit of metabolic inflexibility: Evidence from bed-rest studies. J. Appl. Physiol. 2011, 111, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, J.K.P. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. 2013, 110, 5695–5700. [Google Scholar] [CrossRef]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Léger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.; Weltman, A.; Delgiorno, C.; Balagopal, P.; Damaso, L.; Killen, K.; Mauras, N. Lifestyle Intervention Improves Fitness Independent of Metformin in Obese Adolescents. Med. Sci. Sports Exerc. 2012, 44, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Templeman, I.; Gonzalez, J.T.; Thompson, D.; Betts, J.A. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc. Nutr. Soc. 2020, 79, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Northeast, R.C.; Vyazovskiy, V.V.; Bechtold, D.A. Eat, sleep, repeat: The role of the circadian system in balancing sleep–wake control with metabolic need. Curr. Opin. Physiol. 2020, 15, 183–191. [Google Scholar] [CrossRef]
- Parr, E.B.; Heilbronn, L.K.; Hawley, J.A. A Time to Eat and a Time to Exercise. Exerc. Sport Sci. Rev. 2020, 48, 4–10. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports 2006, 16, 3–63. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the Real Polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef]
- Schibler, U.; Sassone-Corsi, P. A Web of Circadian Pacemakers. Cell 2002, 111, 919–922. [Google Scholar] [CrossRef]
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.S.; Itoh, T.Q.; Yildirim, E.; Hwangbo, D.-S.; Allada, R. Genetics of Circadian Rhythms. Sleep Med. Clin. 2015, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Murphy, P.J.; Onat, O.E.; Krieger, A.C.; Özçelik, T.; Campbell, S.S.; Young, M.W. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 2017, 169, 203–215.e13. [Google Scholar] [CrossRef]
- Toh, K.L.; Chen, B.; Eddaoudi, M.; Hyde, S.T.; O’Keeffe, M.; Yaghi, O.M. An hPer2 Phosphorylation Site Mutation in Familial Advanced Sleep Phase Syndrome. Science 2001, 291, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Shi, G.; Jones, C.R.; Lipzen, A.; Pennacchio, L.A.; Xu, Y.; Hallows, W.C.; McMahon, T.; Yamazaki, M.; Ptáček, L.J.; et al. A Cryptochrome 2 mutation yields advanced sleep phase in humans. eLife 2016, 5, e16695. [Google Scholar] [CrossRef]
- Rudic, R.D.; McNamara, P.; Curtis, A.-M.; Boston, R.C.; Panda, S.; HogenEsch, J.B.; Fitzgerald, G.A. BMAL1 and CLOCK, Two Essential Components of the Circadian Clock, Are Involved in Glucose Homeostasis. PLoS Biol. 2004, 2, e377. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. 2005, 102, 12071–12076. [Google Scholar] [CrossRef]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nat. Cell Biol. 2010, 466, 627–631. [Google Scholar] [CrossRef]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian Clock Proteins and Immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef]
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian Rhythms in Isolated Brain Regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Trexler, N.A.; Egbejimi, O.; McElfresh, T.A.; Suk, H.Y.; Petterson, L.E.; Shaw, C.A.; Hardin, P.E.; Bray, M.S.; Chandler, M.P.; et al. The Circadian Clock within the Cardiomyocyte Is Essential for Responsiveness of the Heart to Fatty Acids. J. Biol. Chem. 2006, 281, 24254–24269. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, K.-A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the Circadian Clock in the Liver by Feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Storch, K.-F.; Paz, C.; Signorovitch, J.; Raviola, E.; Pawlyk, B.; Li, T.; Weitz, C.J. Intrinsic Circadian Clock of the Mammalian Retina: Importance for Retinal Processing of Visual Information. Cell 2007, 130, 730–741. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef]
- Lee, C.; Etchegaray, J.-P.; Cagampang, F.R.A.; Loudon, A.S.I.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [CrossRef]
- Zheng, B.; Albrecht, U.; Kaasik, K.; Sage, M.; Lu, W.; Vaishnav, S.; Li, Q.; Sun, Z.S.; Eichele, G.; Bradley, A.; et al. Nonredundant Roles of the mPer1 and mPer2 Genes in the Mammalian Circadian Clock. Cell 2001, 105, 683–694. [Google Scholar] [CrossRef]
