Morphometric Analysis of Rat Prostate Development: Roles of MEK/ERK and Rho Signaling Pathways in Prostatic Morphogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Prostate Organ Culture and Imaging
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. Cell Culture
2.5. MTS Cell Proliferation Assay
2.6. MLC-P Measurement by Western Blot
2.7. Statistical Analysis
3. Results
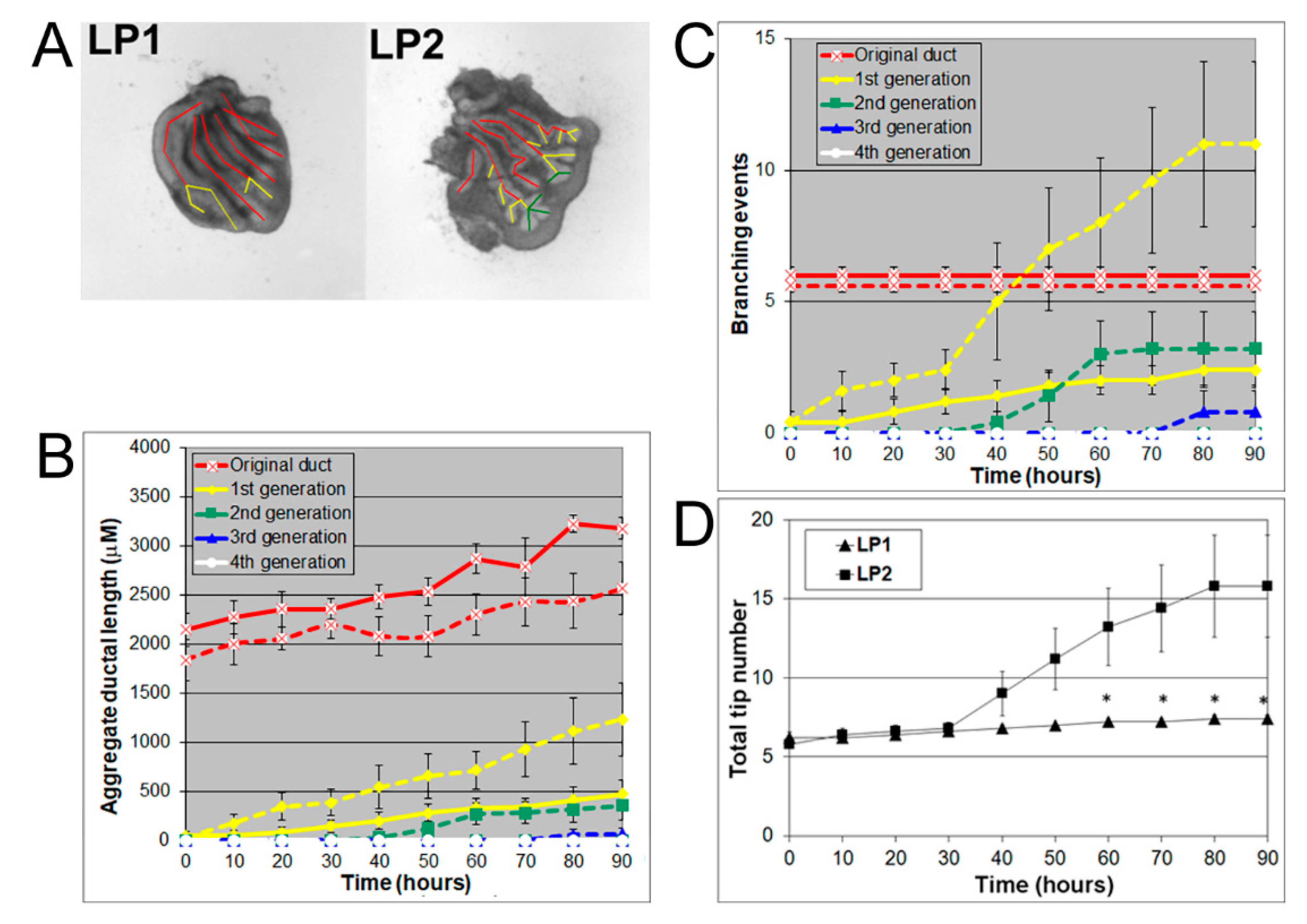
3.1. Prostates Exhibit Distinguishing Lobe-Specific Growth and Branching Patterns
3.2. LP1 Had a Longer Original Ductal Length but Fewer Branching Events and Fewer Tips Compared to LP2
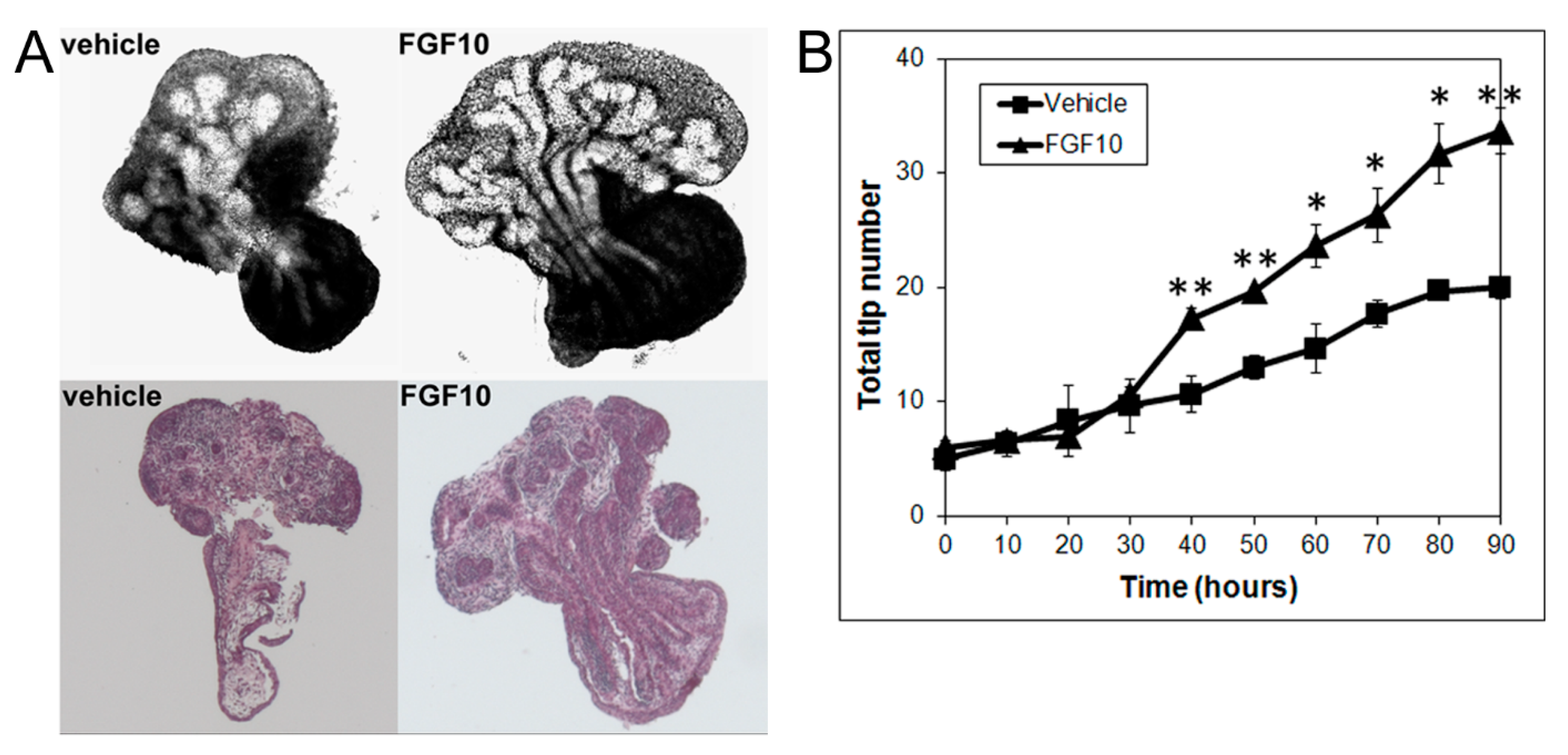
3.3. Effects of MEK Inhibition and FGF10 Supplementation on Ventral or Lateral Prostate Growth and Branching Morphogenesis
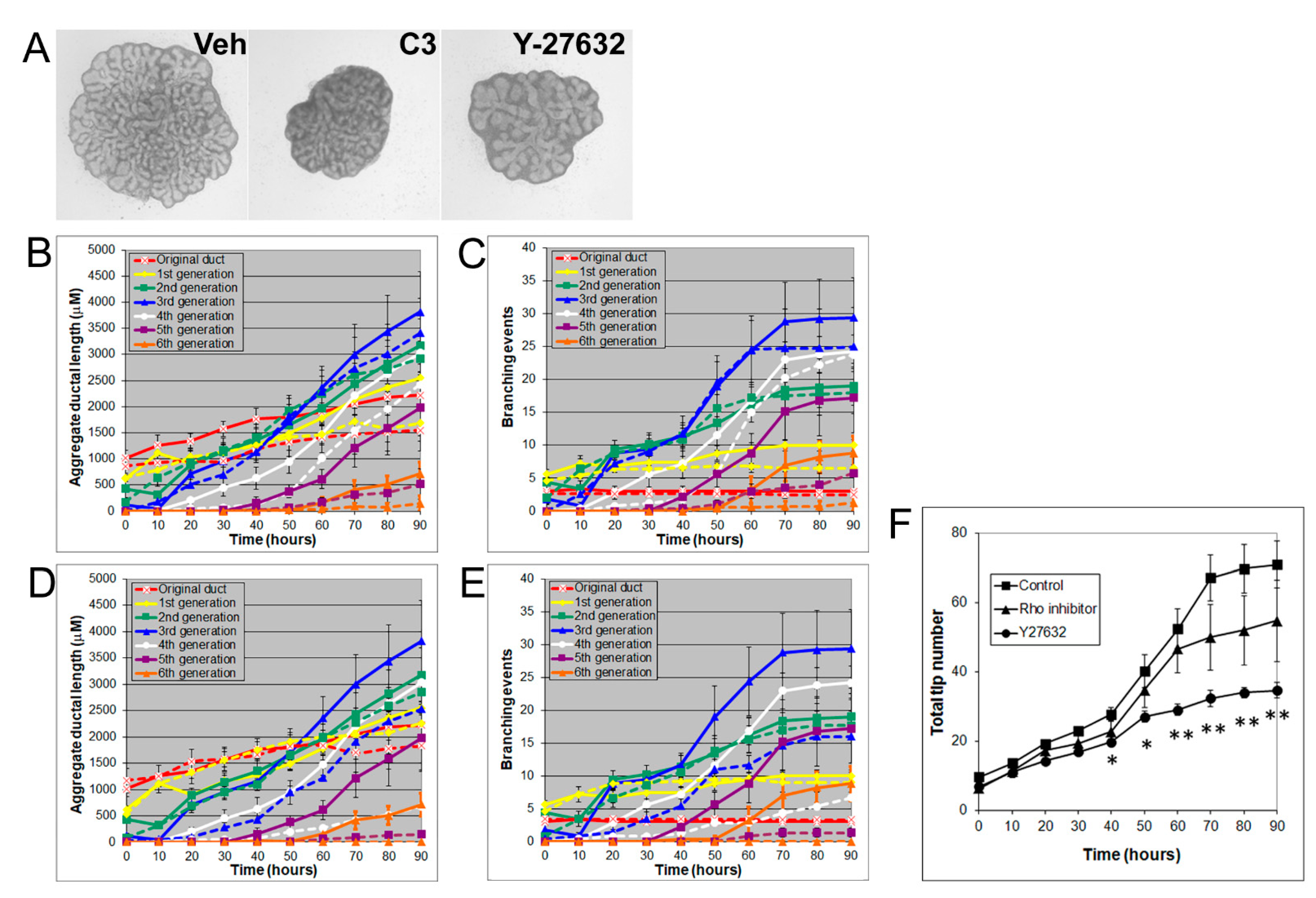
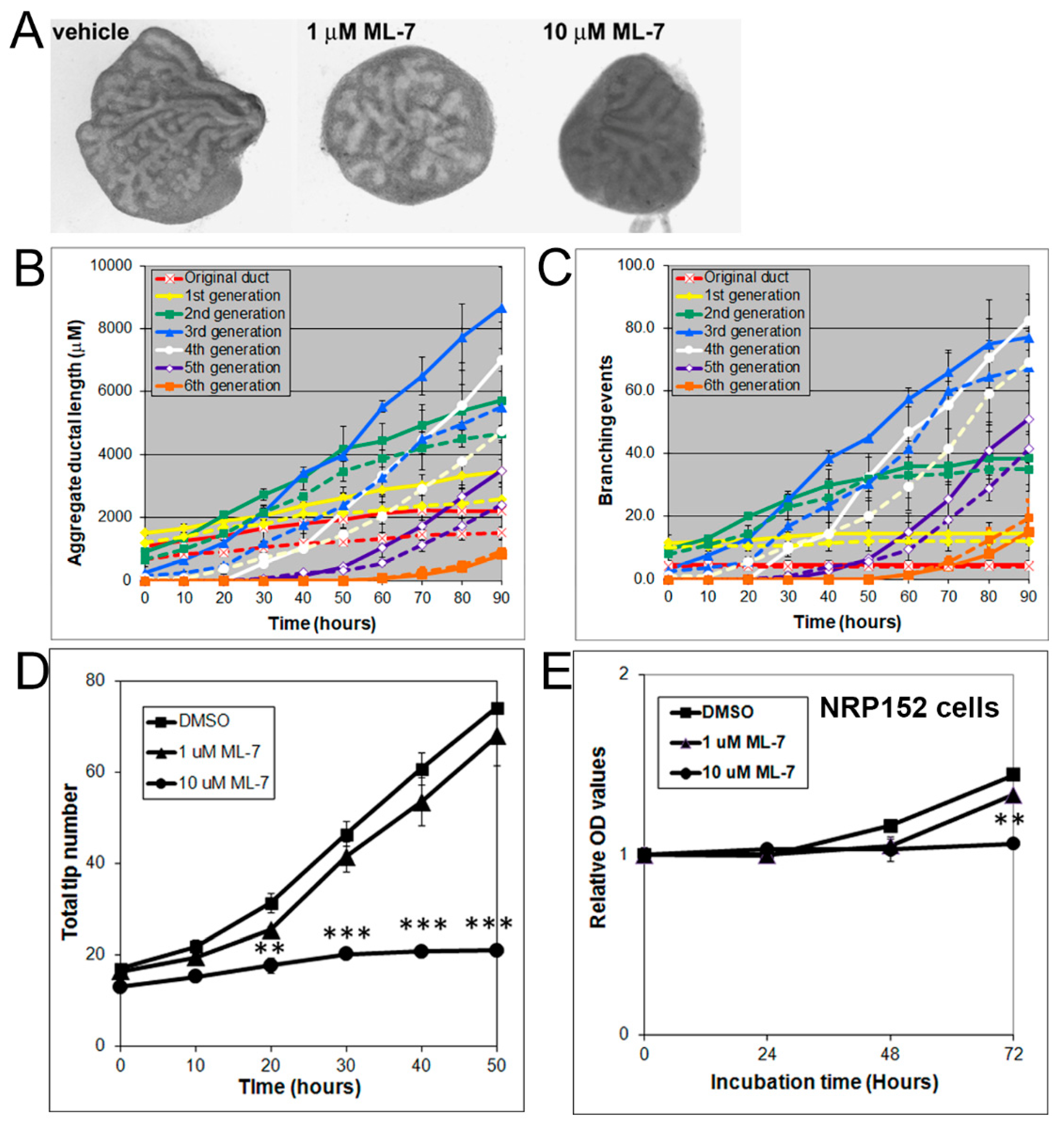
3.4. Effects of Rho, ROCK, and MLCK Inhibitors on Ventral Prostate Growth and Branching Morphogenesis
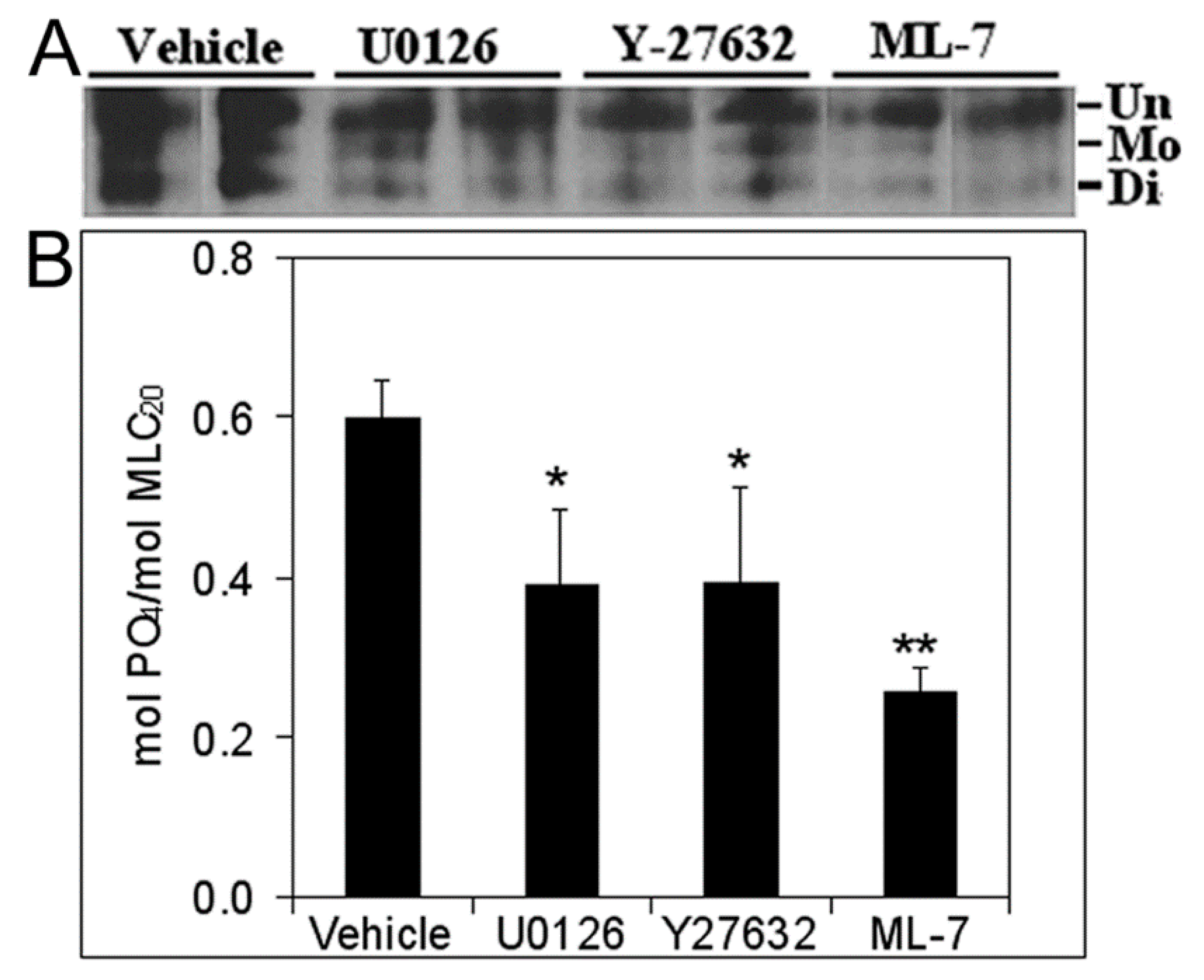
3.5. Inhibition of Cell Proliferation and MLC-P by Inhibitors of MLCK, MEK/ERK, and Rho Kinase
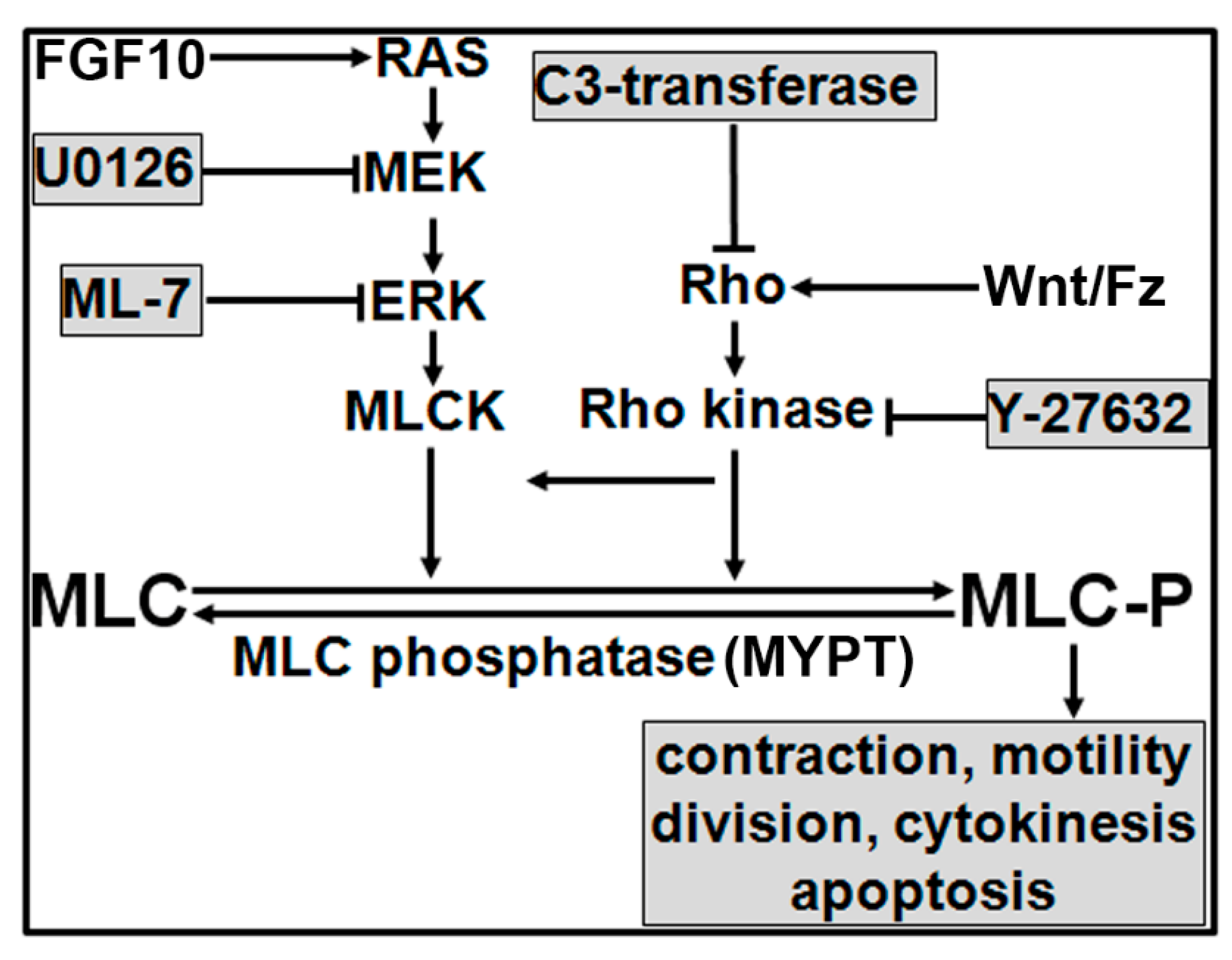
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzo, G. Similarities Between Embryo Development and Cancer Process Suggest New Strategies for Research and Therapy of Tumors: A New Point of View. Front. Cell Dev. Biol. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Yang, Z.; Qin, H. The relationship between early embryo development and tumorigenesis. J. Cell. Mol. Med. 2010, 14, 2697–2701. [Google Scholar] [CrossRef]
