The Design and Preclinical Evaluation of a Single-Label Bimodal Nanobody Tracer for Image-Guided Surgery
Abstract
1. Introduction
2. Materials and Methods
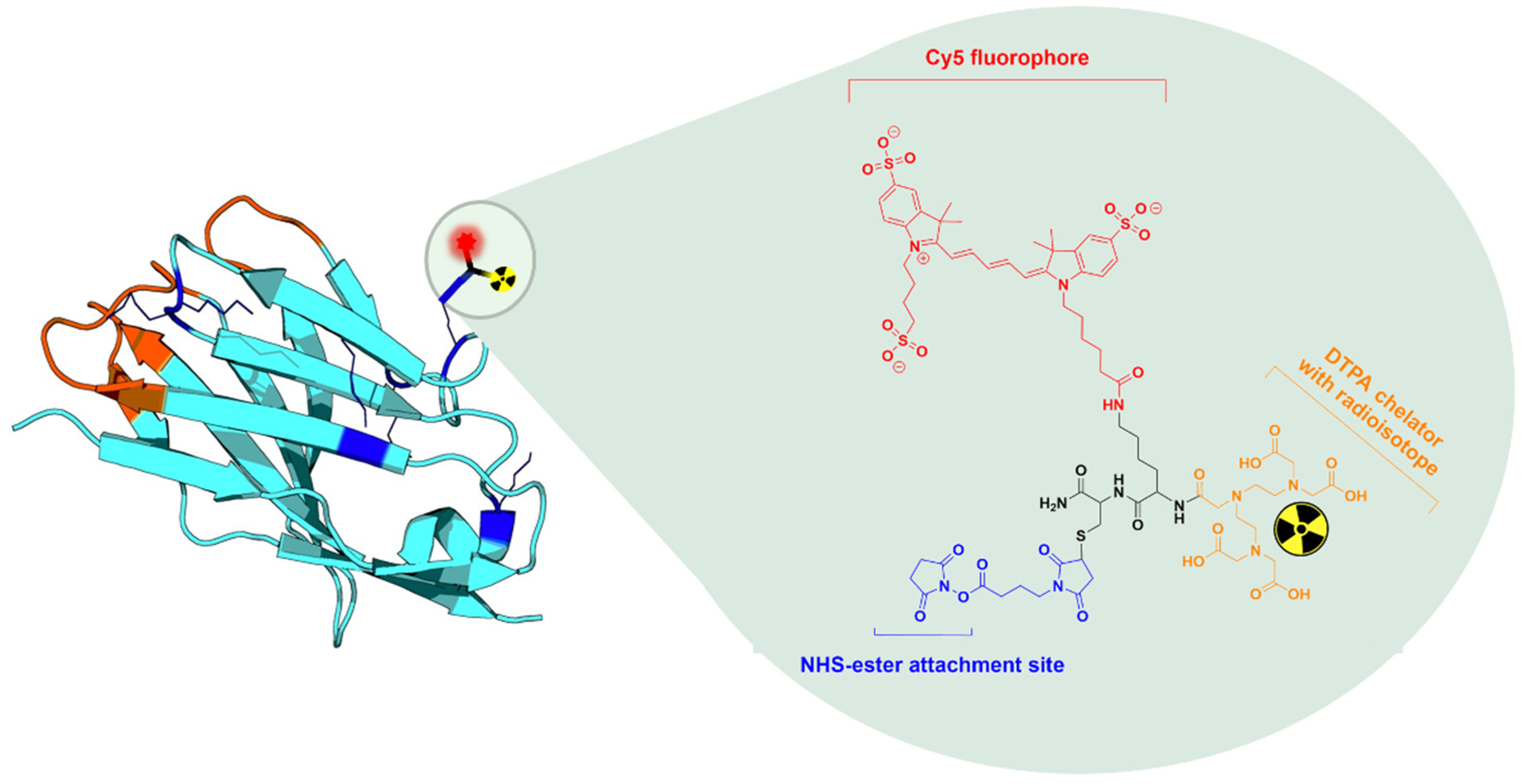
2.1. Production and MSAP-Conjugation of Nanobodies
2.2. Radiolabeling of MSAP-Nanobody Construct
2.3. In Vitro Functionality
2.4. In Vivo Biodistribution and Tumor Targeting Potential Using Bimodal Imaging
2.4.1. Longitudinal Biodistribution Study in Subcutaneous Xenografts
2.4.2. Image-Guided Resection of Intraperitoneally Disseminated Tumor Lesions
2.5. Imaging Protocols
2.6. Statistical Analysis
3. Results
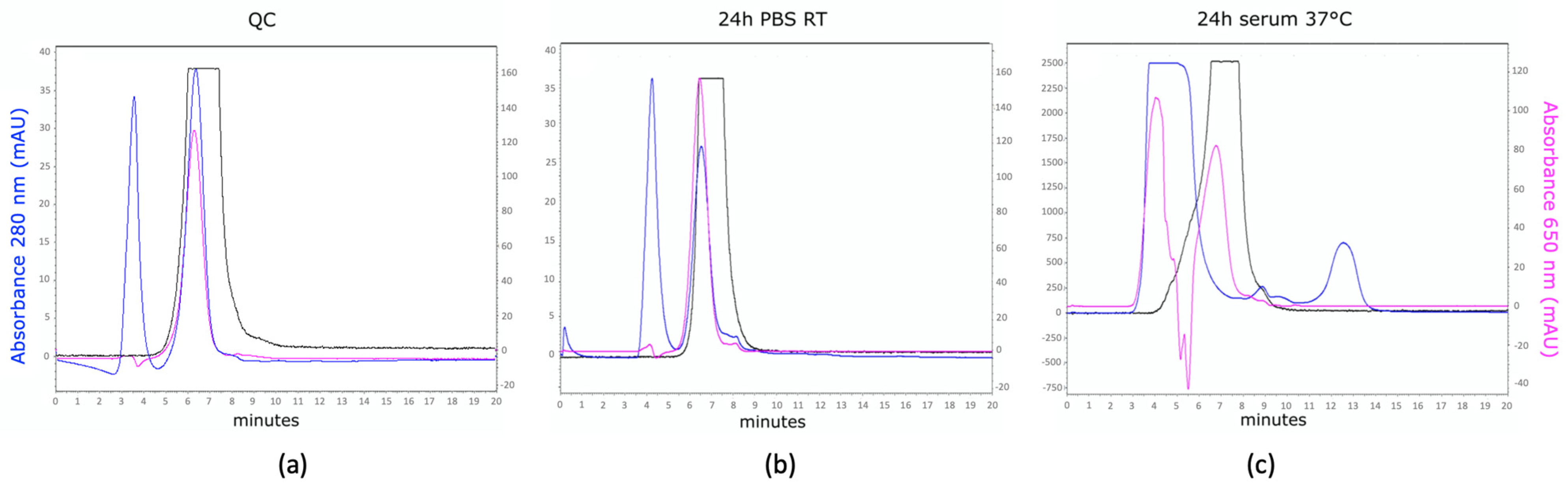
3.1. Production, MSAP Conjugation, and Radiolabeling of Nanobodies
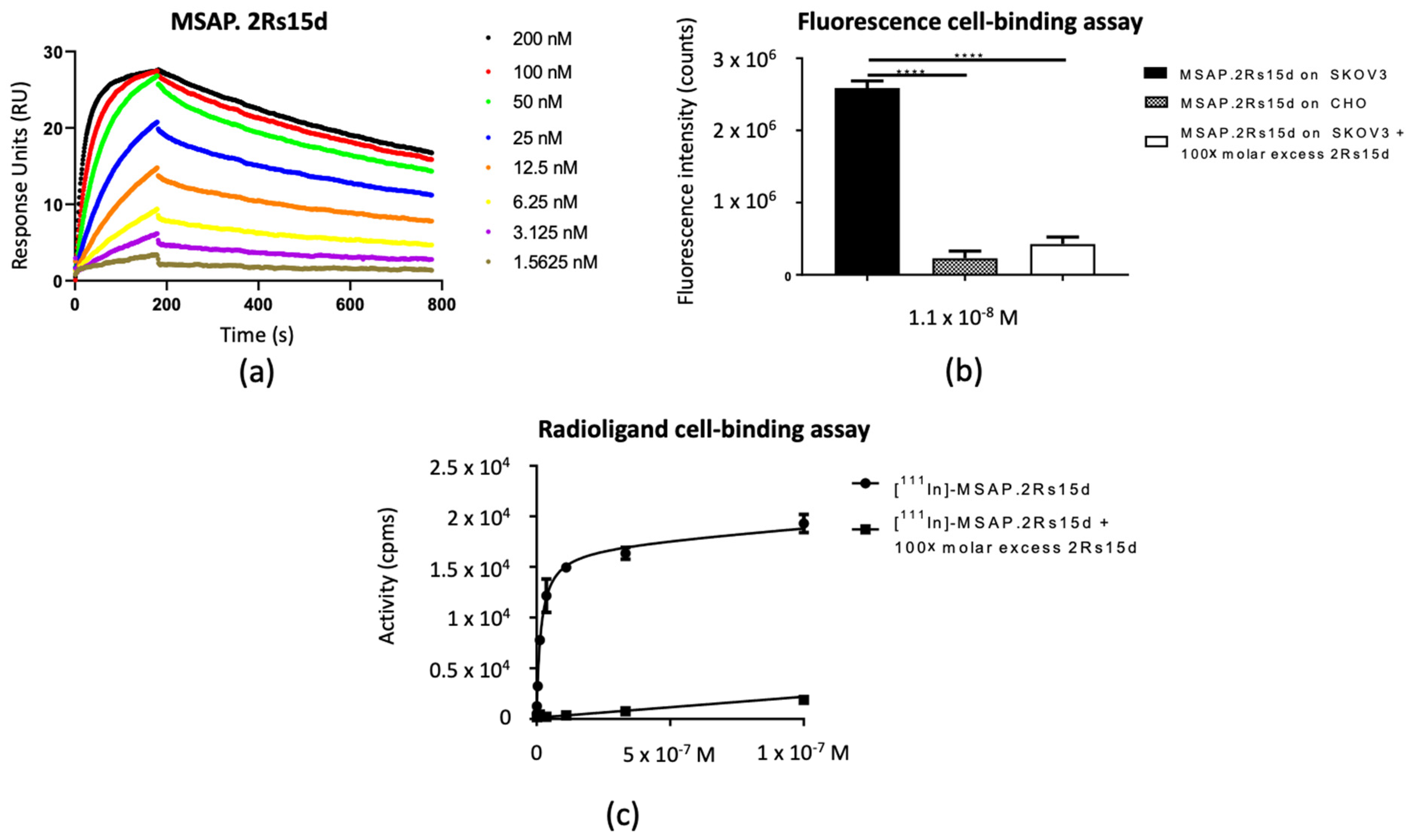
3.2. In Vitro Functionality
3.3. In Vivo Biodistribution and Tumor Targeting Potential Using Bimodal Imaging
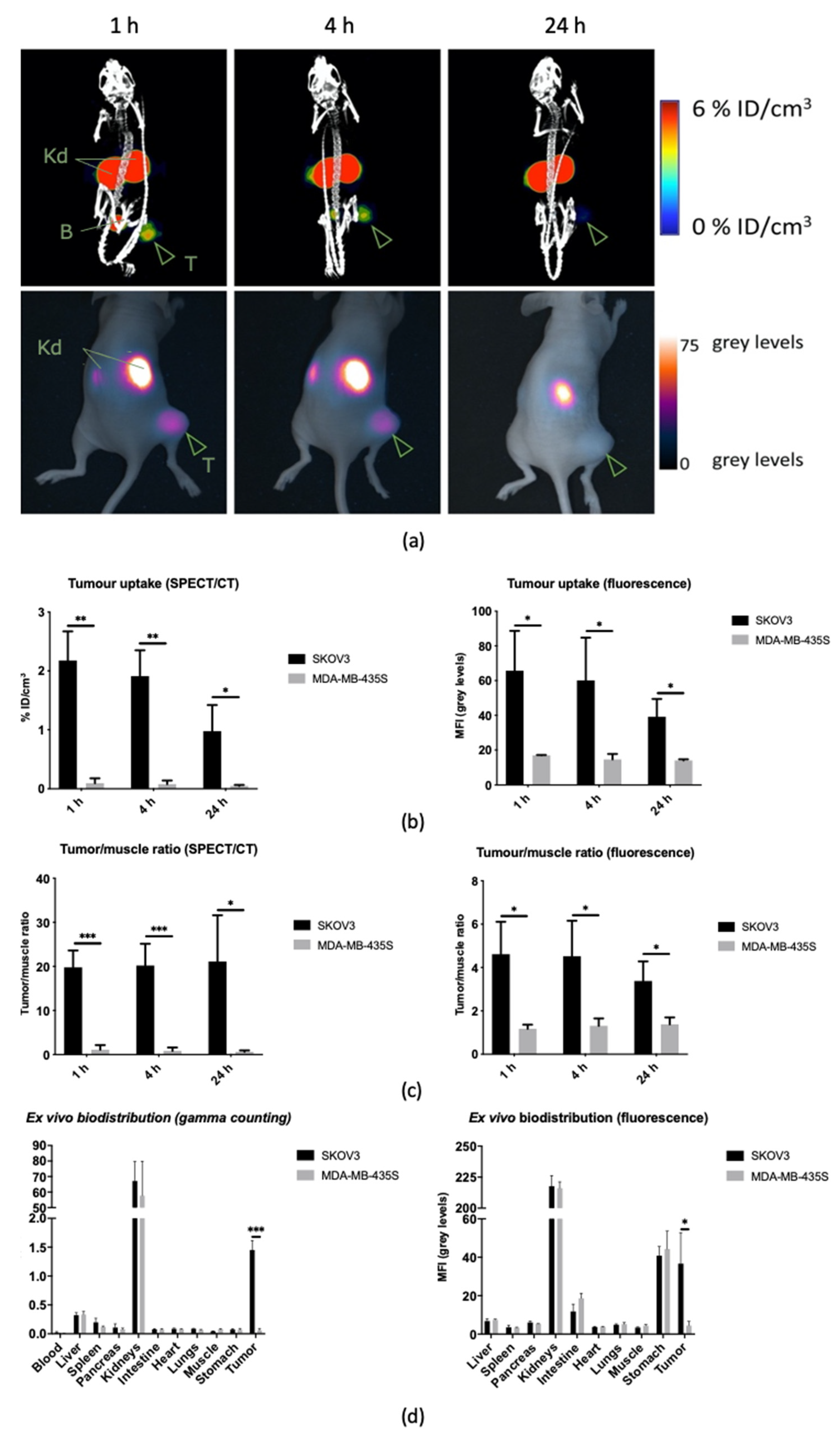
3.3.1. Longitudinal Biodistribution in Subcutaneous Xenografts
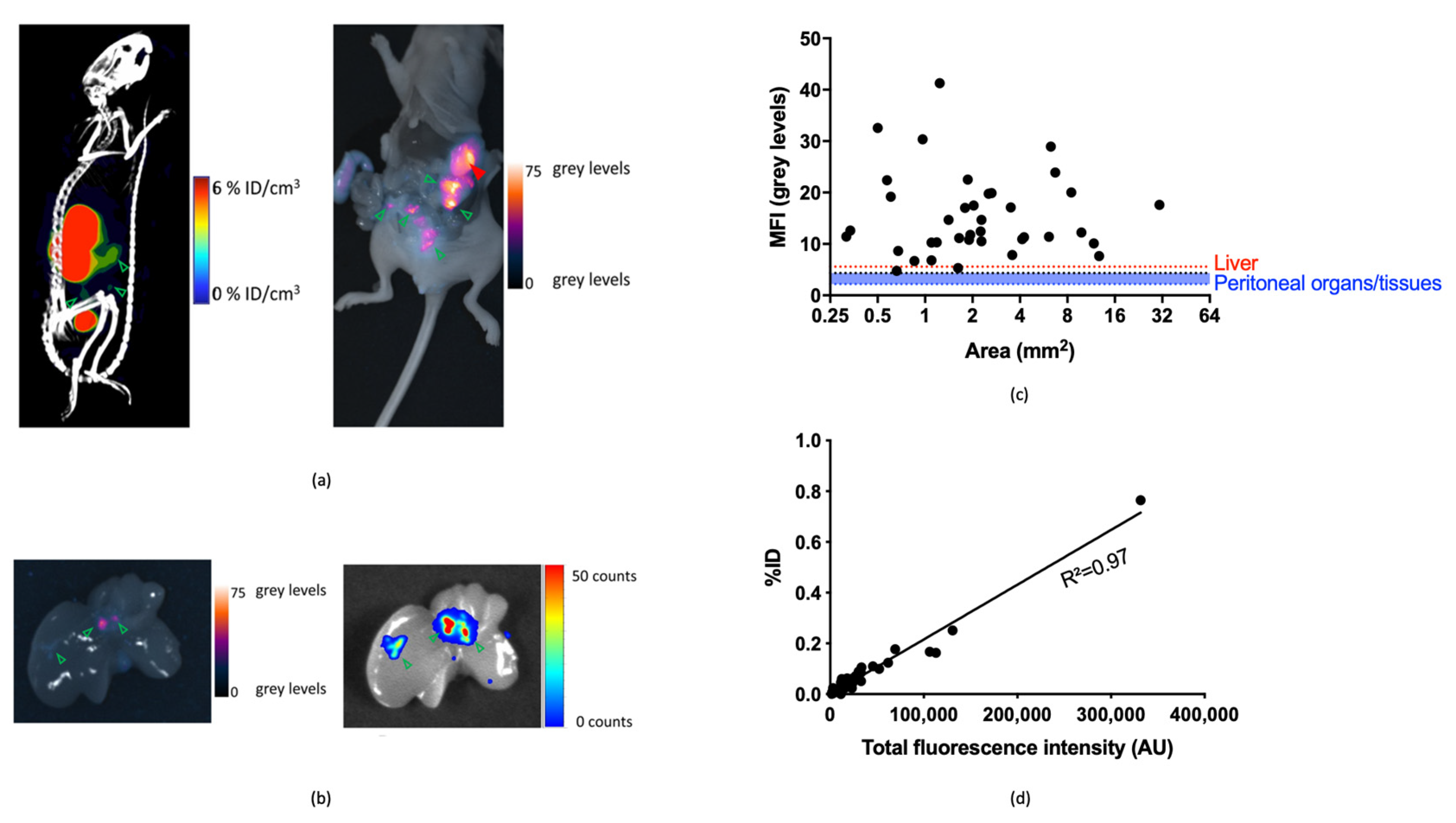
3.3.2. Image-Guided Resection of Intraperitoneally Disseminated Tumor Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernot, S.; Van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Berg, N.S.V.D.; Brouwer, O.R.; Schaafsma, B.E.; Mathéron, H.M.; Klop, W.M.C.; Balm, A.J.M.; Van Tinteren, H.; Nieweg, O.E.; Van Leeuwen, F.W.B.; Olmos, R.A.V. Multimodal Surgical Guidance during Sentinel Node Biopsy for Melanoma: Combined Gamma Tracing and Fluorescence Imaging of the Sentinel Node through Use of the Hybrid Tracer Indocyanine Green–99mTc-Nanocolloid. Radiology 2015, 275, 521–529. [Google Scholar] [CrossRef]
