The Effect of Alendronate on Osteoclastogenesis in Different Combinations of M-CSF and RANKL Growth Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Seeding
2.2. Metabolic Activity and DNA Quantification Measurement
2.3. Carbonic Anhydrase II Activity
2.4. Tartrate-Resistant Acid Phosphatase and Hematoxylin Staining
2.5. Actin Cytoskeleton Staining
2.6. Statistical Analysis
3. Results
| Sample Name | ALN [M] | M-CSF [ng mL−1] | RANKL [ng mL−1] | Sample Name | ALN [M] | M-CSF [ng mL−1] | RANKL [ng mL−1] |
|---|---|---|---|---|---|---|---|
| A10−6+MR | 10−6 | 25 | 30 | A10−8+M | 10−8 | 25 | - |
| A10−6 | 10−6 | - | - | A10−8+R | 10−8 | - | 30 |
| A10−6+M | 10−6 | 25 | - | A10−10+MR | 10−10 | 25 | 30 |
| A10−6+R | 10−6 | - | 30 | A10−10 | 10−10 | - | - |
| A10−7+MR | 10−7 | 25 | 30 | A10−10+M | 10−10 | 25 | - |
| A10−7 | 10−7 | - | - | A10−10+R | 10−10 | - | 30 |
| A10−7+M | 10−7 | 25 | - | A0+MR | - | 25 | 30 |
| A10−7+R | 10−7 | - | 30 | A0 | - | - | - |
| A10−8+MR | 10−8 | 25 | 30 | A0+M | - | 25 | - |
| A10−8 | 10−8 | - | - | A0+R | - | - | 30 |
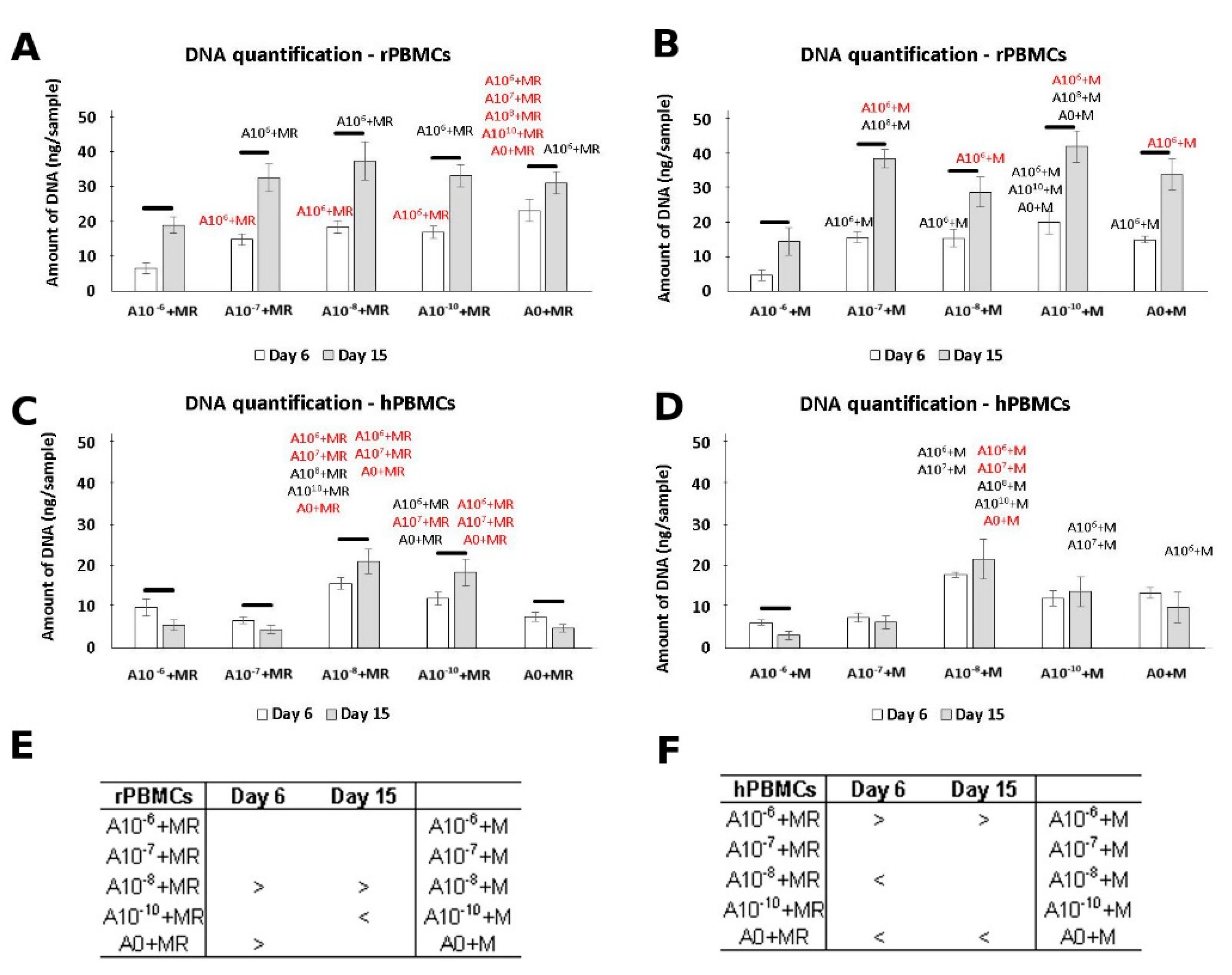
3.1. DNA Quantification
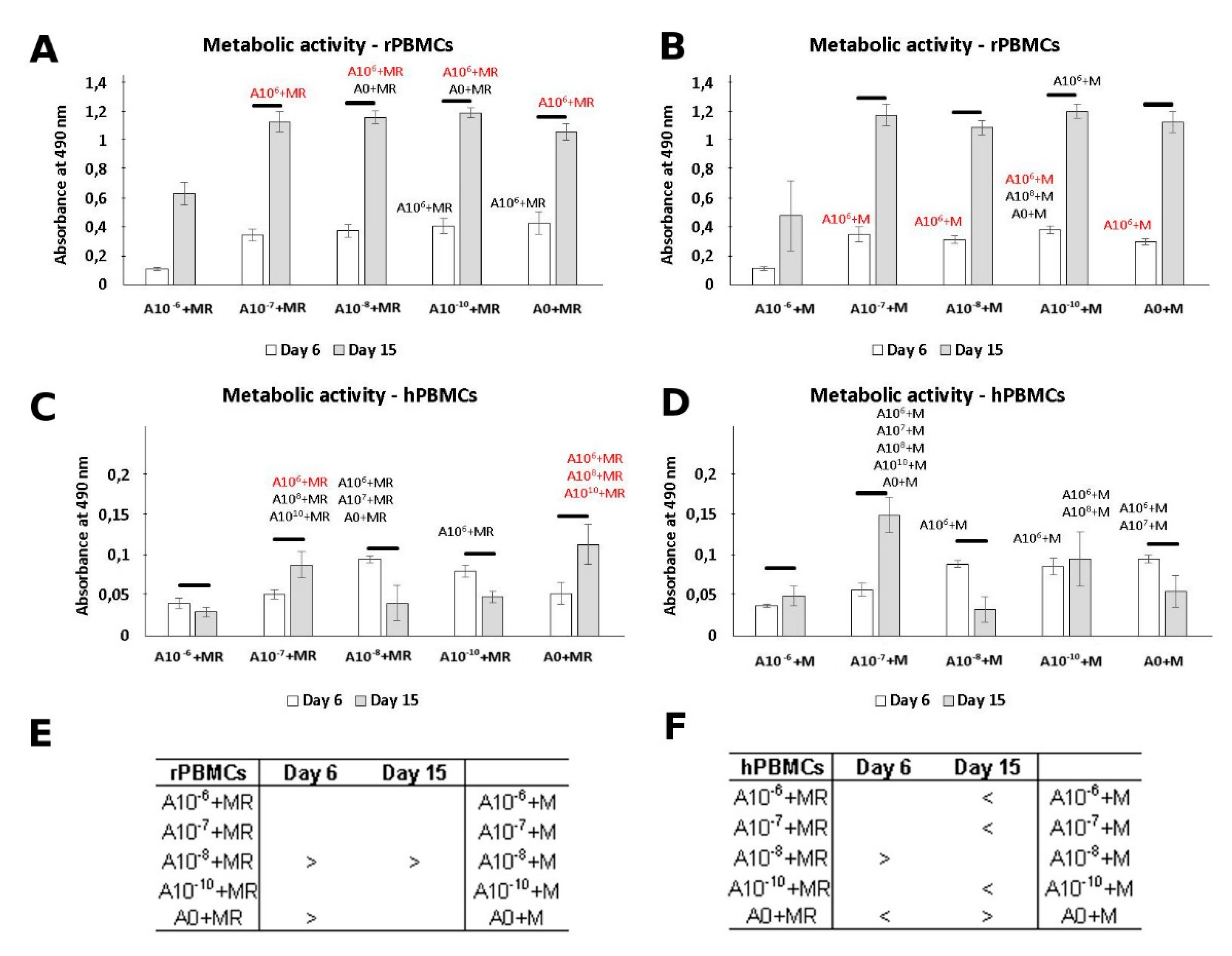
3.2. Metabolic Activity
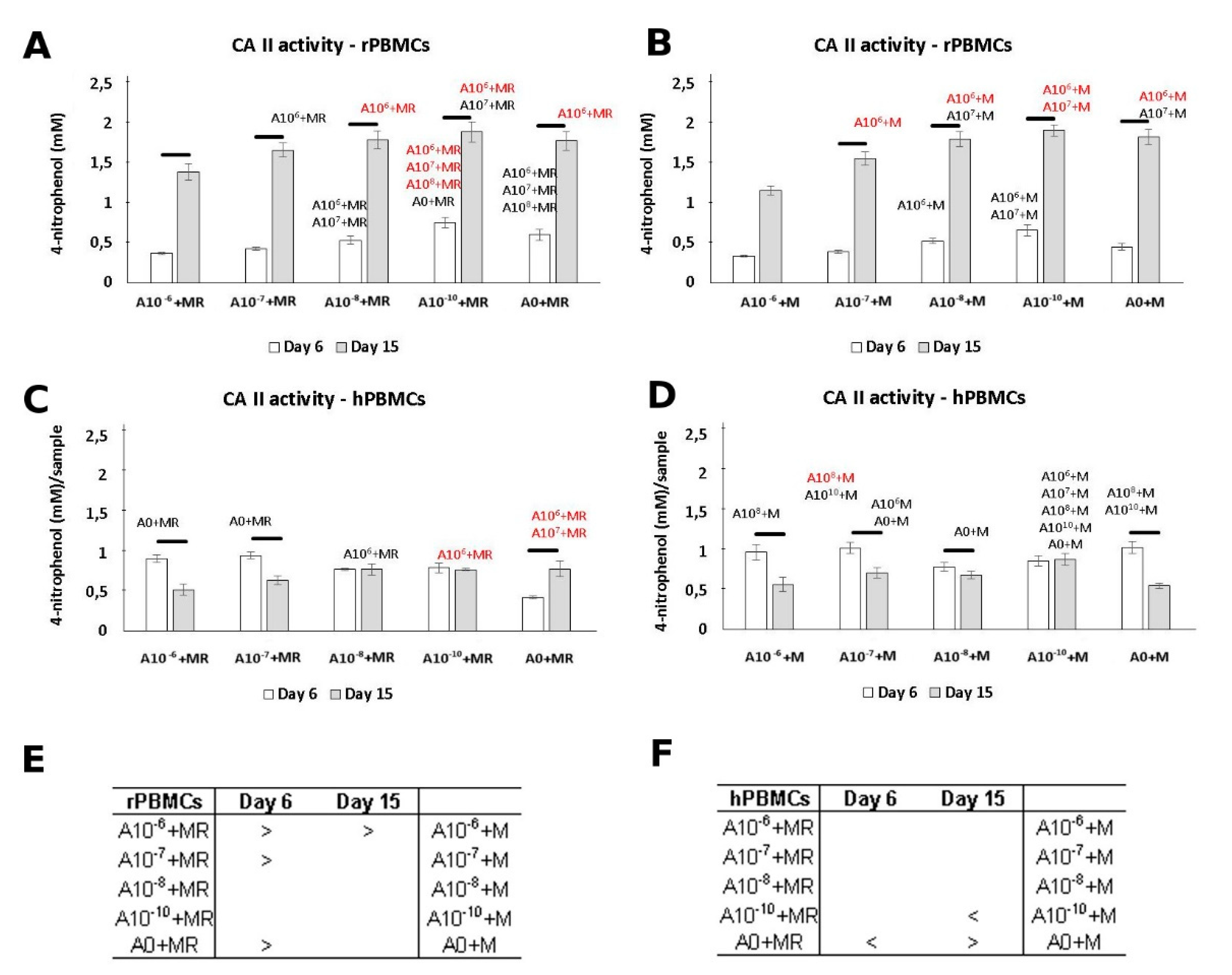
3.3. CA II Activity
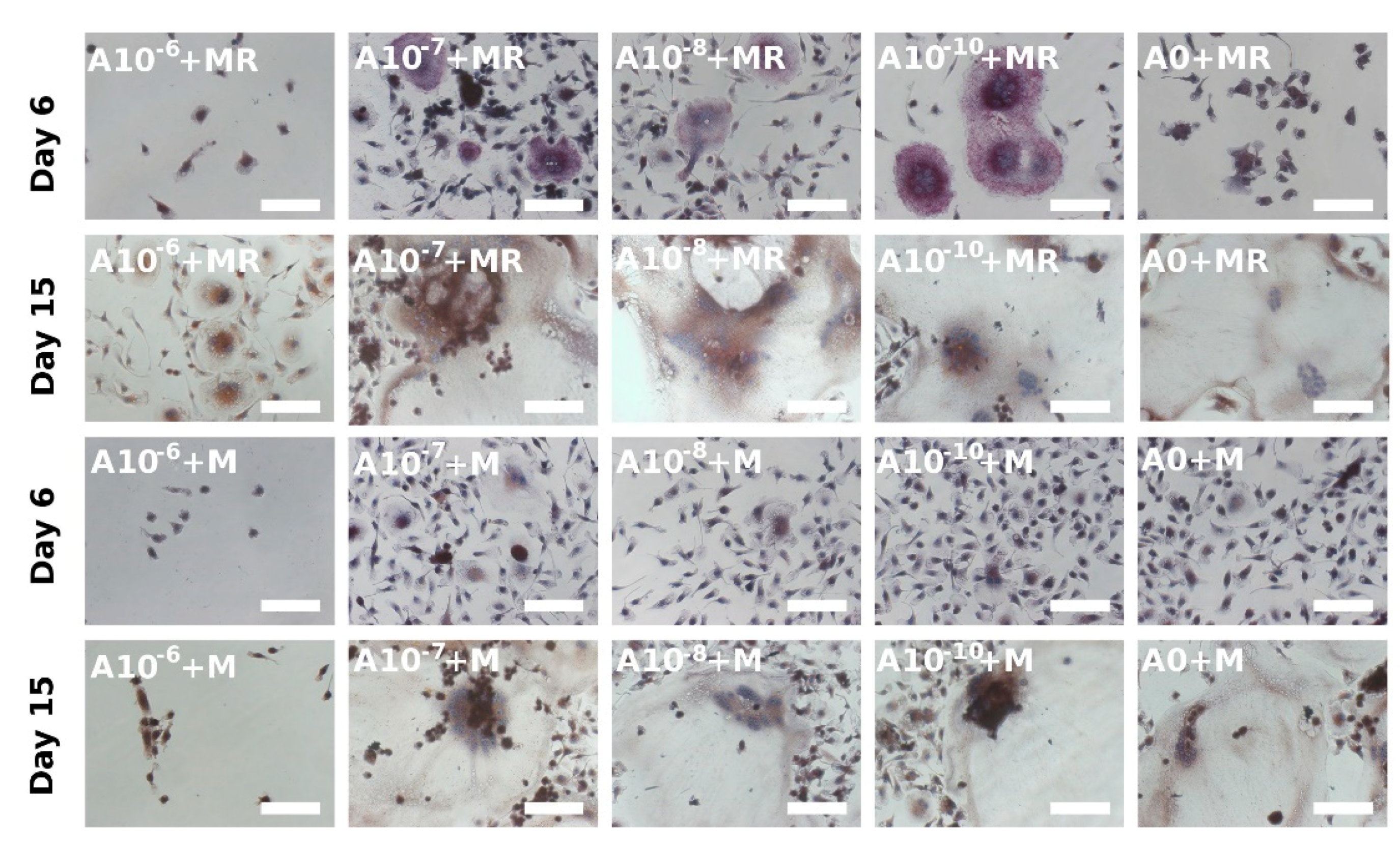
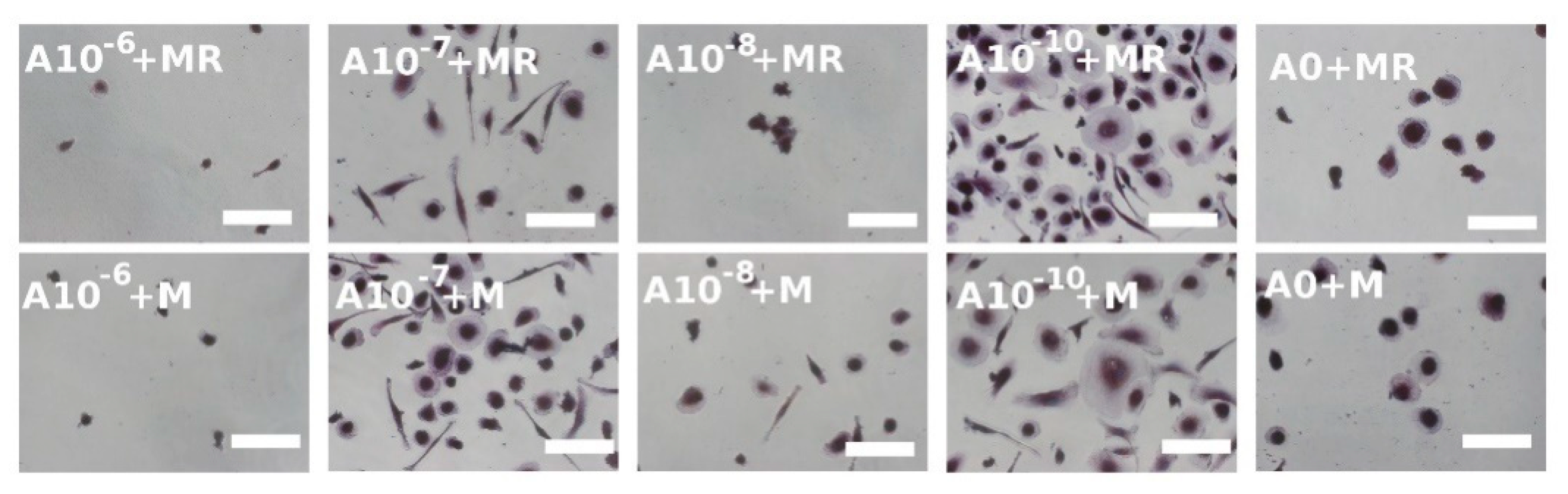
3.4. Visualization of Formed Osteoclasts by TRAP and Hematoxylin Staining
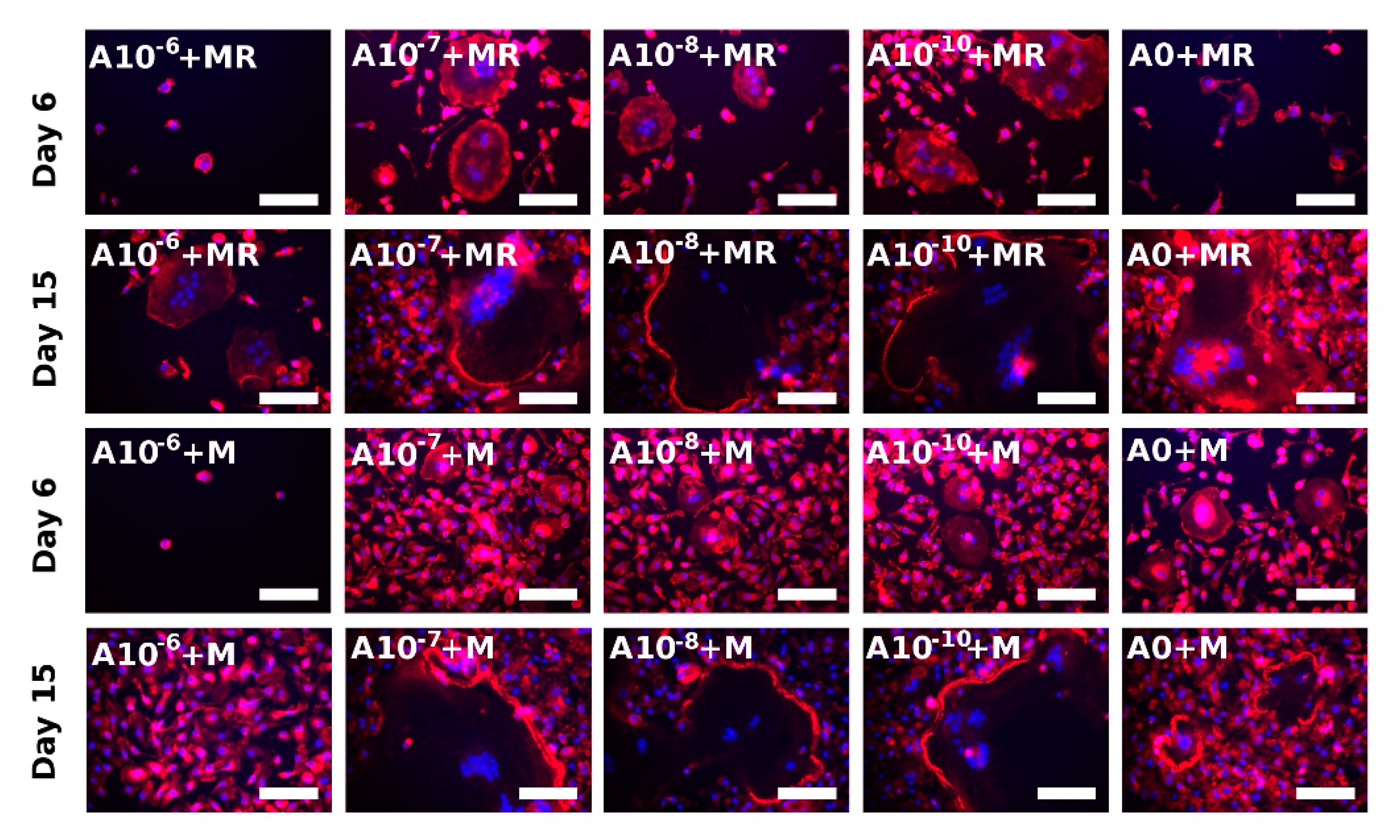
3.5. Actin Ring Formation Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.J.; Kang, W.Y.; Seong, S.J.; Kim, S.Y.; Lim, M.S.; Yoon, Y.R. Follistatin-like 1 promotes osteoclast formation via RANKL-mediated NF-κB activation and M-CSF-induced precursor proliferation. Cell. Signal. 2016, 28, 1137–1144. [Google Scholar] [CrossRef]