- Kume, K.; Zylka, M.J.; Sriram, S.; Shearman, L.P.; Weaver, D.R.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M. mCRY1 and mCRY2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop. Cell 1999, 98, 193–205. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Molina, L.-L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The Orphan Nuclear Receptor REV-ERBα Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Guillaumond, F.; Dardente, H.; Giguère, V.; Cermakian, N. Differential Control of Bmal1 Circadian Transcription by REV-ERB and ROR Nuclear Receptors. J. Biol. Rhythm. 2005, 20, 391–403. [Google Scholar] [CrossRef]
- Leibetseder, V.; Humpeler, S.; Svoboda, M.; Schmid, D.; Thalhammer, T.; Zuckermann, A.; Marktl, W.; Ekmekcioglu, C. Clock Genes Display Rhythmic Expression in Human Hearts. Chronobiol. Int. 2009, 26, 621–636. [Google Scholar] [CrossRef]
- Polidarová, L.; Soták, M.; Sládek, M.; Pácha, J.; Sumová, A. Temporal Gradient in the Clock Gene and Cell-Cycle Checkpoint KinaseWee1Expression along the Gut. Chronobiol. Int. 2009, 26, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, Y.; Sothern, R.B.; Guan, Y.; Chan, P. Chronobiological Analysis of Circadian Patterns in Transcription of Seven Key Clock Genes in Six Peripheral Tissues in Mice. Chronobiol. Int. 2007, 24, 793–820. [Google Scholar] [CrossRef]
- Zvonic, S.; Ptitsyn, A.A.; Conrad, S.A.; Scott, L.K.; Floyd, Z.E.; Kilroy, G.; Wu, X.; Goh, B.C.; Mynatt, R.L.; Gimble, J.M. Characterization of Peripheral Circadian Clocks in Adipose Tissues. Diabetes 2006, 55, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Bozek, K.; Relógio, A.; Kielbasa, S.M.; Heine, M.; Dame, C.; Kramer, A.; Herzel, H. Regulation of Clock-Controlled Genes in Mammals. PLoS ONE 2009, 4, e4882. [Google Scholar] [CrossRef]
- Oster, H.; Damerow, S.; Kiessling, S.; Jakubcakova, V.; Abraham, D.; Tian, J.; Hoffmann, M.W.; Eichele, G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006, 4, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Son, G.H.; Cha, H.K.; Chung, S.; Kim, K. Multimodal Regulation of Circadian Glucocorticoid Rhythm by Central and Adrenal Clocks. J. Endocr. Soc. 2018, 2, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Pazienza, V.; Vinciguerra, M. Clock Genes and Clock-Controlled Genes in the Regulation of Metabolic Rhythms. Chronobiol. Int. 2012, 29, 227–251. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Yang, S.-C.; Tseng, H.-L.; Hwang, L.-L.; Chen, C.-T.; Shieh, K.-R. Abnormal expressions of circadian-clock and circadian clock-controlled genes in the livers and kidneys of long-term, high-fat-diet-treated mice. Int. J. Obes. 2009, 34, 227–239. [Google Scholar] [CrossRef]
- Vogel, M.; Braungardt, T.; Meyer, W.; Schneider, W. The effects of shift work on physical and mental health. J. Neural Transm. 2012, 119, 1121–1132. [Google Scholar] [CrossRef]
- Wang, X.-S.; Armstrong, M.E.G.; Cairns, B.J.; Key, T.J.; Travis, R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. 2011, 61, 78–89. [Google Scholar] [CrossRef]
- Sack, R.L.; Blood, M.L.; Lewy, A.J. Melatonin Rhythms in Night Shift Workers. Sleep 1992, 15, 434–441. [Google Scholar] [CrossRef]
- Roden, M.; Koller, M.; Pirich, K.; Vierhapper, H.; Waldhauser, F. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am. J. Physiol. Integr. Comp. Physiol. 1993, 265, R261–R267. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Cajochen, C.; Pache, M.; Flammer, J.; Wirz-Justice, A. Thermoregulatory effects of melatonin in relation to sleepiness. Chronobiol. Int. 2006, 23, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.C.B.; Filho, F.F.D.; Schnorr, C.C.; Bertoletti, O.A.; Bottega, G.B.; Rodrigues, T.D.C. Night shift work, short sleep and obesity. Diabetol. Metab. Syndr. 2020, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.L.; Roehrs, T.; Richardson, G.; Walsh, J.K.; Roth, T. Shift Work Sleep Disorder: Prevalence and Consequences Beyond that of Symptomatic Day Workers. Sleep 2004, 27, 1453–1462. [Google Scholar] [CrossRef]
- Åkerstedt, T. Shift work and disturbed sleep/wakefulness. Occup. Med. 2003, 53, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bidlingmaier, M.; Petru, R.; Gil, F.P.; Loerbroks, A.; Angerer, P. Impact of shift work on the diurnal cortisol rhythm: A one-year longitudinal study in junior physicians. J. Occup. Med. Toxicol. 2018, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manenschijn, L.; Van Kruysbergen, R.G.P.M.; De Jong, F.H.; Koper, J.W.; Van Rossum, E.F.C. Shift Work at Young Age Is Associated with Elevated Long-Term Cortisol Levels and Body Mass Index. J. Clin. Endocrinol. Metab. 2011, 96, E1862–E1865. [Google Scholar] [CrossRef] [PubMed]