- Prins, G.S.; Putz, O. Molecular signaling pathways that regulate prostate gland development. Differentiation 2008, 76, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Cooke, P.S.; Birch, L.; Donjacour, A.A.; Yalcinkaya, T.M.; Siiteri, P.K.; Cunha, G.R. Androgen receptor expression and 5 alpha-reductase activity along the proximal-distal axis of the rat prostatic duct. Endocrinology 1992, 130, 3066–3073. [Google Scholar] [CrossRef]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef]
- Cunha, G.R.; Donjacour, A.A.; Cooke, P.S.; Mee, S.; Bigsby, R.M.; Higgins, S.J.; Sugimura, Y. The endocrinology and developmental biology of the prostate. Endocr. Rev. 1987, 8, 338–362. [Google Scholar] [CrossRef]
- Price, D. Comparative aspects of development and structure in the prostate. Natl. Cancer Inst. Monogr. 1963, 12, 1–27. [Google Scholar] [PubMed]
- Thomson, A.A.; Marker, P.C. Branching Morphogenesis of the Prostate. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6542/ (accessed on 20 October 2021).
- Hayashi, N.; Sugimura, Y.; Kawamura, J.; Donjacour, A.A.; Cunha, G.R. Morphological and functional heterogeneity in the rat prostatic gland. Biol. Reprod. 1991, 45, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Timms, B.G.; Mohs, T.J.; Didio, L.J. Ductal budding and branching patterns in the developing prostate. J. Urol. 1994, 151, 1427–1432. [Google Scholar] [CrossRef]