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Silverman, S.L.; Kupperman, E.S.; Bukata, S.V.; Members of IOF Fracture Working Group. Fracture healing: A consensus report from the Interna-tional Osteoporosis Foundation Fracture Working Group. Osteoporos. Int. 2016, 27, 2197–2206. [Google Scholar] [CrossRef]
- Namkung-Matthai, H.; Appleyard, R.; Jansen, J.; Lin, J.H.; Maastricht, S.; Swain, M.; Mason, R.; Murrell, G.; Diwan, A.; Diamond, T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 2001, 28, 80–86. [Google Scholar] [CrossRef]
- Nancollas, G.; Tang, R.; Phipps, R.; Henneman, Z.; Gulde, S.; Wu, W.; Mangood, A.; Russell, R.; Ebetino, F. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone 2006, 38, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Rogers, M.J.; Coxon, F.P.; Crockett, J.C. Cytosolic Entry of Bisphosphonate Drugs Requires Acidification of Vesicles after Fluid-Phase Endocytosis. Mol. Pharmacol. 2006, 69, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Halasy-Nagy, J.; Rodan, G.; Reszka, A. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 2001, 29, 553–559. [Google Scholar] [CrossRef]
- Dunford, J.E.; Kwaasi, A.A.; Rogers, M.J.; Barnett, B.L.; Ebetino, F.H.; Russell, R.G.G.; Oppermann, U.; Kavanagh, K.L. Structure–Activity Relationships Among the Nitrogen Containing Bisphosphonates in Clinical Use and Other Analogues: Time-Dependent Inhibition of Human Farnesyl Pyrophosphate Synthase. J. Med. Chem. 2008, 51, 2187–2195. [Google Scholar] [CrossRef]
