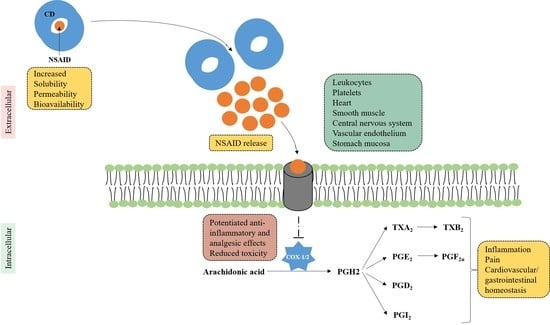
Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review
Abstract
1. Introduction
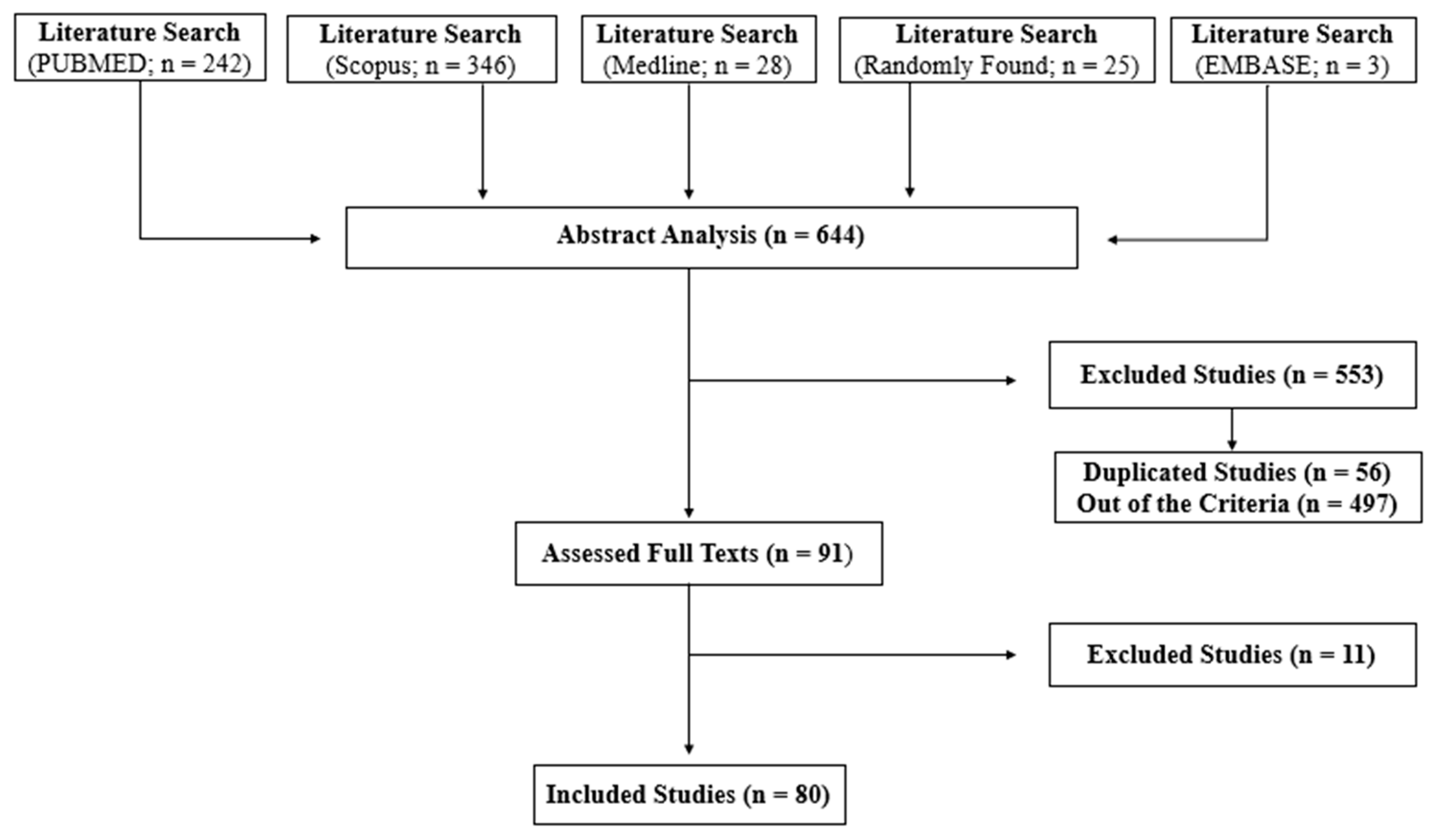
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Ptaschinski, C.; Lukacs, N.W. Acute and Chronic Inflammation Induces Disease Pathogenesis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Straub, R.H.; Cutolo, M. Glucocorticoids and chronic inflammation. Rheumatology 2016, 55, ii6–ii14. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Theken, K.N. Variability in analgesic response to non-steroidal anti-inflammatory drugs. Prostaglandins Other Lipid Mediat. 2018, 139, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Arias, L.H.M.; González, A.M.; Fadrique, R.S.; Vazquez, E.S. Cardiovascular Risk of Nonsteroidal Anti-inflammatory Drugs and Classical and Selective Cyclooxygenase-2 Inhibitors: A Meta-analysis of Observational Studies. J. Clin. Pharmacol. 2019, 59, 55–73. [Google Scholar] [CrossRef]
- Alessandri, A.L.; Sousa, L.P.; Lucas, C.D.; Rossi, A.G.; Pinho, V.; Teixeira, M.M. Resolution of inflammation: Mechanisms and opportunity for drug development. Pharmacol. Ther. 2013, 139, 189–212. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Krause, R.W.M. A vision for cyclodextrin nanoparticles in drug delivery systems and pharmaceutical applications. Nanomed. 2014, 9, 877–894. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F.; et al. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- JASP, T. JASP (Version 0.12. 2) [computer Software]; Eric-Jan Wagenmakers, University of Amsterdam: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lahiani-Skiba, M.; Youm, I.; Bounoure, F.; Skiba, M. Improvement in the water solubility and stability of 4ASA by the use of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2010, 69, 327–331. [Google Scholar] [CrossRef]
- Dahiya, S.; Kaushik, A.; Pathak, K. Improved Pharmacokinetics of Aceclofenac Immediate Release Tablets Incorporating ist Inclusion Complex with Hydroxypropyl-β-Cyclodextrin. Sci. Pharm. 2015, 83, 501–510. [Google Scholar] [CrossRef]
- Kasliwal, N.; Negi, J.S. Development, characterization and performance evaluation of oro-dispersible tablet containing aceclofenac hydroxypropyl-β-cyclodextrin binary system. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 215–224. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Xiao, M.; Zhang, Z.; Li, W.; Bai, J. Intercalation of Aceclofenac/Sulfobutyl Ether-β-cyclodextrin Complex into Layered Double Hydroxides through Swelling/Restoration Reaction and Its Controlled-Release Properties. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Sharma, G.; Kaur, M.; Raza, K.; Thakur, K.; Katare, O.P. Aceclofenac–β-cyclodextrin-vesicles: A dual carrier approach for skin with enhanced stability, efficacy and dermatokinetic profile. RSC Adv. 2016, 6, 20713–20727. [Google Scholar] [CrossRef]
- Samal, H.B.; Debata, J.; Naveen Kumar, N.; Sneha, S.; Patra, P.K. Solubility and dissolution improvement of Aceclofenac using B- Cyclodextrin. Int. J. Drug Dev. Res. 2012, 1, 39–43. [Google Scholar]
- Ranpise, N.S.; Kulkarni, N.S.; Mair, P.D.; Ranade, A.N. Improvement of water solubility and in vitro dissolution rate of aceclofenac by complexation with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. Pharm. Dev. Technol. 2010, 15, 64–70. [Google Scholar] [CrossRef]
- Parra, A.; Clares, B.; Rosselló, A.; Garduño-Ramírez, M.L.; Abrego, G.; García, M.L.; Calpena, A.C. Ex vivo permeation of carprofen from nanoparticles: A comprehensive study through human, porcine and bovine skin as anti-inflammatory agent. Int. J. Pharm. 2016, 501, 10–17. [Google Scholar] [CrossRef]
- Cannavà, C.; Tommasini, S.; Stancanelli, R.; Cardile, V.; Cilurzo, F.; Giannone, I.; Puglisi, G.; Ventura, C.A. Celecoxib-loaded PLGA/cyclodextrin microspheres: Characterization and evaluation of anti-inflammatory activity on human chondrocyte cultures. Colloids Surf. B Biointerfaces 2013, 111, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Mennini, N.; Furlanetto, S.; Cirri, M.; Mura, P. Quality by design approach for developing chitosan-Ca-alginate microspheres for colon delivery of celecoxib-hydroxypropyl-β-cyclodextrin-PVP complex. Eur. J. Pharm. Biopharm. 2012, 80, 67–75. [Google Scholar] [CrossRef]
- Rescifina, A.; Surdo, E.; Cardile, V.; Avola, R.; Graziano, A.C.E.; Stancanelli, R.; Tommasini, S.; Pistarà, V.; Ventura, C.A. Gemcitabine anticancer activity enhancement by water soluble celecoxib/sulfobutyl ether-β-cyclodextrin inclusion complex. Carbohydr. Polym. 2019, 206, 792–800. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U.; Batool, F.; Malik, N.S.; Rehman, M. Novel β-cyclodextrin nanosponges by chain growth condensation for solubility enhancement of dexibuprofen: Characterization and acute oral toxicity studies. J. Drug Deliv. Sci. Technol. 2021, 61, 102089. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U. Synthesis of β-cyclodextrin hydrogel nanoparticles for improving the solubility of dexibuprofen: Characterization and toxicity evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1873–1884. [Google Scholar] [CrossRef]
- Hamdan, I.I.; El-Sabawi, D.; Jalil, M.A. Potential interaction between zinc ions and a cyclodextrin-based diclofenac formulation. Drug Dev. Ind. Pharm. 2016, 42, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Singla, N.; Daniels, S.E.; Lacouture, P.G.; Min, L.H.; Reyes, C.R.; Carr, D.B. Cardiovascular safety of hydroxypropyl-β-cyclodextrin–diclofenac in the management of acute postsurgical pain: A pooled analysis of 2 randomized, double-blind, placebo- and active comparator–controlled phase III clinical trials. J. Clin. Anesth. 2016, 31, 249–258. [Google Scholar] [CrossRef]
- Hamilton, D.A.; Ernst, C.C.; Kramer, W.G.; Madden, D.; Lang, E.; Liao, E.; Lacouture, P.G.; Ramaiya, A.; Carr, D.B. Pharmacokinetics of Diclofenac and Hydroxypropyl-β-Cyclodextrin (HPβCD) Following Administration of Injectable HPβCD-Diclofenac in Subjects with Mild to Moderate Renal Insufficiency or Mild Hepatic Impairment. Clin. Pharmacol. Drug Dev. 2017, 7, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Fathalla, Z.; Moharram, H.; Ali, T.F.S.; Pierscionek, B. Cyclodextrin Enhances Corneal Tolerability and Reduces Ocular Toxicity Caused by Diclofenac. Oxidative Med. Cell. Longev. 2018, 2018, 5260976. [Google Scholar] [CrossRef]
- Klaewklod, A.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Li, L. Characterization of supramolecular gels based on β-cyclodextrin and polyethyleneglycol and their potential use for topical drug delivery. Mater. Sci. Eng. C 2015, 50, 242–250. [Google Scholar] [CrossRef]
- Lenik, J.; Wesoły, M.; Ciosek, P.; Wróblewski, W. Evaluation of taste masking effect of diclofenac using sweeteners and cyclodextrin by a potentiometric electronic tongue. J. Electroanal. Chem. 2016, 780, 153–159. [Google Scholar] [CrossRef]