- Van Leeuwen, F.W.; Schottelius, M.; Brouwer, O.R.; Vidal-Sicart, S.; Achilefu, S.; Klode, J.; Wester, H.-J.; Buckle, T. Trending: Radioactive and Fluorescent Bimodal/Hybrid Tracers as Multiplexing Solutions for Surgical Guidance. J. Nucl. Med. 2019, 61, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oglio, P.; de Vries, H.M.; Mazzone, E.; KleinJan, G.H.; Donswijk, M.L.; van der Poel, H.G.; Horenblas, S.; van Leeuwen, F.W.; Brouwer, O.R. Hybrid Indocyanine Green–99mTc-nanocolloid for Single-photon Emission Computed Tomography and Combined Radio- and Fluorescence-guided Sentinel Node Biopsy in Penile Cancer: Results of 740 Inguinal Basins Assessed at a Single Institution. Eur. Urol. 2020, 78, 865–872. [Google Scholar] [CrossRef] [PubMed]
- De Gooyer, J.M.; Elekonawo, F.M.; Bos, D.L.; Van Der Post, R.S.; Pèlegrin, A.; Framery, B.; Cailler, F.; Vahrmeijer, A.L.; De Wilt, J.H.; Rijpkema, M. Multimodal CEA-Targeted Image-Guided Colorectal Cancer Surgery using 111In-Labeled SGM-101. Clin. Cancer Res. 2020, 26, 5934–5942. [Google Scholar] [CrossRef] [PubMed]
- Hekman, M.C.; Rijpkema, M.; Muselaers, C.H.; Oosterwijk, E.; De Kaa, C.A.H.-V.; Boerman, O.C.; Oyen, W.J.; Langenhuijsen, J.F.; Mulders, P.F. Tumor-targeted Dual-modality Imaging to Improve Intraoperative Visualization of Clear Cell Renal Cell Carcinoma: A First in Man Study. Theranostics 2018, 8, 2161–2170. [Google Scholar] [CrossRef]
- Van Leeuwen, F.W.; Valdés-Olmos, R.; Buckle, T.; Vidal-Sicart, S. Hybrid surgical guidance based on the integration of radionuclear and optical technologies. Br. J. Radiol. 2016, 89, 20150797. [Google Scholar] [CrossRef] [PubMed]
