Overwinter Changes in the Lipid Profile of Young-of-the-Year Striped Bass (Morone saxatilis) in Freshwater Ponds
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Diet
2.2. Sample Selection for Lipid Analysis
2.3. Lipid Analytical Methods
2.4. Data Display and Statistical Analysis
3. Results
3.1. Survival, Temperature, and Dissolved Oxygen
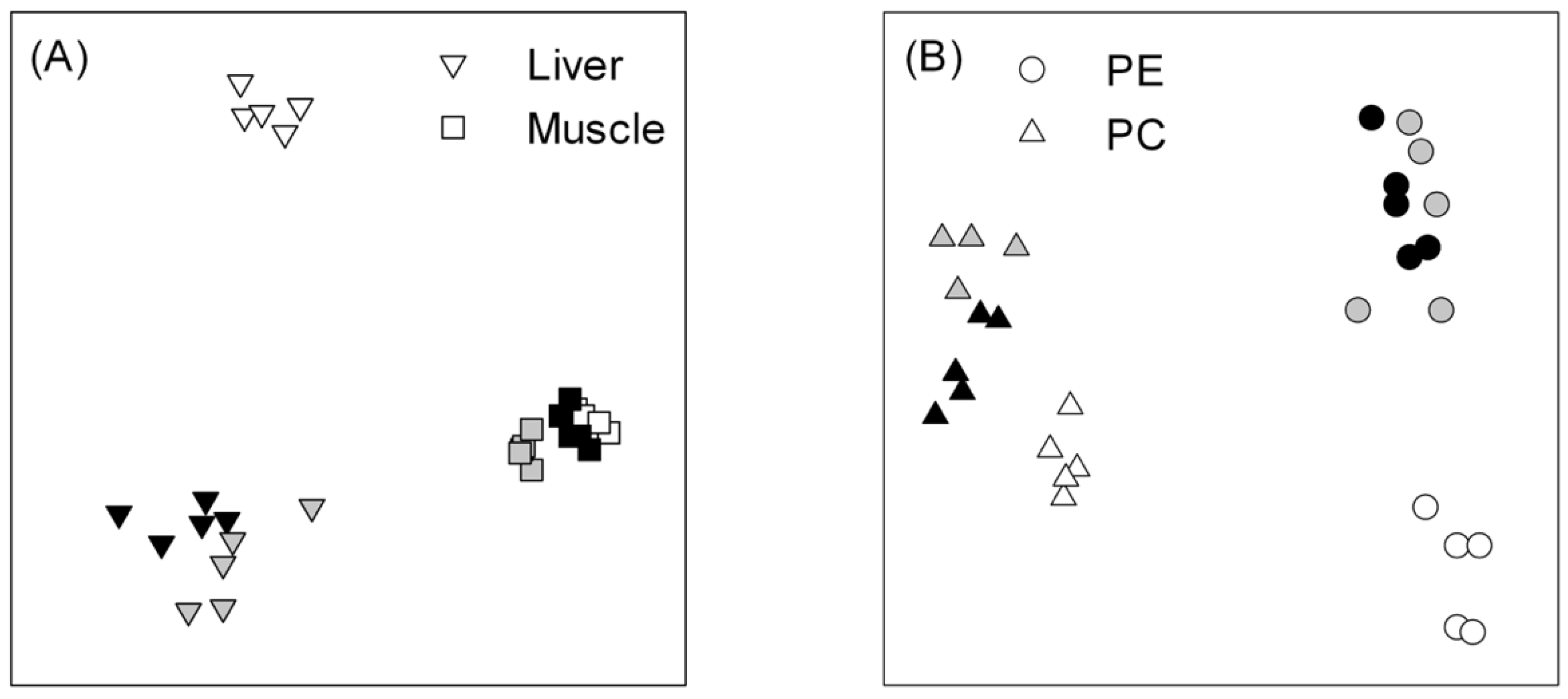
3.2. Total Lipid and Lipid Classes in the Liver and White Muscle Tissues
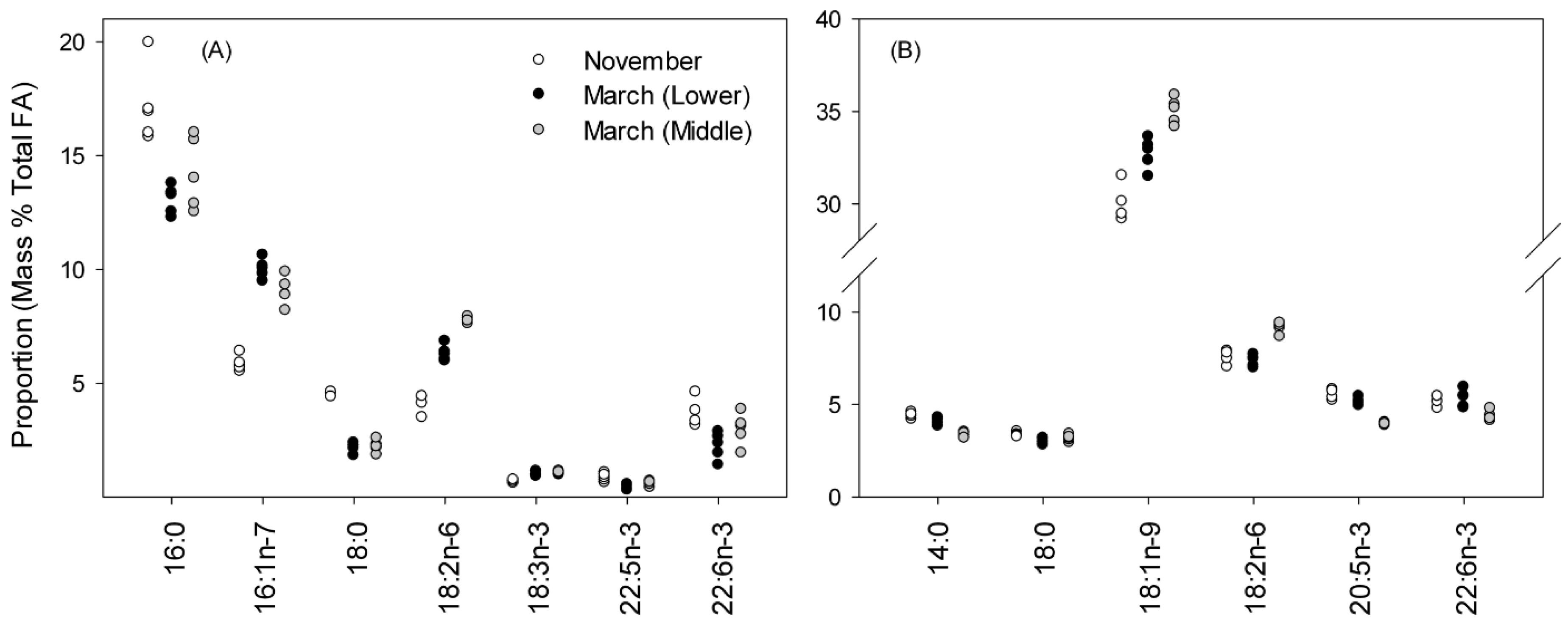
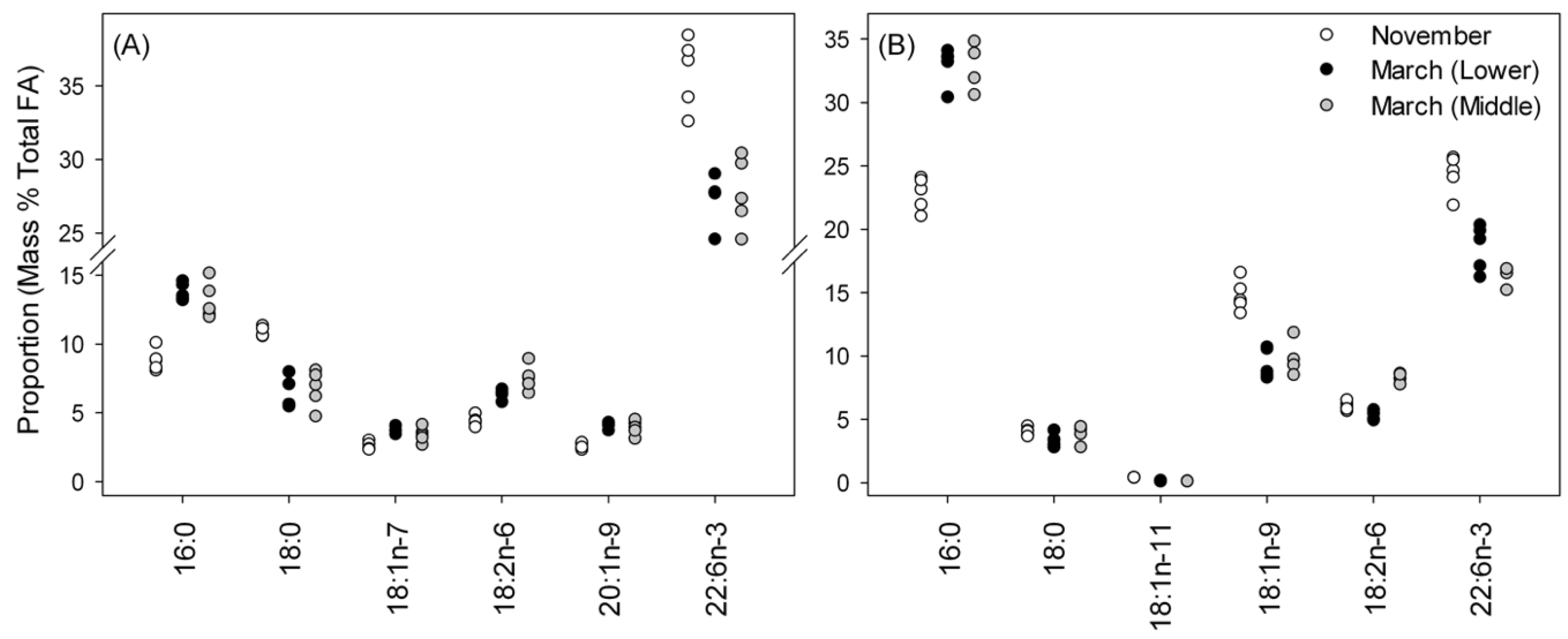
3.3. Fatty-Acid Profiles for Liver and Muscle TAG
3.4. Fatty-Acid Profiles in Phospholipids (PE and PC Classes) in Muscle
4. Discussion
4.1. Thermal Acclimation Overwinter
4.2. Food Scarcity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Post, J.R.; Evans, D.O. Size-Dependent Overwinter Mortality of Young-of-the-Year Yellow Perch (Perca flavescens): Laboratory, In Situ Enclosure, and Field Experiments. Can. J. Fish. Aquat. Sci. 1989, 46, 1958–1968. [Google Scholar] [CrossRef]
- Schultz, E.T.; Conover, D.O. Latitudinal Differences in Somatic Energy Storage: Adaptive Responses to Seasonality in an Estuarine Fish (Atherinidae: Menidia menidia). Oecologia 1997, 109, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Biro, P.A.; Morton, A.E.; Post, J.R.; Parkinson, E.A. Over-Winter Lipid Depletion and Mortality of Age-0 Rainbow Trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 2004, 61, 1513–1519. [Google Scholar] [CrossRef]
- Bradford, R.G.; Chaput, G. Status of Striped Bass (Morone saxatilis) in the Gulf of St. Lawrence in 1996 and Revised Estimates of Spawner Abundance for 1994 and 1995. In Atlantic Fisheries Research Document; Department of Fisheries and Oceans: Ottawa, ON, Canada, 1997. [Google Scholar]