- Mora, M.J.; Longhi, M.R.; Granero, G.E. Synthesis and characterization of binary and ternary complexes of diclofenac with a methyl-β-CD and monoethanolamine and in vitro transdermal evaluation. Eur. J. Med. Chem. 2010, 45, 4079–4088. [Google Scholar] [CrossRef]
- Vieira, A.C.; Serra, A.C.; Carvalho, R.A.; Gonsalves, A.; Figueiras, A.; Veiga, F.J.; Basit, A.W.; Gonsalves, A.M.D.R. Microwave synthesis and in vitro stability of diclofenac-β-cyclodextrin conjugate for colon delivery. Carbohydr. Polym. 2013, 93, 512–517. [Google Scholar] [CrossRef]
- Sherje, A.P.; Kulkarni, V.; Murahari, M.; Nayak, U.Y.; Bhat, P.; Suvarna, V.; Dravyakar, B. Inclusion Complexation of Etodolac with Hydroxypropyl-beta-cyclodextrin and Auxiliary Agents: Formulation Characterization and Molecular Modeling Studies. Mol. Pharm. 2017, 14, 1231–1242. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.; Mostafa, D.M.; Makram, T.S.; Ali, R.M. Host–guest system of etodolac in native and modified β-cyclodextrins: Preparation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2012, 77, 121–134. [Google Scholar] [CrossRef]
- Singh, I.; Kumar, P.; Pahuja, S.; Tung, V.; Arora, S. Development and pharmacological evaluation of cyclodextrin complexes of etoricoxib. Acta Pol. Pharm.-Drug Res. 2011, 68, 279–284. [Google Scholar]
- Ammar, H.O.; Makram, T.S.; Mosallam, S. Effect of Polymers on the Physicochemical Properties and Biological Performance of Fenoprofen Calcium Dihydrate-Triacetyl-β-Cyclodextrin Complex. Pharmaceutics 2017, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- AlShehri, S.; Imam, S.S.; Altamimi, M.A.; Hussain, A.; Shakeel, F.; AlShehri, A. Stimulatory Effects of Soluplus® on Flufenamic Acid β-Cyclodextrin Supramolecular Complex: Physicochemical Characterization and Pre-clinical Anti-inflammatory Assessment. AAPS PharmSciTech 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Ishiguro, T.; Morishita, E.; Iohara, D.; Hirayama, F.; Wada, K.; Motoyama, K.; Arima, H.; Uekama, K. Some pharmaceutical and inclusion properties of 2-hydroxybutyl-β-cyclodextrin derivative. Int. J. Pharm. 2011, 419, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.A.; Joshi, P.N.; Jain, D.D.; Singh, K. Preparation and Evaluation of N-Trimethyl Chitosan Nanoparticles of Flurbiprofen for Ocular Delivery. Curr. Eye Res. 2019, 44, 575–582. [Google Scholar] [CrossRef]
- Wang, H.-B.; Yang, F.-F.; Gai, X.-M.; Cheng, B.-C.; Li, J.-Y.; Pan, H.; Yang, X.-G.; Pan, W.-S. A pH-independent instantaneous release of flurbiprofen: A study of the preparation of complexes, their characterization and in vitro/in vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1460–1471. [Google Scholar] [CrossRef]
- Vega, E.; Egea, M.A.; Garduño-Ramirez, M.L.; Garcia, M.L.; Sanchez, E.; Espina, M.; Calpena, A.C. Flurbiprofen PLGA-PEG nanospheres: Role of hydroxy-β-cyclodextrin on ex vivo human skin permeation and in vivo topical anti-inflammatory efficacy. Colloids Surf. B Biointerfaces 2013, 110, 339–346. [Google Scholar] [CrossRef]
- Li, D.X.; Han, M.J.; Balakrishnan, P.; Yan, Y.D.; Oh, D.H.; Joe, J.H.; Seo, Y.; Kim, J.O.; Park, S.M.; Yong, C.S.; et al. Enhanced oral bioavailability of flurbiprofen by combined use of micelle solution and inclusion compound. Arch. Pharmacal Res. 2010, 33, 95–101. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Wang, X.; Zhang, W.; Lin, C.; Chen, F.; Yang, X.; Pan, W. Drug-in-cyclodextrin-in-liposomes: A novel drug delivery system for flurbiprofen. Int. J. Pharm. 2015, 492, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, W.; Luo, Q.; Zhang, X. Enhanced bioavailability of orally administered flurbiprofen by combined use of hydroxypropyl-cyclodextrin and poly(alkyl-cyanoacrylate) nanoparticles. Eur. J. Drug Metab. Pharmacokinet. 2013, 39, 61–67. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast Dissolving Oral Drug Delivery System Based on Electrospun Nanofibrous Webs of Cyclodextrin/Ibuprofen Inclusion Complex Nanofibers. Mol. Pharm. 2019, 16, 4387–4398. [Google Scholar] [CrossRef]
- Volkova, T.; Surov, A.; Terekhova, I. Metal–organic frameworks based on β-cyclodextrin: Design and selective entrapment of non-steroidal anti-inflammatory drugs. J. Mater. Sci. 2020, 55, 13193–13205. [Google Scholar] [CrossRef]
- Rehman, K.; Amin, M.; Muda, S. Influence of Beta-cyclodextrin and Chitosan in the Formulation of a Colon-Specific Drug Delivery System. Drug Res. 2013, 63, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Rinaldi, F.; Esposito, S.; Di Marzio, L.; Carafa, M. Niosomes encapsulating ibuprofen–cyclodextrin complexes: Preparation and characterization. Curr. Drug Targets 2013, 14, 1070–1078. [Google Scholar] [CrossRef]
- Li, H.; Lv, N.; Li, X.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Stoddart, J.F.; Gref, R.; et al. Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Ferris, D.P.; Holcroft, J.M.; Kandela, I.; Stern, C.L.; Nassar, M.S.; Botros, Y.Y.; Stoddart, J.F. Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies. Mol. Pharm. 2017, 14, 1831–1839. [Google Scholar] [CrossRef]
- Felton, L.A.; Popescu, C.; Wiley, C.; Esposito, E.X.; Lefèvre, P.; Hopfinger, A.J. Experimental and Computational Studies of Physicochemical Properties Influence NSAID-Cyclodextrin Complexation. AAPS PharmSciTech 2014, 15, 872–881. [Google Scholar] [CrossRef]
- Mohamed, M.A.H.; Mahmoud, A.A. Formulation of Indomethacin Eye Drops via Complexation with Cyclodextrins. Curr. Eye Res. 2010, 36, 208–216. [Google Scholar] [CrossRef]
- Ribeiro-Rama, A.C.; Figueiredo, I.V.; Veiga, F.J.; Castel-Branco, M.M.; Cabrita, A.M.S.; Caramona, M.M. Hepatic and renal toxicities of indomethacin acid, salt form and complexed forms with hydroxypropyl-β-cyclodextrin on Wistar rats after oral administration. Fundam. Clin. Pharmacol. 2010, 25, 599–607. [Google Scholar] [CrossRef]
- Vranić, E.; Uzunović, A. Dissolution Studies of Physical Mixtures of Indomethacin with Alpha- and Gamma-Cyclodextrins. Bosn. J. Basic Med. Sci. 2010, 10, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Bragagni, M.; Mennini, N.; Mura, P. Development of a new delivery system consisting in “drug-in cyclodextrin-in nanostructured lipid carriers” for ketoprofen topical delivery. Eur. J. Pharm. Biopharm. 2012, 80, 46–53. [Google Scholar] [CrossRef]
- Grecu, M.; Năstasă, V.; Ilie, C.; Miron, L.D.; Mareş, M. Comparative assessment of effectiveness of ketoprofen and ketoprofen/beta-cyclodextrin complex in two experimental models of inflammation in rats. Lab. Anim. 2014, 48, 20–26. [Google Scholar] [CrossRef]
- Moutasim, M.Y.; ElMeshad, A.N.; El-Nabarawi, M.A. A pharmaceutical study on lornoxicam fast disintegrating tablets: Formulation and in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2017, 7, 450–459. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.; Mahmoud, A.A.; Makram, T.S.; Noshi, S.H. Topical liquid crystalline gel containing lornoxicam/cyclodextrin complex. J. Incl. Phenom. Macrocycl. Chem. 2011, 73, 161–175. [Google Scholar] [CrossRef]