- Tsuboi, K.; Hasegawa, T.; Yamamoto, T.; Sasaki, M.; Hongo, H.; De Freitas, P.H.L.; Shimizu, T.; Takahata, M.; Oda, K.; Michigami, T.; et al. Effects of drug discontinuation after short-term daily alendronate administration on osteoblasts and osteocytes in mice. Histochem. Cell Biol. 2016, 146, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.C.; Mönkkönen, J.; Blackburn, G.M.; Russell, R.G.G.; Rogers, M.J. Clodronate and Liposome-Encapsulated Clodronate Are Metabolized to a Toxic ATP Analog, Adenosine 5′-(β,γ-Dichloromethylene) Triphosphate, by Mammalian Cells In Vitro. J. Bone Miner. Res. 1997, 12, 1358–1367. [Google Scholar] [CrossRef]
- Hodge, J.M.; Collier, F.M.; Pavlos, N.J.; Kirkland, M.A.; Nicholson, G.C. M-CSF Potently Augments RANKL-Induced Resorption Activation in Mature Human Osteoclasts. PLoS ONE 2011, 6, e21462. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Koperski, K.; Schumacher, M.; Gelinsky, M. Relevance of osteoclast-specific enzyme activities in cell-based in vitro resorption assays. Eur. Cells Mater. 2017, 33, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, C.I.A.; Gabbai-Armelin, P.R.; Lopez-Perez, P.M.; Ulrich, D.J.O.; Jansen, J.A.; Renno, A.C.M.; Van Den Beucken, J.J.J.P. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018, 8, 15398. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Ozdemir, H.; Ozer, H.; Eren, K. Alendronate enhances osseous healing in a rat calvarial defect model. Arch. Oral Biol. 2012, 57, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Buzgo, M.; Filova, E.; Staffa, A.M.; Rampichova, M.; Doupnik, M.; Vocetkova, K.; Lukasova, V.; Kolcun, R.; Lukas, D.; Necas, A.; et al. Needleless emulsion electrospinning for the regulated delivery of susceptible proteins. J. Tissue Eng. Regen. Med. 2018, 12, 583–597. [Google Scholar] [CrossRef]
