Structural Rearrangements of a Dodecameric Ketol-Acid Reductoisomerase Isolated from a Marine Thermophilic Methanogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of M. thermolithotrophicus
2.2. Native Purification of KARI
2.3. High-Resolution Clear Native (hrCN) PAGE and Gel Filtration Experiments
2.4. Mass Spectrometry Protein Identification
2.5. Crystallisation
2.6. X-ray Data Collection and Model Refinement/Validation
2.7. Sequences Alignment and Phylogenetic Analysis
3. Results
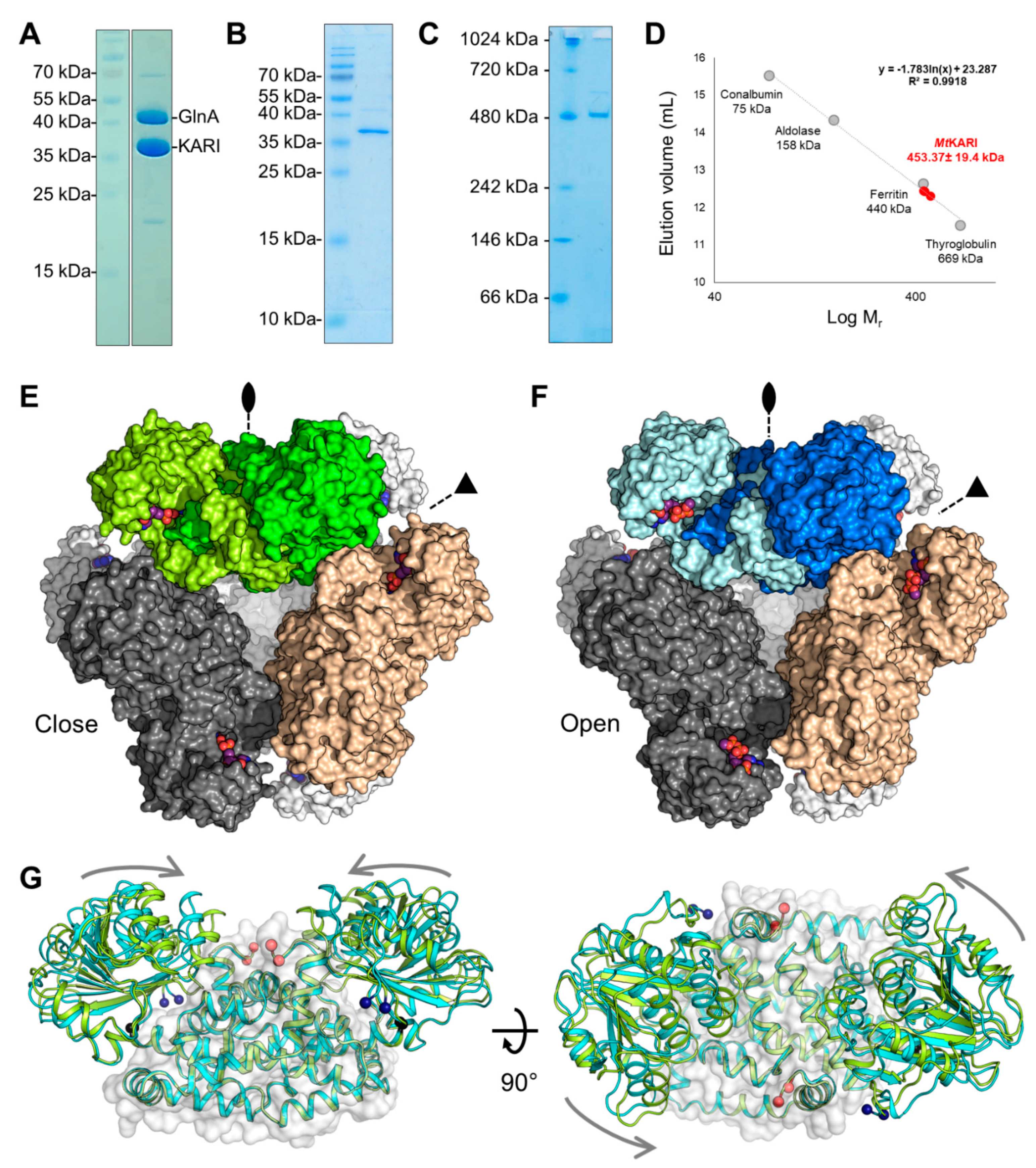
3.1. Purification and Structural Characterisation of the Open/Closed Conformation of MtKARI
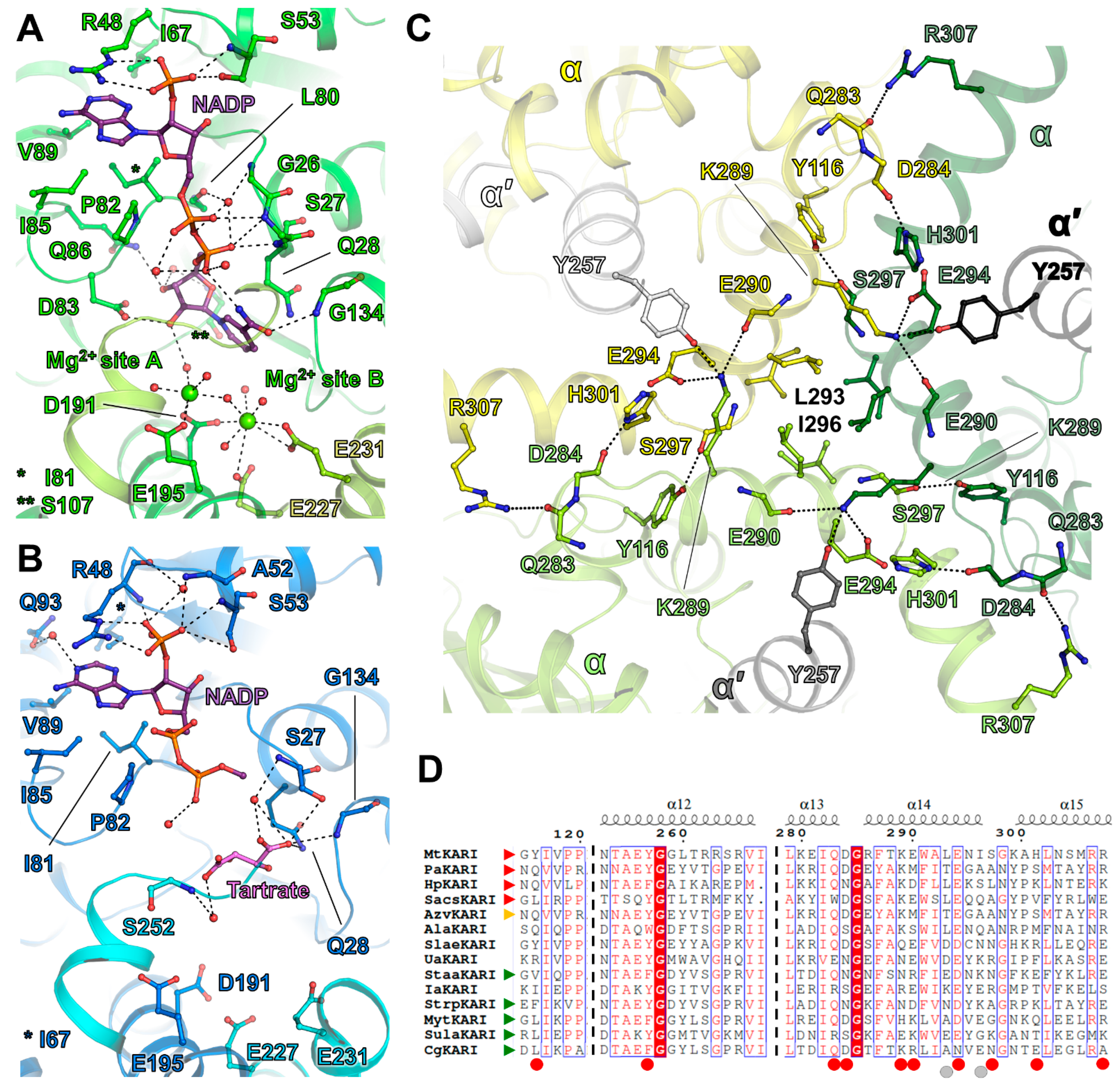
3.2. Substrate Coordination in MtKARI Structures
3.3. A Stable Dodecameric State Is Maintained by a Few Anchoring Residues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumas, R.; Biou, V.; Halgand, F.; Douce, R.; Duggleby, R.G. Enzymology, Structure, and Dynamics of Acetohydroxy Acid Isomeroreductase. Acc. Chem. Res. 2001, 34, 399–408. [Google Scholar] [CrossRef]
- Bayaraa, T.; Kurz, J.L.; Patel, K.M.; Hussein, W.M.; Bilyj, J.K.; West, N.P.; Schenk, G.; McGeary, R.P.; Guddat, L.W. Discovery, Synthesis and Evaluation of a Ketol-Acid Reductoisomerase Inhibitor. Chemistry 2020, 26, 8958–8968. [Google Scholar] [CrossRef]
- Yu, M.J.; Wu, J.; Chen, S.L. Mechanism and Inhibitor Exploration with Binuclear Mg Ketol-Acid Reductoisomerase: Targeting the Biosynthetic Pathway of Branched-Chain Amino Acids. ChemBioChem 2020, 21, 381–391. [Google Scholar] [CrossRef]
- Kandale, A.; Patel, K.; Hussein, W.M.; Wun, S.J.; Zheng, S.; Tan, L.; West, N.P.; Schenk, G.; Guddat, L.W.; McGeary, R.P. Analogues of the Herbicide, N-Hydroxy-N-isopropyloxamate, Inhibit Mycobacterium tuberculosis Ketol-Acid Reductoisomerase and Their Prodrugs Are Promising Anti-TB Drug Leads. J. Med. Chem. 2021, 64, 1670–1684. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, F.; Xu, J.Z.; Zhang, W.G.; Chen, X.L.; Liu, L.M. Improvement of l-Leucine Production in Corynebacterium glutamicum by Altering the Redox Flux. Int. J. Mol. Sci. 2019, 20, 2020. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Hong, J.; Kim, K.J. Crystal Structure and Biochemical Characterization of Ketol-Acid Reductoisomerase from Corynebacterium glutamicum. J. Agric. Food Chem. 2019, 67, 8527–8535. [Google Scholar] [CrossRef]
- Ahn, H.J.; Eom, S.J.; Yoon, H.J.; Lee, B.I.; Cho, H.; Suh, S.W. Crystal structure of class I acetohydroxy acid isomeroreductase from Pseudomonas aeruginosa. J. Mol. Biol. 2003, 328, 505–515. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Ko, T.-P.; Lin, K.-F.; Lin, B.-L.; Huang, C.-H.; Chiang, C.-H.; Horng, J.-C. NADH/NADPH bi-cofactor-utilizing and thermoactive ketol-acid reductoisomerase from Sulfolobus acidocaldarius. Sci. Rep. 2018, 8, 7176. [Google Scholar] [CrossRef] [PubMed]
- Biou, V.; Dumas, R.; Cohen-Addad, C.; Douce, R.; Job, D.; Pebay-Peyroula, E. The crystal structure of plant acetohydroxy acid isomeroreductase complexed with NADPH, two magnesium ions and a herbicidal transition state analog determined at 1.65 A resolution. EMBO J. 1997, 16, 3405–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.M.; Teran, D.; Zheng, S.; Kandale, A.; Garcia, M.; Lv, Y.; Schembri, M.A.; McGeary, R.P.; Schenk, G.; Guddat, L.W. Crystal Structures of Staphylococcus aureus Ketol-Acid Reductoisomerase in Complex with Two Transition State Analogues that Have Biocidal Activity. Chemistry 2017, 23, 18289–18295. [Google Scholar] [CrossRef]
- Kim, G.; Shin, D.; Lee, S.; Yun, J.; Lee, S. Crystal Structure of IlvC, a Ketol-Acid Reductoisomerase, from Streptococcus pneumoniae. Crystals 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Kandale, A.; Wun, S.J.; McGeary, R.P.; Williams, S.J.; Kobe, B.; Sieber, V.; Schembri, M.A.; Schenk, G.; Guddat, L.W. Crystal structure of Mycobacterium tuberculosis ketol-acid reductoisomerase at 1.0 Å resolution—A potential target