- Cunha, G.R.; Ricke, W.; Thomson, A.; Marker, P.C.; Risbridger, G.; Hayward, S.W.; Wang, Y.Z.; Donjacour, A.A.; Kurita, T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 2004, 92, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.A.; Marker, P.C. Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation 2006, 74, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pu, Y.; Hepps, D.; Danielpour, D.; Prins, G.S. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology 2007, 148, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Lipschutz, J.H.; Samid, D.; Cunha, G.R. Phenylacetate is an inhibitor of prostatic growth and development in organ culture. J. Urol. 1996, 155, 1762–1770. [Google Scholar] [CrossRef]
- Lipschutz, J.H.; Foster, B.A.; Cunha, G.R. Differentiation of rat neonatal ventral prostates grown in a serum-free organ culture system. Prostate 1997, 32, 35–42. [Google Scholar] [CrossRef]
- Martikainen, P.; Suominen, J. A morphometric analysis of rat ventral prostate in organ culture. Anat. Rec. 1983, 207, 279–288. [Google Scholar] [CrossRef]
- Huang, L.; Pu, Y.; Hu, W.-Y.; Birch, L.; Luccio-Camelo, D.; Yamaguchi, T.; Prins, G.S. The role of Wnt5a in prostate gland development. Dev. Biol. 2009, 328, 188–199. [Google Scholar] [CrossRef]
- Jarred, R.A.; Cancilla, B.; Prins, G.S.; Thayer, K.A.; Cunha, G.R.; Risbridger, G.P. Evidence that estrogens directly alter androgen-regulated prostate development. Endocrinology 2000, 141, 3471–3477. [Google Scholar] [CrossRef]
- Prins, G.S. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology 1992, 130, 3703–3714. [Google Scholar] [CrossRef]
- Omoto, Y. Estrogen receptor-alpha signaling in growth of the ventral prostate: Comparison of neonatal growth and postcastration regrowth. Endocrinology 2008, 149, 4421–4427. [Google Scholar] [CrossRef]