- Goimil, L.; Jaeger, P.; Ardao, I.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; García-Gonzáles, C.A. Preparation and stability of dexamethasone-loaded polymeric scaffolds for bone regen-eration processed by compressed CO2 foaming. J. CO2 Util. 2018, 24, 89–98. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Goodarzi, A.; Ai, J.; Ai, J. A facile way for development of three-dimensional localized drug delivery system for bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110032. [Google Scholar] [CrossRef] [PubMed]
- Breuil, V.; Cosman, F.; Stein, L.; Horbert, W.; Nieves, J.; Shen, V.; Lindsay, R.; Dempster, D.W. Human Osteoclast Formation and Activity In Vitro: Effects of Alendronate. J. Bone Miner. Res. 1998, 13, 1721–1729. [Google Scholar] [CrossRef]
- Martins, C.A.; Leyhausen, G.; Volk, J.; Geurtsen, W. Effects of Alendronate on Osteoclast Formation and Activity In Vitro. J. Endod. 2015, 41, 45–49. [Google Scholar] [CrossRef]
- Correia, V.D.F.P.; Caldeira, C.L.; Marques, M.M. Cytotoxicity evaluation of sodium alendronate on cultured human periodontal ligament fibroblasts. Dent. Traumatol. 2006, 22, 312–317. [Google Scholar] [CrossRef]
- Coxon, F.P.; Thompson, K.; Roelofs, A.J.; Ebetino, F.H.; Rogers, M.J. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone 2008, 42, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Töyräs, A.; Ollikainen, J.; Taskinen, M.; Mönkkönen, J. Inhibition of mevalonate pathway is involved in alendronate-induced cell growth inhibition, but not in cytokine secretion from macrophages in vitro. Eur. J. Pharm. Sci. 2003, 19, 223–230. [Google Scholar] [CrossRef]