for anti-tuberculosis drug discovery. FEBS J. 2016, 283, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chang, Y.C.; Lin, B.L.; Lin, K.F.; Huang, C.H.; Hsieh, D.L.; Ko, T.P.; Tsai, M.D. Use of Cryo-EM To Uncover Structural Bases of pH Effect and Cofactor Bispecificity of Ketol-Acid Reductoisomerase. J. Am. Chem. Soc. 2019, 141, 6136–6140. [Google Scholar] [CrossRef]
- Tyagi, R.; Duquerroy, S.; Navaza, J.; Guddat, L.W.; Duggleby, R.G. The crystal structure of a bacterial class II ketol-acid reductoisomerase: Domain conservation and evolution. Protein Sci. 2005, 14, 3089–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann-Chen, S.; Cahn, J.K.B.; Arnold, F.H. Uncovering rare NADH-preferring ketol-acid reductoisomerases. Metab. Eng. 2014, 26, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann-Chen, S.; Flock, T.; Cahn, J.K.B.; Snow, C.D.; Brustad, E.M.; McIntosh, J.A.; Meinhold, P.; Zhang, L.; Arnold, F.H. General approach to reversing ketol-acid reductoisomerase cofactor dependence from NADPH to NADH. Proc. Natl. Acad. Sci. USA 2013, 110, 10946–10951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahn, J.K.B.; Brinkmann-Chen, S.; Spatzal, T.; Wiig, J.A.; Buller, A.R.; Einsle, O.; Hu, Y.; Ribbe, M.W.; Arnold, F.H. Cofactor specificity motifs and the induced fit mechanism in class I ketol-acid reductoisomerases. Biochem. J. 2015, 468, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Cahn, J.K.B.; Baumschlager, A.; Brinkmann-Chen, S.; Arnold, F.H. Mutations in adenine-binding pockets enhance catalytic properties of NAD(P)H-dependent enzymes. Protein Eng. Des. Sel. 2016, 29, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Cahn, J.K.B.; Werlang, C.A.; Baumschlager, A.; Brinkmann-Chen, S.; Mayo, S.L.; Arnold, F.H. A General Tool for Engineering the NAD/NADP Cofactor Preference of Oxidoreductases. ACS Synth. Biol. 2017, 6, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Chang, Y.-C.; Lin, B.-L.; Huang, C.-H.; Tsai, M.-D. Temperature-Resolved Cryo-EM Uncovers Structural Bases of Temperature-Dependent Enzyme Functions. J. Am. Chem. Soc. 2019, 141, 19983–19987. [Google Scholar] [CrossRef] [Green Version]