- Bramhane, D.M.; Saindane, N.S.; Vavia, P.R. Inclusion complexation of weakly acidic NSAID with β-cyclodextrin: Selection of arginine, an amino acid, as a novel ternary component. J. Incl. Phenom. Macrocycl. Chem. 2010, 69, 453–460. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Kaomongkolgit, R.; Opanasopit, P. Cyclodextrin-based oral dissolving films formulation of taste-masked meloxicam. Pharm. Dev. Technol. 2017, 23, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. Formulation and evaluation of meloxicam oral disintegrating tablet with dissolution enhanced by combination of cyclodextrin and ion exchange resins. Drug Dev. Ind. Pharm. 2014, 41, 1006–1016. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-loaded solvent exchange-induced in situ forming beta-cyclodextrin gel and microparticle for periodontal pocket delivery. Mater. Sci. Eng. C 2020, 117, 111275. [Google Scholar] [CrossRef] [PubMed]
- Jafar, M.; Salahuddin, M.; Kayed, T.S.; Ahmad, N.; Al-Eid, H.A.; Al-Qarros, A.H. Buoyant in situ Gels of Meloxicam-b-Cyclodextrin-Triethanolamine Ternary Complex for Oral Delivery; From a Box-Behnken Experimental Design to in vivo Activity Detail. Asian J. Chem. 2017, 29, 1275–1284. [Google Scholar] [CrossRef]
- Ainurofiq, A.; Choiri, S. Development and optimization of a meloxicam/β-cyclodextrin complex for orally disintegrating tablet using statistical analysis. Pharm. Dev. Technol. 2016, 23, 464–475. [Google Scholar] [CrossRef]
- Shende, P.K.; Gaud, R.; Bakal, R.; Patil, D. Effect of inclusion complexation of meloxicam with β-cyclodextrin- and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surf. B Biointerfaces 2015, 136, 105–110. [Google Scholar] [CrossRef]
- Radia, O.; Rogalska, E.; Moulay-Hassane, G. Preparation of meloxicam–β-cyclodextrin–polyethylene glycol 6000 ternary system: Characterization, in vitro and in vivo bioavailability. Pharm. Dev. Technol. 2011, 17, 632–637. [Google Scholar] [CrossRef]
- Rasool, B.K.A.; Gareeb, R.H.; Fahmy, S.A.; Rasool, A.A.A. Meloxicam β-cyclodextrin transdermal gel: Physicochemical charac-terization and in vitro dissolution and diffusion studies. Curr Drug Deliv. 2011, 8, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.S.; Kumar, T.G.; Manisha, P.; Preeti, Y.; Kumar, S.S. Development of meloxicam formulations utilizing ternary com-plexation for solubility enhancement. Pak. J. Pharm Sci. 2011, 24, 533–538. [Google Scholar]
- Janovsky, M.; Doležal, T.; Prochazkova, M.; Sliva, J.; Kršiak, M. Influence on analgesic activity and serum levels after meloxicam complexation with beta-cyclodextrin in mice and rats. Arzneimittelforschung 2010, 60, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Rojanarata, T.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Sila-On, W.; Opanasopit, P. Improvement of drug loading onto ion exchange resin by cyclodextrin inclusion complex. Drug Dev. Ind. Pharm. 2012, 39, 1672–1680. [Google Scholar] [CrossRef]
- Tang, P.; Sun, Q.; Zhao, L.; Pu, H.; Yang, H.; Zhang, S.; Gan, R.; Gan, N.; Li, H. Mesalazine/hydroxypropyl-β-cyclodextrin/chitosan nanoparticles with sustained release and enhanced anti-inflammation activity. Carbohydr. Polym. 2018, 198, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Elkordy, A.A.; Ashoore, A.; Essa, E.A. Complexation of naproxen with beta-cyclodextrin with and without poloxamer 407 to enhance drug dissolution. J. Appl. Pharm. 2012, 3, 614–627. [Google Scholar]
- Shelley, H.; Grant, M.; Smith, F.T.; Abarca, E.M.; Babu, R.J. Improved Ocular Delivery of Nepafenac by Cyclodextrin Complexation. AAPS PharmSciTech 2018, 19, 2554–2563. [Google Scholar] [CrossRef]
- Shelley, H.; Rodriguez-Galarza, R.M.; Duran, S.H.; Abarca, E.M.; Babu, R.J. In Situ Gel Formulation for Enhanced Ocular Delivery of Nepafenac. J. Pharm. Sci. 2018, 107, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Veiga, B.; Diaz-Rodriguez, P.; Alvarez-Lorenzo, C.; Loftsson, T.; Sigurdsson, H.H. In Vitro and Ex Vivo Evaluation of Nepafenac-Based Cyclodextrin Microparticles for Treatment of Eye Inflammation. Nanomaterials 2020, 10, 709. [Google Scholar] [CrossRef]
- Auda, S.H. Nimesulide/Methyl β-Cyclodextrin Inclusion Complexes: Physicochemical Characterization, Solubility, Dissolution, and Biological Studies. Drug Dev. Res. 2014, 75, 68–75. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Aguzzi, C.; Viseras, C. Hybrid systems based on “drug-in cyclodextrin-in nanoclays” for improving oxaprozin dissolution properties. Int. J. Pharm. 2016, 509, 8–15. [Google Scholar] [CrossRef]
- Maestrelli, F.; Cirri, M.; Mennini, N.; Zerrouk, N.; Mura, P. Improvement of oxaprozin solubility and permeability by the combined use of cyclodextrin, chitosan, and bile components. Eur. J. Pharm. Biopharm. 2011, 78, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Mura, P.; Cirri, M.; Mennini, N.; Ghelardini, C.; Mannelli, L.D.C. Development and characterization of fast dissolving tablets of oxaprozin based on hybrid systems of the drug with cyclodextrins and nanoclays. Int. J. Pharm. 2017, 531, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Mennini, N.; Maestrelli, F.; Cirri, M.; Mura, P. Analysis of physicochemical properties of ternary systems of oxaprozin with randomly methylated-ß-cyclodextrin and l -arginine aimed to improve the drug solubility. J. Pharm. Biomed. Anal. 2016, 129, 350–358. [Google Scholar] [CrossRef]
- Mennini, N.; Cirri, M.; Maestrelli, F.; Mura, P. Comparison of liposomal and NLC (nanostructured lipid carrier) formulations for improving the transdermal delivery of oxaprozin: Effect of cyclodextrin complexation. Int. J. Pharm. 2016, 515, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Maestrelli, F.; Cecchi, M.; Bragagni, M.; Almeida, A. Development of a new delivery system consisting in ‘drug–in cyclodextrin–in PLGA nanoparticles. J. Microencapsul. 2010, 27, 479–486. [Google Scholar] [CrossRef]
- Mishra, M.; Chawla, V.; Chawla, P. Pectin, beta-cyclodextrin, chitosan and albumin based gastroprotective systems for piroxicam maleate: Synthesis, characterization and biological evaluation. Int. J. Biol. Macromol. 2019, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannidou, E.; Ferrari, M.; Deligianni, A.-D.; Bouropoulos, N.; Andreadis, D.A.; Sorrenti, M.; Catenacci, L.; Nazari, K.; Arshad, M.S.; Chang, M.-W.; et al. In Vitro and Ex Vivo Evaluation of Tablets Containing Piroxicam-Cyclodextrin Complexes for Buccal Delivery. Pharmaceutics 2019, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Bouchal, F.; Skiba, M.; Chaffai, N.; Hallouard, F.; Fatmi, S.; Lahiani-Skiba, M. Fast dissolving cyclodextrin complex of piroxicam in solid dispersion Part I: Influence of β-CD and HPβ-CD on the dissolution rate of piroxicam. Int. J. Pharm. 2015, 478, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.; Bouchal, F.; Boukhris, T.; Bounoure, F.; Fessi, H.; Fatmi, S.; Chaffai, N. Pharmacokinetic study of an oral piroxicam formulation containing different molar ratios of β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 311–314. [Google Scholar] [CrossRef]