- Huang, L.; Pu, Y.; Alam, S.; Birch, L.; Prins, G.S. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J. Androl. 2004, 25, 330–337. [Google Scholar] [CrossRef]
- Prins, G.S.; Chang, W.Y.; Wang, Y.; van Breemen, R.B. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology 2002, 143, 3628–3640. [Google Scholar] [CrossRef][Green Version]
- Vezina, C.M.; Allgeier, S.H.; Fritz, W.A.; Moore, R.W.; Strerath, M.; Bushman, W.; Peterson, R.E. Retinoic acid induces prostatic bud formation. Dev. Dyn. 2008, 237, 1321–1333. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, L.; Birch, L.; Prins, G.S. Androgen regulation of prostate morphoregulatory gene expression: Fgf10-dependent and -independent pathways. Endocrinology 2007, 148, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pu, Y.; Alam, S.; Birch, L.; Prins, G.S. The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: Lobe-specific suppression by neonatal estrogens. Dev. Biol. 2005, 278, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Donjacour, A.A.; Thomson, A.A.; Cunha, G.R. FGF-10 plays an essential role in the growth of the fetal prostate. Dev. Biol. 2003, 261, 39–54. [Google Scholar] [CrossRef]
- Kuslak, S.L.; Marker, P.C. Fibroblast growth factor receptor signaling through MEK-ERK is required for prostate bud induction. Differentiation 2007, 75, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.A.; Cunha, G.R. Prostatic growth and development are regulated by FGF10. Development 1999, 126, 3693–3701. [Google Scholar] [CrossRef]