- Huber, H.; Thomm, M.; König, H.; Thies, G.; Stetter, K.O. Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch. Microbiol. 1982, 132, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Wagner, T.; Koch, J.; Ermler, U.; Shima, S. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe-4S] clusters for reduction. Science 2017, 357, 699–703. [Google Scholar] [CrossRef] [Green Version]
- Engilberge, S.; Wagner, T.; Santoni, G.; Breyton, C.; Shima, S.; Franzetti, B.; Riobé, F.; Maury, O.; Girard, E. Protein crystal structure determination with the crystallophore, a nucleating and phasing agent. J. Appl. Cryst. 2019, 52, 722–731. [Google Scholar] [CrossRef]
- Lemaire, O.N.; Infossi, P.; Ali Chaouche, A.; Espinosa, L.; Leimkühler, S.; Giudici-Orticoni, M.T.; Méjean, V.; Iobbi-Nivol, C. Small membranous proteins of the TorE/NapE family, crutches for cognate respiratory systems in Proteobacteria. Sci. Rep. 2018, 8, 13576. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Cryst. D Biol. Cryst. 2011, 67, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkóczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Cryst. D Struct. Biol. 2019, 75, 861–877. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Cryst. D Biol. Cryst. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, V.B.; Arendall, W.B., 3rd; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Cryst. D Biol. Cryst. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [Green Version]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Chunduru, S.K.; Mrachko, G.T.; Calvo, K.C. Mechanism of ketol acid reductoisomerase. Steady-state analysis and metal ion requirement. Biochemistry 1989, 28, 486–493. [Google Scholar] [CrossRef] [PubMed]
| MtKARI Close State | MtKARI Open State | |
|---|---|---|