- Folli, S.; Wagnieres, G.; Pelegrin, A.; Calmes, J.M.; Braichotte, D.; Buchegger, F.; Chalandon, Y.; Hardman, N.; Heusser, C.; Givel, J.C. Immunophotodiagnosis of colon carcinomas in patients injected with fluoresceinated chimeric antibodies against carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA 1992, 89, 7973–7977. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Stammes, M.A.; De Roos, I.H.; Walsum, M.S.-V.; Visser, G.W.; Van Dongen, G.A. Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets. EJNMMI Res. 2011, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Hekman, M.C.; Rijpkema, M.; Bos, D.L.; Oosterwijk, E.; Goldenberg, D.M.; Mulders, P.F.; Boerman, O.C. Detection of Micrometastases Using SPECT/Fluorescence Dual-Modality Imaging in a CEA-Expressing Tumor Model. J. Nucl. Med. 2017, 58, 706–710. [Google Scholar] [CrossRef]
- Tsai, W.K.; Zettlitz, K.A.; Tavaré, R.; Kobayashi, N.; Reiter, R.E.; Wu, A.M. Dual-Modality ImmunoPET/Fluorescence Imaging of Prostate Cancer with an Anti-PSCA Cys-Minibody. Theranostics 2018, 8, 5903–5914. [Google Scholar] [CrossRef]
- Bunschoten, A.; Buckle, T.; Visser, N.L.; Kuil, J.; Yuan, H.; Josephson, L.; Vahrmeijer, A.L.; van Leeuwen, F.W. Multimodal interventional molecular imaging of tumor margins and distant metastases by targeting alphavbeta3 integrin. Chembiochem 2012, 13, 1039–1045. [Google Scholar] [CrossRef]
- Garanger, E.; Aikawa, E.; Reynolds, F.; Weissleder, R.; Josephson, L. Simplified syntheses of complex multifunctional nanomaterials. Chem. Commun. 2008, 39, 4792–4794. [Google Scholar] [CrossRef] [PubMed]
- Kuil, J.; Buckle, T.; Yuan, H.; Berg, N.S.V.D.; Oishi, S.; Fujii, N.; Josephson, L.; Van Leeuwen, F.W.B. Synthesis and Evaluation of a Bimodal CXCR4 Antagonistic Peptide. Bioconjugate Chem. 2011, 22, 859–864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buckle, T.; Kuil, J.; Berg, N.S.V.D.; Bunschoten, A.; Lamb, H.J.; Yuan, H.; Josephson, L.; Jonkers, J.; Borowsky, A.D.; Van Leeuwen, F.W.B. Use of a Single Hybrid Imaging Agent for Integration of Target Validation with In Vivo and Ex Vivo Imaging of Mouse Tumor Lesions Resembling Human DCIS. PLoS ONE 2013, 8, e48324. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, H.; Rice, W.L.; Kumar, A.T.N.; Goergen, C.J.; Jokivarsi, K.; Josephson, L. The PEG-Fluorochrome Shielding Approach for Targeted Probe Design. J. Am. Chem. Soc. 2012, 134, 19338–19341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bunschoten, A.; Van Willigen, D.M.; Buckle, T.; Berg, N.S.V.D.; Welling, M.M.; Spa, S.J.; Wester, H.-J.; Van Leeuwen, F.W.B. Tailoring Fluorescent Dyes To Optimize a Hybrid RGD-Tracer. Bioconjugate Chem. 2016, 27, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Buckle, T.; van Willigen, D.M.; Spa, S.J.; Hensbergen, A.W.; van der Wal, S.; de Korne, C.M.; Welling, M.M.; van der Poel, H.G.; Hardwick, J.C.H.; van Leeuwen, F.W.B. Tracers for Fluorescence-Guided Surgery: How Elongation of the Polymethine Chain in Cyanine Dyes Alters the Pharmacokinetics of a Dual-Modality c[RGDyK] Tracer. J. Nucl. Med. 2018, 59, 986–992. [Google Scholar] [CrossRef]
- Boonstra, M.C.; van Driel, P.B.; van Willigen, D.M.; Stammes, M.A.; Prevoo, H.A.; Tummers, Q.R.; Mazar, A.P.; Beekman, F.J.; Kuppen, P.J.; van de Velde, C.J.; et al. uPAR-targeted multimodal tracer for pre- and intraoperative imaging in cancer surgery. Oncotarget 2015, 6, 14260–14273. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.C.; Van Driel, P.B.; Keereweer, S.; Prevoo, H.A.; Stammes, M.A.; Baart, V.M.; Lowik, C.W.; Mazar, A.P.; van de Velde, C.J.; Vahrmeijer, A.L.; et al. Preclinical uPAR-targeted multimodal imaging of locoregional oral cancer. Oral Oncol. 