- Leese, G.; Chattington, P.; Fraser, W.; Vora, J.; Edwards, R.; Williams, G. Short-term night-shift working mimics the pituitary-adrenocortical dysfunction in chronic fatigue syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 1867–1870. [Google Scholar] [CrossRef][Green Version]
- Zanquetta, M.M.; Seraphim, P.M.; Sumida, D.H.; Cipolla-Neto, J.; Machado, U.F. Calorie restriction reduces pinealectomy-induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J. Pineal Res. 2003, 35, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Diaz, B.; Blázquez, E. Effect of Pinealectomy on Plasma Glucose, Insulin and Glucagon Levels in the Rat. Horm. Metab. Res. 1986, 18, 225–229. [Google Scholar] [CrossRef]
- Mellado, C.; Rodríguez, V.; Diego, J.G.; Alvarez, E.; Blázquez, E. Effect of Pinealectomy and of Diabetes on Liver Insulin and Glucagon Receptor Concentrations in the Rat. J. Pineal Res. 1989, 6, 295–306. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef]
- Harris, R.B.S. Leptin—Muchmorethan asatietysignal. Annu. Rev. Nutr. 2000, 20, 45–75. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, A.M. Endocrine Regulation of Energy Metabolism: Review of Pathobiochemical and Clinical Chemical Aspects of Leptin, Ghrelin, Adiponectin, and Resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Rohner-Jeanrenaud, F.; Jeanrenaud, B. The discovery of leptin and its impact in the understanding of obesity. Eur. J. Endocrinol. 1996, 135, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Lemoine, P.; Arnaud-Briant, V.; Dreyfus, M. Prevalence and consequences of sleep disorders in a shift worker population. J. Psychosom. Res. 2002, 53, 577–583. [Google Scholar] [CrossRef]
- Ulhôa, M.A.; Marqueze, E.C.; Burgos, L.G.A.; Moreno, C.R.C. Shift Work and Endocrine Disorders. Int. J. Endocrinol. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Meng, H.; Zhu, L.; Kord-Varkaneh, H.; Santos, H.O.; Tinsley, G.M.; Fu, P. Effects of intermittent fasting and energy-restricted diets on lipid profile: A systematic review and meta-analysis. Nutrition 2020, 77, 110801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, B.; Heilbronn, L.K. Intermittent fasting: What questions should we be asking? Physiol. Behav. 2020, 218, 112827. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, A.K. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Gasmi, M.; Sellami, M.; Denham, J.; Padulo, J.; Kuvacic, G.; Selmi, W.; Khalifa, R. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition 2018, 51–52, 29–37. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proc. Nutr. Soc. 2017, 76, 361–368. [Google Scholar]
- Lettieri-Barbato, D.; Cannata, S.M.; Casagrande, V.; Ciriolo, M.R.; Aquilano, K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS ONE 2018, 13, e0195912. [Google Scholar] [CrossRef]
- Elamin, M.; Ruskin, D.N.; Masino, S.A.; Sacchetti, P. Ketogenic Diet Modulates NAD+-Dependent Enzymes and Reduces DNA Damage in Hippocampus. Front. Cell. Neurosci. 2018, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Chapnik, N.; Genzer, Y.; Froy, O. Relationship between FGF21 and UCP1 levels under time-restricted feeding and high-fat diet. J. Nutr. Biochem. 2017, 40, 116–121. [Google Scholar] [CrossRef]
- Mendoza, J. Circadian Clocks: Setting Time By Food. J. Neuroendocr. 2007, 19, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Luiten, P.; Ter Horst, G.; Steffens, A. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog. Neurobiol. 1987, 28, 1–54. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Bruinstroop, E.; Yi, C.; Klieverik, L.; La Fleur, S.; Fliers, E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann. N. Y. Acad. Sci. 2010, 1212, 114–129. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014, 10, 466–475. [Google Scholar] [CrossRef]
- Guillemin, R. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J. Endocrinol. 2005, 184, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Arimura, A.; Kastin, A.J. Hypothalamic Regulatory Hormones: At least nine substances from the hypothalamus control the secretion of pituitary hormones. Science 1973, 179, 341–350. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Gerich, J.; Rizza, R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab. Rev. 1988, 4, 17–30. [Google Scholar] [CrossRef]