- Hurst, T.P.; Conover, D.O. Winter Mortality of Young-of-the-Year Hudson River Striped Bass (Morone saxatilis): Size-Dependent Patterns and Effects on Recruitment. Can. J. Fish. Aquat. Sci. 1998, 55, 1122–1130. [Google Scholar] [CrossRef]
- Hurst, T.P. Causes and Consequences of Winter Mortality in Fishes. J. Fish Biol. 2007, 71, 315–345. [Google Scholar] [CrossRef]
- Woodhead, P.M.J. The Death of North Sea Fish during the Winter of 1962/63, Particularly with Reference to the Sole, Solea vulgaris. Helgolander Wiss. Meeresunters 1964, 10, 283–300. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Bennett, W.A.; McCauley, R.W. Temperature Tolerances of North American Freshwater Fishes Exposed to Dynamic Changes in Temperature. Environ. Biol. Fish. 2000, 58, 237–275. [Google Scholar] [CrossRef]
- Horning II, W.B.; Pearson, R.E. Growth Temperature Requirements and Lower Lethal Temperatures for Juvenile Smallmouth Bass (Micropterus dolomieui). J. Fish. Res. Board Can. 1973, 30, 1226–1230. [Google Scholar] [CrossRef]
- Thompson, J.M.; Bergersen, E.P.; Carlson, C.A.; Kaeding, L.R. Role of Size, Condition, and Lipid Content in the Overwinter Survival of Age−0 Colorado Squawfish. Trans. Am. Fish. Soc. 1991, 120, 346–353. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The Effect of Temperature and Fish Size on Growth, Feed Intake, Food Conversion Efficiency and Stomach Evacuation Rate of Atlantic Salmon Post-Smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Hurst, T.P.; Schultz, E.T.; Conover, D.O. Seasonal Energy Dynamics of Young-of-the-Year Hudson River Striped Bass. Trans. Am. Fish. Soc. 2000, 129, 145–157. [Google Scholar] [CrossRef]