- Mattson, A.M.; Begun, D.L.; Molstad, D.H.H.; Meyer, M.A.; Oursler, M.J.; Westendorf, J.J.; Bradley, E.W. Deficiency in the phosphatase PHLPP1 suppresses osteoclast-mediated bone resorption and enhances bone formation in mice. J. Biol. Chem. 2019, 294, 11772–11784. [Google Scholar] [CrossRef] [PubMed]
- Sophocleous, A.; Idris, A.I. Rodent models of osteoporosis. BoneKEy Rep. 2014, 3, 614. [Google Scholar] [CrossRef]
- Adachi, J.D.; Saag, K.G.; Delmas, P.D.; Liberman, U.A.; Emkey, R.D.; Seeman, E.; Lane, N.E.; Kaufman, J.-M.; Poubelle, P.E.E.; Hawkins, F.; et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: A randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001, 44, 202–211. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Bisphosphonates for the treatment of osteoporosis: Insights for clinicians. Ther. Adv. Chronic Dis. 2010, 1, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.D.; Graham, R.; Russell, R.G.; Fleisch, H. Diphosphonates Inhibit Formation of Calcium Phosphate Crystals in vitro and Pathological Calcification in vivo. Science 1969, 165, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Bisphosphonates Alendronate and Ibandronate Inhibit Artery Calcification at Doses Comparable to Those That Inhibit Bone Resorption. Arter. Thromb. Vasc. Biol. 2001, 21, 817–824. [Google Scholar] [CrossRef]
- Bernhardt, A.; Schumacher, M.; Gelinsky, M. Formation of Osteoclasts on Calcium Phosphate Bone Cements and Polystyrene Depends on Monocyte Isolation Conditions. Tissue Eng. Part C Methods 2015, 21, 160–170. [Google Scholar] [CrossRef]