- Gerich, J.E.; Charles, M.A.; Grodsky, G.M. Regulation of Pancreatic Insulin and Glucagon Secretion. Annu. Rev. Physiol. 1976, 38, 353–388. [Google Scholar] [CrossRef] [PubMed]
- Elrick, H.; Stimmler, L.; Hlad, C.J.; Arai, Y. Plasma Insulin Response to Oral and Intravenous Glucose Administration1. J. Clin. Endocrinol. Metab. 1964, 24, 1076–1082. [Google Scholar] [CrossRef]
- Barthel, A.; Schmoll, D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Metab. 2003, 285, E685–E692. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E.; Langlois, M.; Noacco, C.; Karam, J.H.; Forsham, P.H. Lack of Glucagon Response to Hypoglycemia in Diabetes: Evidence for an Intrinsic Pancreatic Alpha Cell Defect. Science 1973, 182, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Almeda-Valdes, P.; Patterson, B.W.; Okunade, A.L.; Imai, S.-I.; Mittendorfer, B.; Klein, S. Diurnal Variation in Insulin Sensitivity of Glucose Metabolism Is Associated With Diurnal Variations in Whole-Body and Cellular Fatty Acid Metabolism in Metabolically Normal Women. J. Clin. Endocrinol. Metab. 2014, 99, E1666–E1670. [Google Scholar] [CrossRef]
- Carrasco-Benso, M.P.; Rivero-Gutierrez, B.; Lopez-Minguez, J.; Anzola, A.; Diez-Noguera, A.; Madrid, J.A.; Lujan, J.A.; Martínez-Augustin, O.; Scheer, F.A.J.L.; Garaulet, M. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016, 30, 3117–3123. [Google Scholar] [CrossRef]
- Kalsbeek, A.; la Fleur, S.; Fliers, E. Circadian control of glucose metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [PubMed]
- Hara, R.; Wan, K.; Wakamatsu, H.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 2001, 6, 269–278. [Google Scholar] [CrossRef]
- Akhtar, R.A.; Reddy, A.B.; Maywood, E.S.; Clayton, J.D.; King, V.M.; Smith, A.G.; Gant, T.W.; Hastings, M.H.; Kyriacou, C.P. Circadian Cycling of the Mouse Liver Transcriptome, as Revealed by cDNA Microarray, Is Driven by the Suprachiasmatic Nucleus. Curr. Biol. 2002, 12, 540–550. [Google Scholar] [CrossRef]
- Kornmann, B.; Schaad, O.; Bujard, H.; Takahashi, J.S.; Schibler, U. System-Driven and Oscillator-Dependent Circadian Transcription in Mice with a Conditionally Active Liver Clock. PLoS Biol. 2007, 5, e34. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.-F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. 2008, 105, 15172–15177. [Google Scholar] [CrossRef]
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; Nusinow, D.A.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Blaauw, B.; Dyar, K.A. The functional significance of the skeletal muscle clock: Lessons from Bmal1 knockout models. Skelet. Muscle 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Paschos, G.K.; Ibrahim, S.A.; Song, W.-L.; Kunieda, T.; Grant, G.R.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Basse, A.L.; Dalbram, E.; Larsson, L.; Gerhart-Hines, Z.; Zierath, J.R.; Treebak, J.T. Skeletal Muscle Insulin Sensitivity Show Circadian Rhythmicity Which Is Independent of Exercise Training Status. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Gomez-Abellan, P.; Gomez-Santos, C.; Madrid, J.A.; Milagro-Yoldi, F.I.; Campión-Zabalza, J.; Martinez, J.A.; Garaulet, M. Circadian Expression of Adiponectin and Its Receptors in Human Adipose Tissue. Endocrinology 2010, 151, 115–122. [Google Scholar] [CrossRef]
- Shea, S.A.; Hilton, M.F.; Orlova, C.; Ayers, R.T.; Mantzoros, C.S. Independent Circadian and Sleep/Wake Regulation of Adipokines and Glucose in Humans. J. Clin. Endocrinol. Metab. 2005, 90, 2537–2544. [Google Scholar] [CrossRef]
- Sadacca, L.A.; Lamia, K.A.; Delemos, A.S.; Blum, B.; Weitz, C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 2010, 54, 120–124. [Google Scholar] [CrossRef]
- Lee, J.; Moulik, M.; Fang, Z.; Saha, P.; Zou, F.; Xu, Y.; Nelson, D.L.; Ma, K.; Moore, D.D.; Yechoor, V.K. Bmal1 and -Cell Clock Are Required for Adaptation to Circadian Disruption, and Their Loss of Function Leads to Oxidative Stress-Induced -Cell Failure in Mice. Mol. Cell. Biol. 2013, 33, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dang, F.; Zhang, D.; Yuan, Y.; Zhang, C.; Wu, Y.; Wang, Y.; Liu, Y. Glucagon-CREB/CRTC2 Signaling Cascade Regulates Hepatic BMAL1 Protein. J. Biol. Chem. 2015, 290, 2189–2197. [Google Scholar] [CrossRef]