- Russell, N.R.; Fish, J.D.; Wootton, R.J. Feeding and Growth of Juvenile Sea Bass: The Effect of Ration and Temperature on Growth Rate and Efficiency. J. Fish Biol. 1996, 49, 206–220. [Google Scholar] [CrossRef]
- Bar, N. Physiological and Hormonal Changes during Prolonged Starvation in Fish. Can. J. Fish. Aquat. Sci. 2014, 71, 1447–1458. [Google Scholar] [CrossRef]
- van Dijk, P.L.M.; Hardewig, I.; HOlker, F. Energy Reserves during Food Deprivation and Compensatory Growth in Juvenile Roach: The Importance of Season and Temperature. J. Fish Biol. 2005, 66, 167–181. [Google Scholar] [CrossRef]
- Kooka, K.; Yamamura, O. Winter Energy Allocation and Deficit of Juvenile Walleye Pollock Theragra chalcogramma in the Doto Area, Northern Japan. Environ. Biol. Fish. 2012, 94, 389–402. [Google Scholar] [CrossRef]
- Adams, S.M. Ecological Role of Lipids in the Health and Success of Fish Populations. In Lipids in Freshwater Ecosystems; Arts, M.T., Wainman, B.C., Eds.; Springer: New York, NY, USA, 1999; pp. 132–160. [Google Scholar]
- Bar, N.; Volkoff, H. Adaptation of the Physiological, Endocrine, and Digestive System Functions to Prolonged Food Deprivation in Fish. In Comparative Physiology of Fasting, Starvation, and Food Limitation; McCue, M.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 69–89. ISBN 9783642290565. [Google Scholar]
- Post, J.R.; Parkinson, E.A. Energy Allocation Strategy in Young Fish: Allometry and Survival. Ecology 2001, 82, 1040–1051. [Google Scholar] [CrossRef]
- Hurst, T.P.; Conover, D.O. Seasonal and Interannual Variation in the Allometry of Energy Allocation in Juvenile Striped Bass. Ecology 2003, 84, 3360–3369. [Google Scholar] [CrossRef]