- Terunuma, A.; Limgala, R.P.; Park, C.J.; Choudhary, I.; Vogel, J.C. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng. Part A 2010, 16, 1363–1368. [Google Scholar] [CrossRef]
- Gu, L.-Z.; Hu, W.-Y.; Antic, N.; Mehta, R.; Turner, J.R.; de Lanerolle, P. Inhibiting myosin light chain kinase retards the growth of mammary and prostate cancer cells. Eur. J. Cancer 2006, 42, 948–957. [Google Scholar] [CrossRef]
- Fazal, F.; Gu, L.; Ihnatovych, I.; Han, Y.; Hu, W.; Antic, N.; Carreira, F.; Blomquist, J.F.; Hope, T.J.; Ucker, D.S.; et al. Inhibiting myosin light chain kinase induces apoptosis in vitro and in vivo. Mol. Cell. Biol. 2005, 25, 6259–6266. [Google Scholar] [CrossRef]
- Soriano, O.; Alcón-Pérez, M.; Vicente-Manzanares, M.; Castellano, E. The Crossroads between RAS and RHO Signaling Pathways in Cellular Transformation, Motility and Contraction. Genes 2021, 12, 819. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, L.; Prins, G.S. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: Lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev. Biol. 2004, 273, 257–275. [Google Scholar] [CrossRef]
- Bieberich, C.J.; Fujita, K.; He, W.W.; Jay, G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J. Biol. Chem. 1996, 271, 31779–31782. [Google Scholar] [CrossRef]
- Sciavolino, P.J.; Abrams, E.W.; Yang, L.; Austenberg, L.P.; Shen, M.M.; Abate-Shen, C. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev. Dyn. 1997, 209, 127–138. [Google Scholar] [CrossRef]