- Kleinhans, C.; Schmid, F.; Kluger, P. Comparison of osteoclastogenesis and resorption activity of human osteoclasts on tissue culture polystyrene and on natural extracellular bone matrix in 2D and 3D. J. Biotechnol. 2015, 205, 101–110. [Google Scholar] [CrossRef]
- Penolazzi, L.; Lampronti, I.; Borgatti, M.; Khan, M.T.H.; Zennaro, M.; Piva, R.; Gambari, R. Induction of apoptosis of human primary osteoclasts treated with extracts from the medicinal plant Emblica officinalis. BMC Complement. Altern. Med. 2008, 8, 59. [Google Scholar] [CrossRef]
- Bharti, A.C.; Takada, Y.; Shishodia, S.; Aggarwal, B.B. Evidence that receptor activator of nuclear factor (NF)-kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB-independent and TRAF6-dependent mechanism. J. Biol. Chem. 2004, 279, 6065–6076. [Google Scholar] [CrossRef]
- Nakamura, I.; Lipfert, L.; Rodan, G.A.; Duong, L.T. Convergence of αvβ3Integrin–And Macrophage Colony Stimulating Factor–Mediated Signals on Phospholipase Cγ in Prefusion Osteoclasts. J. Cell Biol. 2001, 152, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, S.; Saleem, A.; Yuan, Z.; Emoto, Y.; Prasad, K.V.; Kufe, D. Stimulation of human monocytes with macrophage colony-stimulating factor induces a Grb2-mediated association of the focal adhesion kinase pp125FAK and dynamin. Proc. Natl. Acad. Sci. USA 1995, 92, 6132–6136. [Google Scholar] [CrossRef] [PubMed]
- Thavornyutikarn, B.; Wright, P.F.; Feltis, B.; Kosorn, W.; Turney, T.W. Bisphosphonate activation of crystallized bioglass scaffolds for enhanced bone formation. Mater. Sci. Eng. C 2019, 104, 109937. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Illescas-Montes, R.; Arnett, T.R.; Ruiz, C.; García-Martínez, O. Influence of pH on osteoclasts treated with zoledronate and alendronate. Clin. Oral Investig. 2019, 23, 813–820. [Google Scholar] [CrossRef]