- Griffiths, D.G.; Kirkwood, R.C. Seasonal Variation in Growth, Mortality and Fat Stores of Roach and Perch in Lough Neagh, Northern Ireland. J. Fish Biol. 1995, 47, 537–554. [Google Scholar] [CrossRef]
- Love, R.M. The Chemical Biology of Fishes. 2: Advances 1968–1977; Academic Press: London, UK, 1980; ISBN 9780124558526. [Google Scholar]
- Farkas, T.; Fodor, E.; Kitajka, K.; Halver, J.E. Response of Fish Membranes to Environmental Temperature: Fish Membranes and Temperature. Aquac. Res. 2001, 32, 645–655. [Google Scholar] [CrossRef]
- Dey, I.; Buda, C.; Wiik, T.; Halver, J.E.; Farkas, T. Molecular and Structural Composition of Phospholipid Membranes in Livers of Marine and Freshwater Fish in Relation to Temperature. Proc. Natl. Acad. Sci. USA 1993, 90, 7498–7502. [Google Scholar] [CrossRef]
- Guderley, H. Metabolic Responses to Low Temperature in Fish Muscle. Biol. Rev. 2004, 79, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Hazel, J.R. Thermal Adaptation in Biological Membranes: Is Homeoviscous Adaptation the Explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Wodtke, E. Lipid Adaptation in Liver Mitochondrial Membranes of Carp Acclimated to Different Environmental Temperatures. Biochim. Biophys. Acta—Lipids Lipid Met. 1978, 529, 280–291. [Google Scholar] [CrossRef]
- Scott, W.B.; Scott, M.G. Atlantic fishes of Canada. In Canadian Bulletin of Fisheries and Aquatic Sciences; University of Toronto Press, Canadian Government Publishing Centre: Toronto, ON, Canada, 1988. [Google Scholar]