2017, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Olafsen, T. Antibodies for Molecular Imaging of Cancer. Cancer J. 2008, 14, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R.; Schmidt, F.I.; Ploegh, H.L. Exploiting Nanobodies’ Singular Traits. Annu. Rev. Immunol. 2018, 36, 695–715. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted Nanobody-Based Molecular Tracers for Nuclear Imaging and Image-Guided Surgery. Antibodies 2019, 8, 12. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Cai, W. Nanobody: The “Magic Bullet” for Molecular Imaging? Theranostics 2014, 4, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.; D’Huyvetter, M.; Devoogdt, N.; Frejd, F.Y.; Sorensen, J.; Orlova, A.; Keyaerts, M.; Tolmachev, V. Same-Day Imaging Using Small Proteins: Clinical Experience and Translational Prospects in Oncology. J. Nucl. Med. 2018, 59, 885–891. [Google Scholar] [CrossRef]
- Oliveira, S.; Van Dongen, G.A.; Walsum, M.S.-V.; Roovers, R.C.; Stam, J.C.; Mali, W.; Van Diest, P.J.; Henegouwen, P.M.V.B.E. Rapid Visualization of Human Tumor Xenografts through Optical Imaging with a Near-Infrared Fluorescent Anti–Epidermal Growth Factor Receptor Nanobody. Mol. Imaging 2012, 11, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Van Quathem, J.; Hansen, I.; Bala, G.; Massa, S.; Devoogdt, N.; Xavier, C.; Hernot, S. Effect of Dye and Conjugation Chemistry on the Biodistribution Profile of Near-Infrared-Labeled Nanobodies as Tracers for Image-Guided Surgery. Mol. Pharm. 2017, 14, 1145–1153. [Google Scholar] [CrossRef]