- Keles, G.T.; Topcu, I.; Ekici, Z.; Yentür, A. Evaluation of piroxicam-β-cyclodextrin as a preemptive analgesic in functional endoscopic sinus surgery. Braz. J. Med. Biol. Res. 2010, 43, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Alsarra, I.A.; Ahmed, M.O.; Alanazi, F.K.; Eltahir, K.E.H.; Alsheikh, A.M.; Neau, S.H. Influence of Cyclodextrin Complexation with NSAIDs on NSAID/Cold Stress-Induced Gastric Ulceration in Rats. Int. J. Med. Sci. 2010, 7, 232–239. [Google Scholar] [CrossRef][Green Version]
- Haggag, Y.A.; El-Gizawy, S.A.; El-Din, E.E.Z.; El-Shitany, N.A.; Osman, M.A. Sulindac solid dispersions: Development, characterization and in vivo evaluation of ulcerogenic activity in rats. J. Appl. Pharm. Sci. 2016, 6, 22–31. [Google Scholar] [CrossRef]
- Sherje, A.P.; Patel, F.; Murahari, M.; Suvarna, V.; Patel, K. Study on effect of L-arginine on solubility and dissolution of Zaltoprofen: Preparation and characterization of binary and ternary cyclodextrin inclusion complexes. Chem. Phys. Lett. 2018, 694, 120–128. [Google Scholar] [CrossRef]
- Felson, D.T. Safety of Nonsteroidal Antiinflammatory Drugs. N. Engl. J. Med. 2016, 375, 2595–2596. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.A.; Aiyer, R.; Yu, J.; White, R.S.; Mehta, N.; Gulati, A. Nonopioid versus opioid agents for chronic neuropathic pain, rheumatoid arthritis pain, cancer pain and low back pain. Pain Manag. 2019, 9, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Rogoveanu, O.C.; Streba, C.T.; Vere, C.C.; Petrescu, L.; Trăistaru, R. Superior digestive tract side effects after prolonged treat-ment with NSAIDs in patients with osteoarthritis. J. Med. Life 2015, 8, 458–461. [Google Scholar] [PubMed]
- Kim, D.-H.; Lee, S.-E.; Pyo, Y.-C.; Tran, P.; Park, J.-S. Solubility enhancement and application of cyclodextrins in local drug delivery. J. Pharm. Investig. 2020, 50, 17–27. [Google Scholar] [CrossRef]
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361. [Google Scholar] [CrossRef]
- Santos, P.L.; Brito, R.G.; Quintans, J.S.; Araujo, A.A.; Menezes, I.R.; Brogden, N.K.; Quintans-Junior, L.J. Cyclodextrins as Complexation Agents to Improve the Anti-inflammatory Drugs Profile: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Rizk, M.L.; Zou, L.; Savic, R.M.; Dooley, K.E. Importance of Drug Pharmacokinetics at the Site of Action. Clin. Transl. Sci. 2017, 10, 133–142. [Google Scholar] [CrossRef]
- Lennernäs, H. Regional intestinal drug permeation: Biopharmaceutics and drug development. Eur. J. Pharm. Sci. 2014, 57, 333–341. [Google Scholar] [CrossRef]
- Vastag, G.; Apostolov, S.; Matijević, B. Prediction of lipophilicity and pharmacokinetics of chloroacetamides by chemomet-ric approach. Iran. J. Pharm. Res. 2018. [Google Scholar] [CrossRef]
- Fine-Shamir, N.; Beig, A.; Miller, J.M.; Dahan, A. The solubility, permeability and the dose as key factors in formulation development for oral lipophilic drugs: Maximizing the bioavailability of carbamazepine with a cosolvent-based formulation. Int. J. Pharm. 2020, 582, 119307. [Google Scholar] [CrossRef] [PubMed]
- Jyoti Sen, D.; Patel, J.G. Logarithmic Partition Coefficient Comparison Study and Molecular Weight of Synthesized Prodrugs of Ibuprofen+Paracetamol, Diclofenac Sodium+Paracetamol and Ibuprofen+Diclofenac Sodium. Am. J. Adv. Drug Deliv. 2016, 4. [Google Scholar] [CrossRef]
- Youn, Y.-S.; Lee, J.-H.; Jeong, S.-H.; Shin, B.-S.; Park, E.-S. Pharmaceutical Usefulness of Biopharmaceutics Classification System: Overview and New Trend. J. Pharm. Investig. 2010, 40, 1–7. [Google Scholar] [CrossRef]
- Muankaew, C.; Jansook, P.; Loftsson, T. Evaluation of γ-cyclodextrin effect on permeation of lipophilic drugs: Application of cellophane/fused octanol membrane. Pharm. Dev. Technol. 2017, 22, 562–570. [Google Scholar] [CrossRef]
- Mizera, M.; Muratov, E.N.; Alves, V.M.; Tropsha, A.; Cielecka-Piontek, J. Computer-Aided Discovery of New Solubility-Enhancing Drug Delivery System. Biomolecules 2020, 10, 913. [Google Scholar] [CrossRef]
- Yang, J.; Li, K.; He, D.; Gu, J.; Xu, J.; Xie, J.; Zhang, M.; Liu, Y.; Tan, Q.; Zhang, J. Toward a better understanding of metabolic and pharmacokinetic characteristics of low-solubility, low-permeability natural medicines. Drug Metab. Rev. 2020, 52, 19–43. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.D.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Landy, D.; Sizun, C.; Cézard, C.; Solgadi, A.; Przybylski, C.; De Chaisemartin, L.; Herfindal, L.; Barratt, G.; Legrand, F.-X. Cyclodextrin complexation studies as the first step for repurposing of chlorpromazine. Int. J. Pharm. 2020, 584, 119391. [Google Scholar] [CrossRef] [PubMed]
- Schjerning, A.-M.; Mcgettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]

| Property | α-CD | β-CD | γ-CD |
|---|---|---|---|
| Glucopyranose units | 6 | 7 | 8 |
| Molecular weight (Da) | 972 | 1135 | 1297 |
| Solubility in water (mg/mL) | 145 | 18.5 | 232 |
| Inner diameter (Å) | 5.7 | 7.8 | 9.5 |
| Safety (orally) | High * | Acceptable daily intake of 5 mg/kg/day | High * |
| Chemical structure | 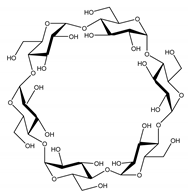 | 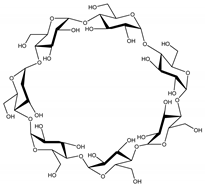 | 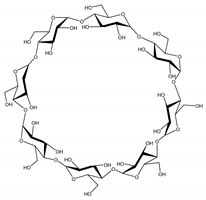 |
| Authors | Inclusion Complex | Study Design | Main Findings |
|---|---|---|---|
| Lahiani-Skiba et al., 2011 [24] | 4-Aminosalicylic acid/α-CD/β-CD/γ-CD/HP-β-CD | Investigated the effect of cyclodextrins on the solubility and chemical stability of 4-Aminosalicylic acid (in vitro). | Complexation with different CDs increased drug solubility, while γ-CD and HP-β-CD significantly increased drug stability 4-fold. |
| Dahiya et al., 2015 [25] | Aceclofenac/HP-β-CD | Investigated the pharmacokinetic parameters of immediate release aceclofenac tablets (in vitro and in vivo). | Tablets prepared with HP-β-CD provided a more rapid onset of pharmacological effects in comparison to the market formulation and pure drug. |
| Kasliwal et al., 2011 [26] | Aceclofenac/HP-β-CD | Formulated oral dispersible rapid-disintegration tablet to mask the bitter taste of aceclofenac (in vivo and in vitro). | The formulated tablets tested in humans revealed considerable taste masking and rapid disintegration. |
| Li et al., 2014 [27] | Aceclofenac/SBE-β-CD | Studied the dissolution profile of the inclusion complex and the controlled-release property of the drug (in vitro). | The formulation enhanced the dissolution profile of aceclofenac and promoted a controlled release of the drug. |
| Sharma et al., 2016 [28] | Aceclofenac/β-CD/liposomes | Evaluated the improvement in stability and transdermal delivery to the inflammatory sites in osteoarthritis (ex vivo). | Achieved better stability permeation and enhanced skin bioavailability of the drug to epidermis and dermis. |