- Kawamoto, T.; Noshiro, M.; Furukawa, M.; Honda, K.K.; Nakashima, A.; Ueshima, T.; Usui, E.; Katsura, Y.; Fujimoto, K.; Honma, S.; et al. Effects of Fasting and Re-Feeding on the Expression of Dec1, Per1, and Other Clock-Related Genes. J. Biochem. 2006, 140, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Dachraoui, M.; Baumert, T.F. Perturbation of the circadian clock and pathogenesis of NAFLD. Metabolism 2020, 111, 154337. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Christensen, M.; Lund, A.; De Heer, J.; Svendsen, B.; Kielgast, U.; Knop, F.K. Regulation of glucagon secretion by incretins. Diabetes Obes. Metab. 2011, 13, 89–94. [Google Scholar] [CrossRef]
- Macdonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51, S434–S442. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Raz, I.; Ahren, B.; Chapnik, N.; Ganz, T.; Menaged, M.; Barnea, M.; Bar-Dayan, Y.; et al. Influences of Breakfast on Clock Gene Expression and Postprandial Glycemia in Healthy Individuals and Individuals With Diabetes: A Randomized Clinical Trial. Diabetes Care 2017, 40, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Manoogian, E.N.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian Clocks and Feeding Time Regulate the Oscillations and Levels of Hepatic Triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Behre, C. Adiponectin: Saving the starved and the overfed. Med. Hypotheses 2007, 69, 1290–1292. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, H.S.; Kim, K.-S.; Choi, W.H.; Ahn, C.W.; Kim, B.T.; Kim, S.M.; Lee, S.Y.; Ahn, S.M.; Kim, Y.K.; et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008, 19, 371–375. [Google Scholar] [CrossRef]
- Anderlová, K.; Kremen, J.; Dolezalová, R.; Housová, J.; Haluzíková, D.; Kunesová, M.; Haluzík, M. The influence of very-low-calorie-diet on serum leptin, soluble leptin receptor, adiponectin and resistin levels in obese women. Physiol. Res. 2006, 55, 277–283. [Google Scholar]
- Cho, Y.; Hong, N.; Kim, K.-W.; Cho, S.J.; Lee, M.; Lee, Y.-H.; Lee, Y.-H.; Kang, E.S.; Cha, B.-S.; Lee, B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Chen, L.; Clark, G.O.; Lee, Y.; Stevens, R.D.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Charron, M.J.; Newgard, C.B.; et al. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl. Acad. Sci. 2010, 107, 4813–4819. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Blair, S.N. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989, 262, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, C.L.; Hackney, A.C.; Garcia, R.; Groff, D.; Evans, E.; Shea, T. The Effects of an Exercise Program in Leukemia Patients. Integr. Cancer Ther. 2009, 8, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Stults-Kolehmainen, M.A.; Sinha, R. The Effects of Stress on Physical Activity and Exercise. Sports Med. 2014, 44, 81–121. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of Exercise Is a Major Cause of Chronic Diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef]
- Moser, O.; Eckstein, M.L.; West, D.J.; Goswami, N.; Sourij, H.; Hofmann, P. Type 1 Diabetes and Physical Exercise: Moving (forward) as an Adjuvant Therapy. Curr. Pharm. Des. 2020, 26, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Roberts, M.D.; Church, T.S. Toward Exercise as Personalized Medicine. Sports Med. 2013, 43, 157–165. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; Boom, L.V.D.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Pediatr. Diabetes 2020, 21, 1375–1393. [Google Scholar] [CrossRef]
- Ohta, M.; Okufuji, T.; Matsushima, Y.; Ikeda, M. The Effect of Lifestyle Modification on Physical Fitness and Work Ability in Different Workstyles. J. UOEH 2004, 26, 411–421. [Google Scholar] [CrossRef]
- Ortlepp, J.R.; Metrikat, J.; Albrecht, M.; Maya-Pelzer, P. Relationship between physical fitness and lifestyle behaviour in healthy young men. Eur. J. Cardiovasc. Prev. Rehabilitation 2004, 11, 192–200. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- Wilson, K. Mobile cell phone technology puts the future of health care in our hands. Can. Med. Assoc. J. 2018, 190, E378–E379. [Google Scholar] [CrossRef]
- Direito, A.; Jiang, Y.; Whittaker, R.; Maddison, R. Smartphone apps to improve fitness and increase physical activity among young people: Protocol of the Apps for IMproving FITness (AIMFIT) randomized controlled trial. BMC Public Health 2015, 15, 635. [Google Scholar] [CrossRef]