- Debie, P.; Vanhoeij, M.; Poortmans, N.; Puttemans, J.; Gillis, K.; Devoogdt, N.; Lahoutte, T.; Hernot, S. Improved Debulking of Peritoneal Tumor Implants by Near-Infrared Fluorescent Nanobody Image Guidance in an Experimental Mouse Model. Mol. Imaging Biol. 2017, 20, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2015, 57, 27–33. [Google Scholar] [CrossRef]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, Preclinical Validation, Dosimetry, and Toxicity of 68Ga-NOTA-Anti-HER2 Nanobodies for iPET Imaging of HER2 Receptor Expression in Cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Spa, S.; Hensbergen, A.; Van Der Wal, S.; Kuil, J.; Van Leeuwen, F. The influence of systematic structure alterations on the photophysical properties and conjugation characteristics of asymmetric cyanine 5 dyes. Dye. Pigment. 2018, 152, 19–28. [Google Scholar] [CrossRef]
- Bala, G.; Crauwels, M.; Blykers, A.; Remory, I.; Marschall, A.L.; Dübel, S.; Dumas, L.; Broisat, A.; Martin, C.; Ballet, S.; et al. Radiometal-labeled anti-VCAM-1 nanobodies as molecular tracers for atherosclerosis–impact of radiochemistry on pharmacokinetics. Biol. Chem. 2019, 400, 323–332. [Google Scholar] [CrossRef] [PubMed]
- De Vlieghere, E.; Carlier, C.; Ceelen, W.; Bracke, M.; De Wever, O. Data on in vivo selection of SK-OV-3 Luc ovarian cancer cells and intraperitoneal tumor formation with low inoculation numbers. Data Brief 2016, 6, 542–549. [Google Scholar] [CrossRef][Green Version]
- Bhaumik, S.; DePuy, J.; Klimash, J. Strategies to minimize background autofluorescence in live mice during noninvasive fluorescence optical imaging. Lab Anim. 2007, 36, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. 18F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. 131I-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef]