- Ho, S.M.; Damassa, D.; Kwan, P.W.; Seto, H.S.; Leav, I. Androgen receptor levels and androgen contents in the prostate lobes of intact and testosterone-treated Noble rats. J. Androl. 1985, 6, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Shain, S.A.; Boesel, R.W. Aging-associated diminished rat prostate androgen receptor content concurrent with decreased androgen dependence. Mech. Ageing Dev. 1977, 6, 219–232. [Google Scholar] [CrossRef]
- Prins, G.S. Differential regulation of androgen receptors in the separate rat prostate lobes: Androgen independent expression in the lateral lobe. J. Steroid Biochem. 1989, 33, 319–326. [Google Scholar] [CrossRef]
- Prins, G.S.; Woodham, C. Autologous regulation of androgen receptor messenger ribonucleic acid in the separate lobes of the rat prostate gland. Biol. Reprod. 1995, 53, 609–619. [Google Scholar] [CrossRef]
- Kling, D.E.; Lorenzo, H.K.; Trbovich, A.M.; Kinane, T.B.; Donahoe, P.K.; Schnitzer, J.J. MEK-1/2 inhibition reduces branching morphogenesis and causes mesenchymal cell apoptosis in fetal rat lungs. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L370–L378. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.E.; Michael, L.; Barnett, M.W.; Davies, J.A. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development 2001, 128, 4329–4338. [Google Scholar] [CrossRef]
- Somlyo, A.V.; Bradshaw, D.; Ramos, S.; Murphy, C.; Myers, C.E.; Somlyo, A.P. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem. Biophys. Res. Commun. 2000, 269, 652–659. [Google Scholar] [CrossRef]
- Somlyo, A.V.; Phelps, C.; Dipierro, C.; Eto, M.; Read, P.; Barrett, M.; Gibson, J.J.; Burnitz, M.C.; Myers, C.; Somlyo, A.P. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB J. 2003, 17, 223–234. [Google Scholar] [CrossRef]