- Cecchini, M.G.; Felix, R.; Fleisch, H.; Cooper, P.H. Effect of bisphosphonates on proliferation and viability of mouse bone marrow-derived macrophages. J. Bone Miner. Res. 2009, 2, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, F.; Zhang, W.; Sexton, B.E.; Windsor, L.J. Effects of alendronate on human osteoblast-like MG63 cells and matrix metalloproteinases. Arch. Oral Biol. 2012, 57, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.-M.; Kim, R.J.; Hah, Y.-S.; Gwark, J.-Y.; Bin Park, H. In vitro effects of alendronate on fibroblasts of the human rotator cuff tendon. BMC Musculoskelet. Disord. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pourgonabadi, S.; Ghorbani, A.; Najarn, Z.T.; Mousavi, S.H. In vitro assessment of alendronate toxic and apoptotic effects on human dental pulp stem cells. Iran. J. Basic Med. Sci. 2018, 21, 905–910. [Google Scholar]
- Mathov, I.; Plotkin, L.I.; Sgarlata, C.L.; Leoni, J.; Bellido, T. Extracellular Signal-Regulated Kinases and Calcium Channels Are Involved in the Proliferative Effect of Bisphosphonates on Osteoblastic Cells In Vitro. J. Bone Miner. Res. 2001, 16, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, M.; Zhou, Y.; Liu, W.; Shen, J.; Shen, Y.; Liu, L. The effect of alendronate on the expression of important cell factors in osteoclasts. Mol. Med. Rep. 2013, 8, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.M.; Fuller, K.; Chambers, T.J. Osteoclast activation: Potent inhibition by the bisphosphonate alendronate through a nonresorptive mechanism. J. Cell. Physiol. 1997, 172, 79–86. [Google Scholar] [CrossRef]