| Samal et al., 2012 [29] | Aceclofenac/β-CD | Evaluated the solubility and dissolution rate of aceclofenac in complex with β-CD (in vitro). | The dissolution rate of aceclofenac was significantly higher compared to pure aceclofenac. |
| Ranpise et al., 2010 [30] | Aceclofenac/β-CD/HP-β-CD | Developed formulations based on inclusion complexes of aceclofenac with β-CD and HP-β-CD and evaluated their anti-inflammatory and analgesic effects (in vivo). | Spray-dried aceclofenac complexed with β-CD presented increased solubility. The formulation presented stronger analgesic and anti-inflammatory effects when compared to the uncomplex drug. |
| Parra et al., 2016 [31] | Carprofen/HP-β-CD/PLGA nanoparticles | Characterized and analyzed the permeability (ex vivo), biomechanical properties, skin irritation, and anti-inflammatory effects (in vivo) of the inclusion complex. | The complex presented high membrane permeability and induced no evident skin irritation. Topically administered nanoparticles demonstrated strong anti-inflammatory activity. |
| Cannavà et al., 2013 [32] | Celecoxib/PLGA/DiMe-β-CD. | Evaluated the influence of the polymeric carriers on the pharmacological activity (ex vivo). | Celecoxib-loaded PLGA/DiMe-β-CD microspheres were more effective than the free drug as an anti-inflammatory agent on human chondrocyte tests. |
| Mennini et al., 2012 [33] | Celecoxib/HP-β-CD/PVP/Chitosan-Ca2+-alginate | Developed a new system for colon delivery of celecoxib for systemic and local therapy (in vitro). | The results demonstrated the effectiveness of the proposed complex loaded in chitosan-Ca2+-alginate microspheres for colon delivery. |
| Rescifina et al., 2019 [34] | Celecoxib/SBE-β-CD | Analyzed the cytotoxicity of celecoxib and its inclusion complex against A549 cell lines and investigated the impact of the combined use of the inclusion complex in the anti-cancer activity of gemcitabine. | Complexation increased the water solubility of celecoxib, which presented limited cytotoxic activity against A549 cells. Complexation significantly increased the cytotoxic activity of this NSAID as well as improved the cytotoxicity of gemcitabine. |
| Jansook et al., 2018 [19] | Celecoxib/γ-CD/Rme-β-CD | Developed celecoxib eye drop formulations containing CD and evaluated drug bioavailability, mucoadhesion, permeability (ex vivo), and in vitro cytotoxicity to the retina cell line. | Formulations containing ternary celecoxib/Rme-β-CD/hyaluronic acid improved mucoadhesion, permeability through membrane barriers (semipermeable membrane, simulated vitreous humor, and scleral tissues), and cytocompatibility with human RPE cell line. |
| Khalid et al., 2020 [35] | Dexibuprofen/β-CD | Prepared and investigated polymeric nanosponges of β-CD to enhance the solubility of dexibuprofen (in vitro). | The polymeric complexation resulted in a 6-fold increase in dexibuprofen solubilization with 89% drug release within 30 min. |
| Khalid et al., 2017 [36] | Dexibuprofen/β-CD | Prepared β-CD hydrogel nanoparticles using 2- Acrylamido-2-methylpropane sulfonic acid and N, N′-Methylene bis(acrylamide). Solubility was investigated in vitro, and acute toxicity was investigated in rats (in vivo). | The β-CD polymer hydrogel nanoparticles presented high solubility and excellent physicochemical characteristics. No evident toxicity was observed, indicating low oral toxicity. |
| Hamdan et al., 2016 [37] | Diclofenac/HP-β-CD | Evaluated effects of the preparation on drug absorption, bioavailability, and dissolution (in vivo and in vitro). | In vivo experiments on rats showed that HP-β-CD had no statistically significant effect on absorption or bioavailability of diclofenac. However, improvement in its in vitro dissolution by HP-β-CD was observed. |
| Gan et al., 2016 [38] | Diclofenac/HP-β-CD | Examined the efficacy and cardiovascular safety of intravenous HP-β-CD-diclofenac (clinical study). | The investigation suggested that the postoperative use of HP-β-CD-diclofenac does not present any additional cardiovascular risk over the placebo. |
| Hamilton et al., 2018 [39] | Diclofenac/HP-β-CD | Evaluated the pharmacokinetics of diclofenac and HP-β-CD in patients with mild or moderate renal insufficiency or mild hepatic impairment (clinical study). | The results suggest that diclofenac-HP-β-CD might be administered to patients with mild or moderate renal insufficiency or mild hepatic impairment without requirement of dose adjustment. |
| Abdelkader et al., 2018 [40] | Diclofenac/α-CD, β-CD, γ-CD, and HP-β-CD | Prepared inclusion complexes and evaluated for corneal permeation, corneal opacity/permeability, and toxicity (ex vivo). | Reduction in ocular toxicity by 3- to 16-fold and comparable corneal permeability to free diclofenac were recorded using γ-CD and HP-β-CD complexes. |
| Klaewklod et al., 2015 [41] | Diclofenac/β-CD/PEG/K2CO3 | Developed and evaluated the release (in vitro) and skin permeability (Franz diffusion cell method) of a diclofenac gel formulation containing β-CD and K2CO3 in PEG. | The release and permeation of diclofenac from the formulation were significantly greater than those from a commercial gel, indicating its usefulness for the topical delivery of drugs. |
| Lenik et al., 2016 [42] | Diclofenac/HP-β-CD | Evaluated taste-masking efficiency of diclofenac complexed with HP-β-CD and different sweeteners using a potentiometric electronic tongue. | Pronounced taste-masking effects were obtained with cyclodextrin, comparable to those obtained with other sweeteners such as acesulfame potassium and sodium saccharin. |
| Mora et al., 2010 [43] | Diclofenac/Me-β-CD/MEA | Characterized diclofenac-Me-β-CD inclusion complex in the presence of MEA. Drug absorption was analyzed through excised human skin (ex vivo). | Diclofenac solubility was increased by the addition of Me-β-CD and further improved in the presence of MEA. Permeability through excised human skin was increased upon drug complexation. |
| Vieira et al., 2013 [44] | Diclofenac-β-CD | Analyzed the stability and colonic delivery of a diclofenac- β-CD conjugate in simulated gastric and small intestinal fluids, and in fecal human material. | The inclusion complex was stable in simulated gastric and small intestinal fluids, efficiently liberating diclofenac in less than 2 h within the human fecal slurry. |
| Sherje et al., 2017 [45] | Etodolac/HP-β-CD/L-arginine | Obtained, characterized, and evaluated, by computational modeling (in silico), the solubility of etodolac/HP-β-CD in the presence of different auxiliary agents. | More stable inclusion complexes were obtained when L-arginine was used as an auxiliary agent. The ternary complex of etodolac with HP-β-CD-L/Arginine improved the solubility of the NSAID. |
| Ammar et al., 2013 [46] | Etodolac/β-CD/Me-β-CD/HP-β-CD | Used several CDs to improve the physicochemical properties of etodolac through complexation (in vitro). | Complexation to CDs enhanced the aqueous solubility and dissolution rate of etodolac. |