- Moore, K.A.; Polte, T.; Huang, S.; Shi, B.; Alsberg, E.; Sunday, M.E.; Ingber, D.E. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. 2005, 232, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Madueke, I.; Hu, W.Y.; Hu, D.; Swanson, S.M.; Vander Griend, D.; Abern, M.; Prins, G.S. The role of WNT10B in normal prostate gland development and prostate cancer. Prostate 2019, 79, 1692–1704. [Google Scholar] [CrossRef]
- Madueke, I.C.; Hu, W.Y.; Huang, L.; Prins, G.S. WNT2 is necessary for normal prostate gland cyto-differentiation and modulates prostate growth in an FGF10 dependent manner. Am. J. Clin. Exp. Urol. 2018, 6, 154–163. [Google Scholar]
- Schlessinger, K.; Hall, A.; Tolwinski, N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009, 23, 265–277. [Google Scholar] [CrossRef]
- Habas, R.; Kato, Y.; He, X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 2001, 107, 843–854. [Google Scholar] [CrossRef]
- Shi, W.; Xu, C.; Gong, Y.; Wang, J.; Ren, Q.; Yan, Z.; Mei, L.; Tang, C.; Ji, X.; Hu, X.; et al. RhoA/Rock activation represents a new mechanism for inactivating Wnt/β-catenin signaling in the aging-associated bone loss. Cell Regen. 2021, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Aaron, L.; Franco, O.E.; Hayward, S.W. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2016, 43, 279–288. [Google Scholar] [CrossRef]