| Data collection | ||
| Wavelength (Å) | 1.73913 | 0.97916 |
| Space group | I23 | I23 |
| Resolution (Å) | 45.92—2.10 (2.21—2.10) | 46.25—2.20 (2.32—2.20) |
| Cell dimensions: a = b = c (Å) | 129.88 | 130.83 |
| Rmerge (%) a | 9.5 (74.4) | 16.3 (52.8) |
| Rpim (%) a | 2.6 (21.2) | 3.9 (16.6) |
| CC1/2 a | 0.999 (0.468) | 0.998 (0.385) |
| I/σI a | 18.3 (3.5) | 23.7 (4.4) |
| Completeness a | 100 (100) | 100 (100) |
| Redundancy a | 14.3 (13.3) | 18.2 (10.9) |
| Nr. unique reflections a | 21,408 (3,090) | 19,073 (2766) |
| Refinement | ||
| Resolution (Å) | 41.07—2.10 | 41.37—2.20 |
| Twinning fraction and operator | 0.32 (-l,-k,-h) | 0.12 (-l,-k,-h) |
| Number of reflections | 21,408 | 19,073 |
| Rwork/Rfree b (%) | 17.59/18.86 | 18.64/21.23 |
| Number of atoms | ||
| Protein | 2542 | 2536 |
| Ligands/ions | 96 | 49 |
| Solvent | 63 | 140 |
| Mean B-value (Å2) | 45.9 | 30.3 |
| Molprobity clashscore, all atoms | 6.64 | 2.53 |
| Ramachandran plot | ||
| Favored regions (%) | 96.32 | 97.23 |
| Outlier regions (%) | 0 | 0 |
| Rmsd c bond lengths (Å) | 0.009 | 0.006 |
| rmsd c bond angles (°) | 1.15 | 0.82 |
| PDB ID code | 7Q03 | 7Q07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemaire, O.N.; Müller, M.-C.; Kahnt, J.; Wagner, T. Structural Rearrangements of a Dodecameric Ketol-Acid Reductoisomerase Isolated from a Marine Thermophilic Methanogen. Biomolecules 2021, 11, 1679. https://doi.org/10.3390/biom11111679
Lemaire ON, Müller M-C, Kahnt J, Wagner T. Structural Rearrangements of a Dodecameric Ketol-Acid Reductoisomerase Isolated from a Marine Thermophilic Methanogen. Biomolecules. 2021; 11(11):1679. https://doi.org/10.3390/biom11111679
Chicago/Turabian StyleLemaire, Olivier Nicolas, Marie-Caroline Müller, Jörg Kahnt, and Tristan Wagner. 2021. "Structural Rearrangements of a Dodecameric Ketol-Acid Reductoisomerase Isolated from a Marine Thermophilic Methanogen" Biomolecules 11, no. 11: 1679. https://doi.org/10.3390/biom11111679