| Singh et al., 2011 [47] | Etoricoxib/β-CD/HP-β-CD | Developed, characterized, and evaluated the solubility and in vivo analgesic activity (tail flick and hot plate) of binary systems of etoricoxib with cyclodextrins. | Etoricoxib showed increased solubility and enhanced analgesic activity upon complexation with β-CD and HP-β-CD. Etoricoxib-HP-β-CD complex showed maximum analgesic effect. |
| Ammar et al., 2017 [48] | Fenoprofen/TA-β-CD | Characterized and investigated (in vivo) the analgesic and anti-inflammatory properties of fenoprofen/TA-β-CD inclusion complex conjugated with other polymers. | Fenoprofen calcium dehydrate/TA-β-CD conjugated with ethyl cellulose polymer showed the most potent and sustained anti-inflammatory and analgesic activities, possibly due to controlled drug release. |
| Alshehri et al., 2020 [49] | Flufenamic acid/β-CD/Soluplus® | Investigated the solubility, drug release profile (in vitro), and in vivo anti-inflammatory activity (carrageenan-induced paw edema in rats) of the inclusion complex. | The solubility and anti-inflammatory effect of flurbiprofen were optimized after incorporation into the inclusion complex. |
| Ishiguro et al., 2011 [50] | Flurbiprofen-2-HB-CD | Prepared HB-β-CDs with different degrees of substitution and characterized their physicochemical and biological properties. The hemolytic activity was evaluated in vitro. | HB-β-CDs acted as fast-dissolving carrier molecules for poorly water-soluble drugs. The hemolytic activity was variable according to the degree of substitution. |
| Shinde et al., 2019 [51] | Flurbiprofen/HP- β-CD/N-TMC | Analyzed TMC nanoparticles containing flurbiprofen/HP-β-CD inclusion complex for drug release, mucoadhesion, and irritation potential (in vitro). | TMC nanoparticles offered prolonged release potential for transmucosal ocular delivery of the NSAID. HET-CAM studies demonstrated their safety for ocular use. |
| Wang et al., 2017 [52] | Flurbiprofen/HP-β-CD | Analyzed the impact of penetration enhancers on permeability (ex vivo) and pharmacokinetics (in vivo) of flurbiprofen formulations. | Flurbiprofen/HP-β-CD percutaneous permeability was significantly accelerated by turpentine. In vivo pharmacokinetic study showed increased Cmax, shortened Tmax, and unchanged bioavailability. |
| Vega et al., 2013 [53] | Flurbiprofen/HP-β-CD/PEG/PLGA | Prepared formulations and evaluated as skin-controlled delivery systems (in vivo and ex vivo). | Greater anti-inflammatory efficacy compared to the control and stronger efficacy in preparations using HP-β-CD. |
| Li et al., 2010 [54] | Flurbiprofen/β-CD | Evaluated the effect of a drug solution containing a surfactant and β-CD on the solubility, bioavailability, and pharmacokinetics of flurbiprofen. | Solubility and plasma exposure (both AUC and Cmax) of flurbiprofen in the mixed system were significantly higher compared to formulations containing surfactant alone, or β-CD alone. |
| Zhang et al., 2015 [55] | Flurbiprofen/β-CD, HP- β-CD and SBE- β-CD/liposomes | Characterized the pharmacokinetic profile (in vivo) of inclusion complexes of the NSAID and β-CD, HP-β-CD, and SBE-β-CD loaded into liposomes. | The delivery systems significantly improved the relative bioavailability of flurbiprofen in Wistar rats, indicating that they are promising delivery systems for poorly soluble drugs. |
| Zhao et al., 2013 [56] | Flurbiprofen/β-CD | Incorporated flurbiprofen/β-CD into PACA nanoparticles and its oral bioavailability was evaluated in rats (in vivo). | The reduced particle size and increased surface area may have contributed to the enhanced oral bioavailability of flurbiprofen in the new formulation. |
| Celebioglu et al., 2019 [57] | Ibuprofen/HP-β-CD | Produced and characterized ibuprofen-HP-β-CD nanofibrous webs and investigated their solubility in water and artificial saliva. | Ibuprofen-HP-β-CD nanofibrous webs presented excellent solubility in both water and artificial saliva, indicating that they have the potential to be used as fast-dissolving oral drug delivery systems. |
| Volkova et al., 2020 [58] | Ibuprofen/β-CD | Prepared and evaluated ibuprofen properties in MOFs based on β-CD and K+ (in vitro). | Ibuprofen-β-CD inclusion complex showed improved aqueous solubility. |
| Rehman et al., 2013 [59] | Ibuprofen/β-CD | Prepared tablets containing variable compositions of polysaccharide matrix and assessed for in vitro drug release in simulated gastrointestinal fluids containing digestive enzymes. | Formulations containing ethyl cellulose and β-CD (1:1) presented better drug release profiles and proved to be the most adequate for drug delivery to the colon. |
| Marianecci et al., 2013 [60] | Ibuprofen/β-CD | Evaluated the permeation properties of ibuprofen-β-CD loaded into non-ionic surfactant vesicles (NSVs) using a two-compartment diffusion cell (in vitro). | Ibuprofen-β-CD-NSV system exhibited significantly improved in vitro drug permeation properties with respect to those of the plain drug suspension. |
| Li et al., 2017 [61] | Ibuprofen/γ-CD/MOF/PAA | Prepared and characterized composites of γ-CD-based MOFs and PAA. The in vitro cytotoxicity was evaluated using J774 macrophages. | Microspheres composed of CD-MOF nanocrystals embedded in PAA exhibited sustained drug release and presented reduced cell toxicity, compared with pure drug or the drug-γ-CD complex. |
| Hartlieb et al., 2017 [62] | Ibuprofen-γ-CD-MOF | Obtained and characterized a pharmaceutical cocrystal of ibuprofen-MOF-CD. Cytotoxicity (in vitro) and pharmacokinetics (in vivo) were determined. | The pharmaceutical cocrystal did not affect viability of the cells, while the in vivo bioavailability was not affected, and the formulation presented 100% longer half-life in blood plasma. |
| Felton et al., 2014 [63] | Ibuprofen, ketoprofen, naproxen, flurbiprofen/β-CD/HP-β-CD | Characterized the properties of different NSAIDs complexed with β-CD and two HP-β-CD derivatives (in silico). | NSAID solubility was significantly increased with increasing CD concentration of both HP-β-CD derivatives, whereas β-CD complexation caused little increase in NSAID solubility. |
| Mohamed et al., 2011 [64] | Indomethacin/HP-β-CD | Investigated the influence of CD incorporation on the solubility pharmacologic effect of indomethacin in injured eyes of albino rabbits (in vivo). | The complex demonstrated sufficient solubility and effectiveness in healing the corneal lesion, with improved anti-inflammatory activity. |
| Ribeiro-Rama et al., 2011 [65] | Indomethacin/HP-β-CD | Investigated the hepatic and renal injuries caused by indomethacin in several formulations including complexes with HP-β-CD (in vivo). | The animals administered with indomethacin in complexed form showed similar hepatic and renal lesions to those administered with the drug in acid or salt forms. |