- Prins, G.S.; Hu, W.Y.; Xie, L.; Shi, G.B.; Hu, D.P.; Birch, L.; Bosland, M.C. Evaluation of Bisphenol A (BPA) Exposures on Prostate Stem Cell Homeostasis and Prostate Cancer Risk in the NCTR-Sprague-Dawley Rat: An NIEHS/FDA CLARITY-BPA Consortium Study. Environ. Health Perspect. 2018, 126, 117001. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Ye, S.H.; Birch, L.; Zhang, X.; Cheong, A.; Lin, H.; Calderon-Gierszal, E.; Groen, J.; Hu, W.Y.; Ho, S.M.; et al. Prostate Cancer Risk and DNA Methylation Signatures in Aging Rats following Developmental BPA Exposure: A Dose-Response Analysis. Environ. Health Perspect. 2017, 125, 077007. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.-Y.; Afradiasbagharani, P.; Lu, R.; Liu, L.; Birch, L.A.; Prins, G.S. Morphometric Analysis of Rat Prostate Development: Roles of MEK/ERK and Rho Signaling Pathways in Prostatic Morphogenesis. Biomolecules 2021, 11, 1829. https://doi.org/10.3390/biom11121829
Hu W-Y, Afradiasbagharani P, Lu R, Liu L, Birch LA, Prins GS. Morphometric Analysis of Rat Prostate Development: Roles of MEK/ERK and Rho Signaling Pathways in Prostatic Morphogenesis. Biomolecules. 2021; 11(12):1829. https://doi.org/10.3390/biom11121829
Chicago/Turabian StyleHu, Wen-Yang, Parivash Afradiasbagharani, Ranli Lu, Lifeng Liu, Lynn A. Birch, and Gail S. Prins. 2021. "Morphometric Analysis of Rat Prostate Development: Roles of MEK/ERK and Rho Signaling Pathways in Prostatic Morphogenesis" Biomolecules 11, no. 12: 1829. https://doi.org/10.3390/biom11121829
APA StyleHu, W.-Y., Afradiasbagharani, P., Lu, R., Liu, L., Birch, L. A., & Prins, G. S. (2021). Morphometric Analysis of Rat Prostate Development: Roles of MEK/ERK and Rho Signaling Pathways in Prostatic Morphogenesis. Biomolecules, 11(12), 1829. https://doi.org/10.3390/biom11121829