- Massa, S.; Vikani, N.; Betti, C.; Ballet, S.; Vanderhaegen, S.; Steyaert, J.; Descamps, B.; Vanhove, C.; Bunschoten, A.; Van Leeuwen, F.W.B.; et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: A versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging 2016, 11, 328–339. [Google Scholar] [CrossRef]
- Massa, S.; Xavier, C.; De Vos, J.; Caveliers, V.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Site-Specific Labeling of Cysteine-Tagged Camelid Single-Domain Antibody-Fragments for Use in Molecular Imaging. Bioconjugate Chem. 2014, 25, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Gainkam, L.O.T.; Caveliers, V.; Devoogdt, N.; Vanhove, C.; Xavier, C.; Boerman, O.; Muyldermans, S.; Bossuyt, A.; Lahoutte, T. Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice. Contrast Media Mol. Imaging 2010, 6, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hensbergen, A.W.; Buckle, T.; van Willigen, D.M.; Schottelius, M.; Welling, M.M.; van der Wijk, F.A.; Maurer, T.; van der Poel, H.G.; van der Pluijm, G.; van Weerden, W.M.; et al. Hybrid Tracers Based on Cyanine Backbones Targeting Prostate-Specific Membrane Antigen: Tuning Pharmacokinetic Properties and Exploring Dye-Protein Interaction. J. Nucl. Med. 2020, 61, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Boy, C.; Pöppel, T.; Kuhn, J.; Klötgen, K.; Dissemond, J.; Schadendorf, D.; Klode, J. Association Between Sentinel Lymph Node Excision With or Without Preoperative SPECT/CT and Metastatic Node Detection and Disease-Free Survival in Melanoma. JAMA 2012, 308, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Robu, S.; Schottelius, M.; Schwamborn, K.; Rauscher, I.; Berg, N.S.V.D.; van Leeuwen, F.W.; Haller, B.; Horn, T.; Heck, M.M.; et al. 99mTechnetium-based Prostate-specific Membrane Antigen–radioguided Surgery in Recurrent Prostate Cancer. Eur. Urol. 2019, 75, 659–666. [Google Scholar] [CrossRef] [PubMed]