| Vranic et al., 2010 [66] | Indomethacin/α-CD/γ-CD | The aim of the study was to compare the dissolution profiles of indomethacin alone and complexed with CDs. | The complexation of indomethacin with α- and γ-cyclodextrins resulted in increased dissolution rates. |
| Cirri et al., 2012 [67] | Ketoprofen/Epi-β-CD/Epi-CM-β-CD | Developed a topical administration system to improve the solubility of ketoprofen. | Demonstrated the usefulness of this new system, with improvement in the dissolution of the drug. |
| Grecu et al., 2014 [68] | Ketoprofen/β-CD | Compared the anti-inflammatory effects of orally administered ketoprofen and ketoprofen/β-CD inclusion complex using two models of experimentally induced acute inflammation in rats (paw edema and peritonitis). | The complexation of ketoprofen with β-CD resulted in increased solubility and bioavailability compared with ketoprofen. In both models of inflammation, ketoprofen/β-CD complex presented a stronger anti-inflammatory activity than ketoprofen. |
| Moutasim et al., 2017 [69] | Lornoxicam/β-CD | Formulated tablets containing lornoxicam-β-CD to increase drug solubility and to mask its bitter taste. Bioequivalence was evaluated in healthy volunteers. | The formulation presented acceptable palatability, enhanced dissolution, and rapid onset of drug action. The pharmacokinetic profile was significantly different from the control tablet. |
| Ammar et al., 2012 [70] | Lornoxicam/β-CD/HP-β-CD | Analyzed the efficacy of lornoxicam by complexation with cyclodextrins in liquid crystalline gel (ex vivo). | The preparation showed high improvement in drug dissolution, superior anti-inflammatory activity, and low skin permeation, being suitable for topical use. |
| Bramhane et al., 2011 [71] | Lornoxicam/β-CD/L-arginine | Evaluated the dissolution of lornoxicam molecular inclusion with β-CD alone and in combination with arginine (in vitro). | The complexation of lornoxicam with β-CD and arginine resulted in significantly higher dissolution compared with the uncomplexed drug and binary systems. |
| Samprasit et al., 2018 [72] | Meloxicam/HP-β-CD | Prepared taste-masked meloxicam oral dissolving films and evaluated in human volunteers. Cytotoxicity was evaluated using human gingival fibroblasts (HGF). | A fast disintegration time of meloxicam was obtained from ethanol system, which presented lower cytotoxicity. The films rapidly dissolved in the mouth and had a less bitter taste than meloxicam. |
| Samprasit et al., 2014 [73] | Meloxicam/HP-β-CD | Developed and characterized oral disintegrating tablets using the combination of ion exchange resin and CD. Solubility (in vitro) and taste (in vivo) were evaluated. | Meloxicam incorporated in a combination of ion exchange resin and HP-β-CD demonstrated a good taste, rapid disintegration, complete solubility, and significant stability. |
| Rein et al., 2020 [74] | Meloxicam/β-CD | Investigated an in situ forming system based on meloxicam in β-CD for periodontitis treatment, aiming sustainable drug release at a periodontal pocket (in vitro). | The developed system comprising 40% β-CD transformed into microparticles extended the drug release to 7 days in the locality of the treatment. |
| Jafar et al., 2017 [75] | Meloxicam-β-CD-TEA | Investigated the properties of a buoyant in situ gel prepared from a ternary complex of meloxicam with β-CD and TEA on solubility, stability, and in vivo anti-inflammatory activity (carrageenan model in mice). | The solubility, stability. and anti-inflammatory activity of meloxicam was successfully increased due to its incorporation in ternary complex with β-CD-TEA, demonstrating improved pharmaceutical and pharmacodynamic properties. |
| Ainurofiq et al., 2016 [76] | Meloxicam/β-CD | Investigated inclusion complexes of meloxicam/β-CD incorporated into orally disintegrating tablets using a quality by design approach. | Although interactions between meloxicam and β-CD occurred under different complexation methods, best solubility and dissolution rate enhancement was achieved with the spray drying method. |
| Shende et al., 2015 [77] | Meloxicam/β-CD -based nanosponges | Investigated inclusion complexes of meloxicam and β-CD-based nanosponges on stability, solubility, drug release (in vitro and in vivo) and analgesic and anti-inflammatory effect (in vivo). | Incorporation of meloxicam into β-CD-based nanosponges improved its biopharmaceutical properties and potentiated its analgesic and anti-inflammatory effects. |
| Radia et al., 2012 [78] | Meloxicam/β-CD. | Investigated the effect of Meloxicam complexation with β-CD on the bioavailability of the drug (in vivo and in vitro). | The oral bioavailability of ML was significantly improved through complexation with β-CD. |
| Rasool et al., 2011 [79] | Meloxicam/β-CD | Developed and evaluated the properties of a meloxicam transdermal gel in complexation with β-CD. | The dissolution properties of the meloxicam-β-CD complexes were superior when compared to meloxicam alone. |
| Awasthi et al., 2011 [80] | Meloxicam/β-CD | Investigated the improvement on meloxicam solubility by preparation of its solid dispersion using β-CD blended with various water-soluble polymer carriers (in vitro). | Meloxicam formulations obtained by solid dispersion technique presented significantly enhanced solubility, highlighting β-CD blended with 0.12% (w/w) HP-methylcellulose polymer. |
| Janovský et al., 2010 [81] | Meloxicam/β-CD | Investigated the influence on analgesic activity and serum levels after meloxicam complexation (in vivo). | Stronger analgesic activity than unmodified meloxicam; Serum levels of meloxicam were significantly higher. |
| Samprasit et al., 2013 [82] | Meloxicam, Piroxicam/β-CD, HP-β-CD | Prepared and characterized inclusion complexes of NSAIDs with either β-CD or HP-β-CD loaded into an anionic exchange resin. | The solubility of both drugs was found to increase with increasing CD concentration. The presence of cyclodextrin in the loading solution resulted in the improvement of drug loading into the resin. |
| Tang et al., 2018 [83] | Mesalazine/HP-β-CD/chitosan nanoparticles | Analyzed the cytotoxic and anti-inflammatory effects of mesalazine/HP-β-CD/chitosan nanoparticles on cytokine stimulated HT-29 cells (in vitro). | The complexed drug more strongly inhibited the production of inflammatory mediators such as NO, PGE2 and IL-8. |
| Elkordy et al., 2012 [84] | Naproxen/β-CD, Poloxamer-407 | Investigated the effects of β-CD, Poloxamer-407 and sorbitol as drug carriers (in vitro). | Different tested mixtures of the drug with β-CD and Poloxamer-407 showed enhancement in drug release. |
| Shelley et al., 2018 (a) [85] | Nepafenac/HP-β-CD | Investigated trans-corneal permeation of nepafenac in a HP-β-CD complex (ex vivo). | The formulation showed a significantly higher drug concentration in the cornea with a permeation rate 18-fold higher than the commercial product. |