- Parasuraman, S.; Sam, A.T.; Yee, S.W.K.; Chuon, B.L.C.; Ren, L.Y. Smartphone usage and increased risk of mobile phone addiction: A concurrent study. Int. J. Pharm. Investig. 2017, 7, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Maher, F.; Vannucci, S.J.; Simpson, I.A. Glucose transporter proteins in brain. FASEB J. 1994, 8, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Mul, J.D.; Stanford, K.I.; Hirshman, M.F.; Goodyear, L.J. Exercise and Regulation of Carbohydrate Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.D. Physiological responses to food intake throughout the day. Nutr. Res. Rev. 2014, 27, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Cajochen, C.; Werth, E.; Wirz-Justice, A. Alteration of Internal Circadian Phase Relationships after Morning versus Evening Carbohydrate-Rich Meals in Humans. J. Biol. Rhythm. 2002, 17, 364–376. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef]
- Burke, L.M.; Kiens, B.; Ivy, J.L. Carbohydrates and fat for training and recovery. J. Sports Sci. 2004, 22, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Sopowski, M.J.; Hampton, S.M.; Ribeiro, D.C.O.; Morgan, L.; Arendt, J. Postprandial Triacylglycerol Responses in Simulated Night and Day Shift: Gender Differences. J. Biol. Rhythm. 2001, 16, 272–276. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Frye, S.M.; Goodson, L.; Wootton, S.A. Effect of meal sequence on postprandial lipid, glucose and insulin responses in young men. Eur. J. Clin. Nutr. 2003, 57, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Moran-Ramos, S.; Guerrero-Vargas, N.N.; Mendez-Hernandez, R.; Basualdo, M.D.C.; Escobar, C.; Buijs, R.M. The suprachiasmatic nucleus drives day-night variations in postprandial triglyceride uptake into skeletal muscle and brown adipose tissue. Exp. Physiol. 2017, 102, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H. Molecular and Integrative Physiology of Intestinal Peptide Transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef]
- Pan, X.; Terada, T.; Irie, M.; Saito, H.; Inui, K.-I. Diurnal rhythm of H+-peptide cotransporter in rat small intestine. Am. J. Physiol. Liver Physiol. 2002, 283, G57–G64. [Google Scholar] [CrossRef]
- Winget, C.M.; Deroshia, C.W.; Holley, D.C. Circadian rhythms and athletic performance. Med. Sci. Sports Exerc. 1985, 17, 498–516. [Google Scholar] [CrossRef]
- Thun, E.; Bjorvatn, B.; Flo, E.; Harris, A.; Pallesen, S. Sleep, circadian rhythms, and athletic performance. Sleep Med. Rev. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Drust, B.; Waterhouse, J.; Atkinson, G.; Edwards, B.; Reilly, T. Circadian Rhythms in Sports Performance—An Update. Chronobiol. Int. 2005, 22, 21–44. [Google Scholar] [CrossRef]
- Smolander, J.; Lindgvist, A.; Kolari, P.; Laitinen, L.A. Circadian variation in peripheral blood flow in relation to core temperature at rest. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993, 67, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Edwards, B.; Bedford, P.; Hughes, A.; Robinson, K.; Nevill, A.; Weinert, D.; Reilly, T. Thermoregulation during mild exercise at different circadian times. Chronobiol. Int. 2004, 21, 253–275. [Google Scholar] [CrossRef]
- Weinert, D.; Waterhouse, J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol. Behav. 1998, 63, 837–843. [Google Scholar] [CrossRef]
- Weinert, D.; Waterhouse, J. The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol. Behav. 2007, 90, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Bessot, N.; Nicolas, A.; Moussay, S.; Gauthier, A.; Sesboüé, B.; Davenne, D. The Effect of Pedal Rate and Time of Day on the Time to Exhaustion from High-Intensity Exercise. Chronobiol. Int. 2006, 23, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.W. Morning–evening differences in response to exhaustive severe-intensity exercise. Appl. Physiol. Nutr. Metab. 2014, 39, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.W. Effect of time of day on aerobic power in exhaustive high-intensity exercise. J. Sports Med. Phys. Fit. 1996, 36, 155–160. [Google Scholar]
- Reilly, T.; Baxter, C. Influence of time of day on reactions to cycling at a fixed high intensity. Br. J. Sports Med. 1983, 17, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Weydahl, A. Chronotype, Physical Activity, and Sport Performance: A Systematic Review. Sports Med. 2017, 47, 1859–1868. [Google Scholar] [CrossRef]
- Facer-Childs, E.; Brandstaetter, R. The Impact of Circadian Phenotype and Time since Awakening on Diurnal Performance in Athletes. Curr. Biol. 2015, 25, 518–522. [Google Scholar] [CrossRef]