- Carano, A.; Teitelbaum, S.L.; Konsek, J.D.; Schlesinger, P.H.; Blair, H.C. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J. Clin. Investig. 1990, 85, 456–461. [Google Scholar] [CrossRef]
- Guo, S.; Ni, Y.; Ben, J.; Xia, Y.; Zhou, T.; Wang, D.; Ni, J.; Bai, H.; Wang, L.; Ma, J.; et al. Class A Scavenger Receptor Exacerbates Osteoclastogenesis by an Interleukin-6-Mediated Mechanism through ERK and JNK Signaling Pathways. Int. J. Biol. Sci. 2016, 12, 1155–1167. [Google Scholar] [CrossRef]
- Kudo, O.; Sabokbar, A.; Pocock, A.; Itonaga, I.; Fujikawa, Y.; Athanasou, N. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 2003, 32, 1–7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedvičáková, V.; Žižková, R.; Buzgo, M.; Rampichová, M.; Filová, E. The Effect of Alendronate on Osteoclastogenesis in Different Combinations of M-CSF and RANKL Growth Factors. Biomolecules 2021, 11, 438. https://doi.org/10.3390/biom11030438
Hedvičáková V, Žižková R, Buzgo M, Rampichová M, Filová E. The Effect of Alendronate on Osteoclastogenesis in Different Combinations of M-CSF and RANKL Growth Factors. Biomolecules. 2021; 11(3):438. https://doi.org/10.3390/biom11030438
Chicago/Turabian StyleHedvičáková, Věra, Radmila Žižková, Matěj Buzgo, Michala Rampichová, and Eva Filová. 2021. "The Effect of Alendronate on Osteoclastogenesis in Different Combinations of M-CSF and RANKL Growth Factors" Biomolecules 11, no. 3: 438. https://doi.org/10.3390/biom11030438
APA StyleHedvičáková, V., Žižková, R., Buzgo, M., Rampichová, M., & Filová, E. (2021). The Effect of Alendronate on Osteoclastogenesis in Different Combinations of M-CSF and RANKL Growth Factors. Biomolecules, 11(3), 438. https://doi.org/10.3390/biom11030438