| Shelley et al., 2018 (b) [86] | Nepafenac/HP-β-CD. | Evaluated the solubility and transcorneal permeation of a gel containing nepafenac-HP-β-CD, in comparison with the commercial nepafenac (ex vivo). | The formulation increased the solubility and ocular release of nepafenac, resulting in a higher concentration of drug in the cornea, sclera and retina compared to the commercial drug |
| Lorenzo-Veiga et al., 2020 [87] | Nepafenac/γ-CD/HP-β-CD | Analyzed CD-based formulations with several polymers to efficiently deliver nepafenac topically to the eye (in vitro and ex vivo). | Formulations with methylcellulose and carboxymethylcellulose polymers showed improved solubility, mucoadhesion, permeability and anti-inflammatory potential. |
| Auda, 2014 [88] | Nimesulide/Me-β-CD | Characterized and evaluated the solubility (in vitro) and the anti-inflammatory effect of the inclusion complex in carrageenan-induced inflammation in rats (in vivo). | The study showed that the inclusion system enhanced the solubility and anti-inflammatory activity of the drug. |
| Mura et al., 2016 [89] | Oxaprozin/Me-β-CD | Used a combined approach based on drug complexation with methylated β-CD and nanoclays to improve the dissolution of oxaprozin (in vitro). | The complexation and clay nanoencapsulation improved the oxaprozin dissolution properties around 100%. |
| Maestrelli et al., 2011 [90] | Oxaprozin-Rme-β-D/chitosan/bile components | Investigated the effect of the combined use of Rme-β-CD, chitosan, and different bile components on solubility and permeability of oxaprozin through Caco-2 cells. | Chitosan and bile acids increased the Rme-β-CD solubilizing power. The addition of CS to oxaprozin-Rme-β-CD systems increased drug permeability through Caco-2 cells. |
| Maestrelli et al., 2017 [91] | Oxaprozin/Rme-β-CD/L-arginine | Investigated a possible enhancement of the Rme-β-CD solubilizing efficacy through a combined approach with arginine in sepiolite nanoclay (SV). Anti-inflammatory activity was assessed using CFA-induced arthritis (in vivo). | The new hybrid nanocomposite was more effective than the respective oxaprozin-Rme-β-CD and oxaprozin-SV systems in improving the drug dissolution properties, as well as the effects on CFA-induced arthritis. |
| Mennini et al., 2016(a) [92] | Oxaprozin/Rme-β-CD/L-arginine | Evaluated the influence of L-arginine on the properties of an oxaprozin/Rme-β-CD inclusion complex, including stability and solubility. | The ternary system presented improved properties, indicating its potential for enhancing safety, bioavailability, and therapeutic efficacy of NSAIDs such as oxaprozin. |
| Mennini et al., 2016(b) [93] | Oxaprozin/Rme-β-CD/Arginine | Characterized and evaluated the in vitro drug permeability of the drug complexed with Rme-β-CD and loaded into liposomes or nanostructured lipid carriers (NLCs). | The combined use of Rme-β-CD and either liposomes or NLCs enabled increases in the drug permeability through artificial membranes and excised human skin. |
| Mura, et al., 2010 [94] | Oxaprozin/β-CD/Me-β-CD/PLGA | Investigated the properties of a formulation based on the combined use of CD and PLGA nanoparticles of oxaprozin (in vitro). | The complex was effective in solubilizing and stabilizing the drug, improving the prolonged-release properties of nanoparticles. |
| Mishra et al., 2019 [95] | Piroxicam/β-CD/Pectin/chitosan/albumin | Studied β-CD-based formulations with several polymers to optimize the delivery and activity of the drug (in vivo). | The drug in the β-CD polymer conjugate was found to possess enhanced analgesic activity and reduced ulcerogenic potential in rats. |
| Kontogiannidou et al., 2019 [96] | Piroxicam/β-CD/HP-β-CD/Me-β-CD | Obtained and characterized piroxicam-CD tablet formulations. The in vitro release profile was analyzed in simulated saliva and the drug permeation studies, across porcine buccal mucosa (ex vivo). | The drug release profile as well as the tissue permeability followed this order: Me-β-CD > HP-β-CD > β-CD. Chitosan significantly increased the transport of the drug compared to their free complexes. Loss of superficial cell layers was attributed to the presence of CD. |
| Bouchal et al., 2015 [97] | Piroxicam/β-CD/HP-β-CD | Compared the dissolution of piroxicam in the absence or presence of CDs. | The drug was faster and completely dissolved in an aqueous medium in the conjugated form compared to the free form. |
| Skiba et al., 2013 [98] | Piroxicam/β-CD | Compared the pharmacokinetic parameters of piroxicam after an oral administration in rabbits (in vivo). | The complexed piroxicam had faster absorption than the free drug but the excess of CD had a negative impact on the permeability of the drug through the biological membrane. |
| Keleş et al., 2010 [99] | Piroxicam/β-CD | Investigated the analgesic efficacy and adverse effects of preoperatively administered piroxicam-β-CD (clinical study). | Preemptive administration of piroxicam-β-CD effectively reduced analgesic consumption, and 40 mg of the complexed drug was effective without side effects. |
| Alsarra et al., 2010 [100] | Piroxicam, indomethacin/β-CD, HP-β-CD | Compared the formation of gastric ulcers in rats treated with isolated and complexed drugs (in vivo). | Complexation with either β-CD or HP-β-CD significantly reduced gastric ulcer formation in rats treated with indomethacin or piroxicam. |
| Haggag et al., 2016 [101] | Sulindac/β-CD/CAP | Characterized and investigated the ability of different polymers to ameliorate sulindac-induced gastric ulcers in rats (in vivo). | β-CD significantly enhanced the solubility of sulindac but had no protective effect against gastric ulcer formation in rats. |
| Sherje et al., 2018 [102] | Zaltoprofen/β-CD/HP-β-CD/L-arginine | Investigated formulations containing CDs and L-arginine for drug stability, dissolution, and solubility (in vitro). | The addition of L-arginine to drug-CD system increased stability, dissolution, and solubility of zaltoprofen. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, G.M.; Santos, V.O.R.e.; Bessa, J.R.; Teles, Y.C.F.; Yahouédéhou, S.C.M.A.; Goncalves, M.S.; Ribeiro-Filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. https://doi.org/10.3390/biom11030361
Miranda GM, Santos VORe, Bessa JR, Teles YCF, Yahouédéhou SCMA, Goncalves MS, Ribeiro-Filho J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules. 2021; 11(3):361. https://doi.org/10.3390/biom11030361
Chicago/Turabian StyleMiranda, Gustavo Marinho, Vitória Ohana Ramos e Santos, Jonatas Reis Bessa, Yanna C. F. Teles, Setondji Cocou Modeste Alexandre Yahouédéhou, Marilda Souza Goncalves, and Jaime Ribeiro-Filho. 2021. "Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review" Biomolecules 11, no. 3: 361. https://doi.org/10.3390/biom11030361
APA StyleMiranda, G. M., Santos, V. O. R. e., Bessa, J. R., Teles, Y. C. F., Yahouédéhou, S. C. M. A., Goncalves, M. S., & Ribeiro-Filho, J. (2021). Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules, 11(3), 361. https://doi.org/10.3390/biom11030361