- Brown, F.M.; Neft, E.E.; LaJambe, C.M. Collegiate Rowing Crew Performance Varies by Morningness-Eveningness. J. Strength Cond. Res. 2008, 22, 1894–1900. [Google Scholar] [CrossRef]
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 2020, 5, 12–14. [Google Scholar] [CrossRef]
- Chtourou, H.; Souissi, N. The Effect of Training at a Specific Time of Day. J. Strength Cond. Res. 2012, 26, 1984–2005. [Google Scholar] [CrossRef]
- Bernard, T.; Giacomoni, M.; Gavarry, O.; Seymat, M.; Falgairette, G. Time-of-day effects in maximal anaerobic leg exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 77, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Souissi, N.; Gauthier, A.; Sesboüé, B.; LaRue, J.; Davenne, D. Circadian Rhythms in Two Types of Anaerobic Cycle Leg Exercise: Force-Velocity and 30-s Wingate Tests. Int. J. Sports Med. 2004, 25, 14–19. [Google Scholar] [CrossRef]
- Racinais, S.; Connes, P.; Bishop, D.; Blonc, S.; Hue, O. Morning Versus Evening Power Output and Repeated-Sprint Ability. Chronobiol. Int. 2005, 22, 1029–1039. [Google Scholar] [CrossRef]
- Racinais, S.; Perrey, S.; Denis, R.; Bishop, D. Maximal power, but not fatigability, is greater during repeated sprints performed in the afternoon. Chronobiol. Int. 2010, 27, 855–864. [Google Scholar] [CrossRef]
- Melhim, A.F. Investigation of Circadian Rhythms in Peak Power and Mean Power of Female Physical Education Students. Int. J. Sports Med. 1993, 14, 303–306. [Google Scholar] [CrossRef]
- Douglas, C.M.; Hesketh, S.J.; Esser, K.A. Time of Day and Muscle Strength: A Circadian Output? Physiology 2021, 36, 44–51. [Google Scholar] [CrossRef]
- Waterhouse, P.J.; Drust, B.; Weinert, D.; Edwards, B.; Gregson, W.; Atkinson, G.; Kao, S.; Aizawa, S.; Reilly, T. The Circadian Rhythm of Core Temperature: Origin and some Implications for Exercise Performance. Chronobiol. Int. 2005, 22, 207–225. [Google Scholar] [CrossRef]
- Sato, S.; Basse, A.L.; Schönke, M.; Chen, S.; Samad, M.; Altıntaş, A.; Laker, R.C.; Dalbram, E.; Barrès, R.; Baldi, P.; et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab. 2019, 30, 92–110.e4. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.D.; Howlett, R.A.; Kim, M.J.; Olfert, I.M.; Hogan, M.C.; McNulty, W.; Hickey, R.P.; Wagner, P.D.; Kahn, C.R.; Giordano, F.J.; et al. Loss of Skeletal Muscle HIF-1α Results in Altered Exercise Endurance. PLoS Biol. 2004, 2, e288. [Google Scholar] [CrossRef] [PubMed]
- Smilios, I.; Pilianidis, T.; Karamouzis, M.; Tokmakidis, S.P. Hormonal Responses after Various Resistance Exercise Protocols. Med. Sci. Sports Exerc. 2003, 35, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Kiyonaga, A.; Arakawa, K.; Tanaka, H.; Shindo, M. Blood pressure and hormonal responses to aerobic exercise. Hypertension 1985, 7, 125–131. [Google Scholar] [CrossRef]
- Raastad, T.; Bjøro, T.; Hallén, J. Hormonal responses to high- and moderate-intensity strength exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 82, 121–128. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.W.; Hirshman, M.F.; Gervino, E.V.; Ocel, J.V.; Forse, R.A.; Hoenig, S.J.; Aronson, D.; Goodyear, L.J.; Horton, E.S. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999, 48, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Front. Horm. Res. 2016, 47, 12–26. [Google Scholar] [CrossRef]
- Maresh, C.M.; Whittlesey, M.J.; Armstrong, L.E.; Yamamoto, L.M.; Judelson, D.A.; Fish, K.E.; Casa, D.J.; Kavouras, S.A.; Castracane, V.D. Effect of Hydration State on Testosterone and Cortisol Responses to Training-Intensity Exercise in Collegiate Runners. Int. J. Sports Med. 2006, 27, 765–770. [Google Scholar] [CrossRef]
- Khani, S.; Tayek, J.A. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin. Sci. 2001, 101, 739–747. [Google Scholar] [CrossRef]
- Djurhuus, C.B.; Gravholt, C.H.; Nielsen, S.; Mengel, A.; Christiansen, J.S.; Schmitz, O.E.; Møller, N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am. J. Physiol. Metab. 2002, 283, E172–E177. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress and the Individual. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Hackney, A.C.; Viru, A. Twenty-four-hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin. Physiol. 1999, 19, 178–182. [Google Scholar] [CrossRef]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocr. 2017, 44, 83–102. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Weltman, J.Y.; Pieper, K.S.; Weltman, A.; Hartman, M.L. Cortisol and Growth Hormone Responses to Exercise at Different Times of Day 1. J. Clin. Endocrinol. Metab. 2001, 86, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Rodenbeck, A.; Huether, G.; Rüther, E.; Hajak, G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci. Lett. 2002, 324, 159–163. [Google Scholar] [CrossRef]