- English, D.P.; Roque, D.M.; Santin, A.D. HER2 Expression Beyond Breast Cancer: Therapeutic Implications for Gynecologic Malignancies. Mol. Diagn. Ther. 2013, 17, 85–99. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debie, P.; Declerck, N.B.; van Willigen, D.; Huygen, C.M.; De Sloovere, B.; Mateusiak, L.; Bridoux, J.; Puttemans, J.; Devoogdt, N.; van Leeuwen, F.W.B.; et al. The Design and Preclinical Evaluation of a Single-Label Bimodal Nanobody Tracer for Image-Guided Surgery. Biomolecules 2021, 11, 360. https://doi.org/10.3390/biom11030360
Debie P, Declerck NB, van Willigen D, Huygen CM, De Sloovere B, Mateusiak L, Bridoux J, Puttemans J, Devoogdt N, van Leeuwen FWB, et al. The Design and Preclinical Evaluation of a Single-Label Bimodal Nanobody Tracer for Image-Guided Surgery. Biomolecules. 2021; 11(3):360. https://doi.org/10.3390/biom11030360
Chicago/Turabian StyleDebie, Pieterjan, Noemi B. Declerck, Danny van Willigen, Celine M. Huygen, Bieke De Sloovere, Lukasz Mateusiak, Jessica Bridoux, Janik Puttemans, Nick Devoogdt, Fijs W. B. van Leeuwen, and et al. 2021. "The Design and Preclinical Evaluation of a Single-Label Bimodal Nanobody Tracer for Image-Guided Surgery" Biomolecules 11, no. 3: 360. https://doi.org/10.3390/biom11030360
APA StyleDebie, P., Declerck, N. B., van Willigen, D., Huygen, C. M., De Sloovere, B., Mateusiak, L., Bridoux, J., Puttemans, J., Devoogdt, N., van Leeuwen, F. W. B., & Hernot, S. (2021). The Design and Preclinical Evaluation of a Single-Label Bimodal Nanobody Tracer for Image-Guided Surgery. Biomolecules, 11(3), 360. https://doi.org/10.3390/biom11030360