- Buman, M.P.; Phillips, B.A.; Youngstedt, S.D.; Kline, C.E.; Hirshkowitz, M. Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America. Poll. Sleep Med. 2014, 15, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Florini, J.R. Hormonal control of muscle growth. Muscle Nerve 1987, 10, 577–598. [Google Scholar] [CrossRef]
- Gore, D.C.; Jahoor, F.; Wolfe, R.R.; Herndon, D.N. Acute Response of Human Muscle Protein to Catabolic Hormones. Ann. Surg. 1993, 218, 679–684. [Google Scholar] [CrossRef]
- Bird, S.P.; Tarpenning, K.M. Influence of Circadian Time Structure on Acute Hormonal Responses to a Single Bout of Heavy-Resistance Exercise in Weight-Trained Men. Chronobiol. Int. 2004, 21, 131–146. [Google Scholar] [CrossRef]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J. Search for the feeding-entrainable circadian oscillator: A complex proposition. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R1524–R1526. [Google Scholar] [CrossRef]
- Borer, K.T.; Wuorinen, E.C.; Lukos, J.R.; Denver, J.W.; Porges, S.W.; Burant, C.F. Two Bouts of Exercise before Meals, but Not after Meals, Lower Fasting Blood Glucose. Med. Sci. Sports Exerc. 2009, 41, 1606–1614. [Google Scholar] [CrossRef]
- Edinburgh, R.M.; Bradley, H.E.; Abdullah, N.-F.; Robinson, S.L.; Chrzanowski-Smith, O.J.; Walhin, J.-P.; Joanisse, S.; Manolopoulos, K.N.; Philp, A.; Hengist, A.; et al. Lipid Metabolism Links Nutrient-Exercise Timing to Insulin Sensitivity in Men Classified as Overweight or Obese. J. Clin. Endocrinol. Metab. 2019, 105, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Toghi-Eshghi, S.R.; Yardley, J.E. Morning (Fasting) vs Afternoon Resistance Exercise in Individuals With Type 1 Diabetes: A Randomized Crossover Study. J. Clin. Endocrinol. Metab. 2019, 104, 5217–5224. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Coyle, E.F. Carbohydrate ingestion during prolonged exercise: Effects on metabolism and performance. Exerc. Sport Sci. Rev. 1991, 19, 1–40. [Google Scholar] [CrossRef]
- Jeukendrup, A.; Brouns, F.; Wagenmakers, A.J.M.; Saris, W.H.M. Carbohydrate-Electrolyte Feedings Improve 1 h Time Trial Cycling Performance. Int. J. Sports Med. 1997, 18, 125–129. [Google Scholar] [CrossRef]
- Coyle, E.F. Timing and method of increased carbohydrate intake to cope with heavy training, competition and recovery. J. Sports Sci. 1991, 9, 29–52. [Google Scholar] [CrossRef]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep. J. Neuroendocr. 2003, 15, 432–437. [Google Scholar] [CrossRef]
- Middleton, B.; Arendt, J.; Stone, B. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J. Sleep Res. 1996, 5, 69–76. [Google Scholar] [CrossRef]
- Khalsa, S.B.S.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A Phase Response Curve to Single Bright Light Pulses in Human Subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Maury, E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 1597. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haupt, S.; Eckstein, M.L.; Wolf, A.; Zimmer, R.T.; Wachsmuth, N.B.; Moser, O. Eat, Train, Sleep—Retreat? Hormonal Interactions of Intermittent Fasting, Exercise and Circadian Rhythm. Biomolecules 2021, 11, 516. https://doi.org/10.3390/biom11040516
Haupt S, Eckstein ML, Wolf A, Zimmer RT, Wachsmuth NB, Moser O. Eat, Train, Sleep—Retreat? Hormonal Interactions of Intermittent Fasting, Exercise and Circadian Rhythm. Biomolecules. 2021; 11(4):516. https://doi.org/10.3390/biom11040516
Chicago/Turabian StyleHaupt, Sandra, Max L. Eckstein, Alina Wolf, Rebecca T. Zimmer, Nadine B. Wachsmuth, and Othmar Moser. 2021. "Eat, Train, Sleep—Retreat? Hormonal Interactions of Intermittent Fasting, Exercise and Circadian Rhythm" Biomolecules 11, no. 4: 516. https://doi.org/10.3390/biom11040516
APA StyleHaupt, S., Eckstein, M. L., Wolf, A., Zimmer, R. T., Wachsmuth, N. B., & Moser, O. (2021). Eat, Train, Sleep—Retreat? Hormonal Interactions of Intermittent Fasting, Exercise and Circadian Rhythm. Biomolecules, 11(4), 516. https://doi.org/10.3390/biom11040516