- Bradford, R.G.; Halfyard, E.A. Overview of 2013 Bay of Fundy Striped Bass Biology and General Status; Department of Fisheries and Oceans Canada, Science Branch, Maritime Region: Dartmouth, NS, Canada, 2015. [Google Scholar]
- Tonning, K.A. Overwinter Mortality of Young-of-the-Year Striped Bass (Morone saxatilis) in Freshwater Ponds in Nova Scotia’. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2019. [Google Scholar]
- Manríquez-Hernández, J.; Breau, H.M.; Duston, J. Acute Toxicity of Salt Cavern Brine on Early Life Stages of Striped Bass (Morone saxatilis). Arch. Environ. Contam. Toxicol. 2020, 78, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Budge, S.M.; Iverson, S.J.; Koopman, H.N. Studying Trophic Ecology in Marine Ecosystems Using Fatty Acids: A Primer on Analysis and Interpretation. Mar. Mamm. Sci. 2006, 22, 759–801. [Google Scholar] [CrossRef]
- Kupke, I.R.; Yamamura, O.; Ohkubo, N.; Honda, S. Quantitative High-Performance Thin-Layer Chromatography of Lipids in Plasma and Liver Homogenates after Direct Application of 0.5 ul Samples to the Silica-Gel Layer. J. Chrom. 1978, 146, 261–271. [Google Scholar] [CrossRef]
- Hilditch, T.P.; Rigg, J.G. 416. Experiments on the Direct Esterification of Higher Fatty Acids with Glycerol and with Ethylene Glycol. J. Chem. Soc. 1935, 1774–1778. [Google Scholar] [CrossRef]
- Schultz, E.T.; Conover, D.O. The Allometry of Energy Reserve Depletion: Test of a Mechanism for Size-Dependent Winter Mortality. Oecologia 1999, 119, 474–483. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Lee, R.E.; Costanzo, J.P. Membrane Adaptation in Phospholipids and Cholesterol in the Widely Distributed, Freeze-Tolerant Wood Frog, Rana sylvatica. J. Comp. Physiol. B 2014, 184, 371–383. [Google Scholar] [CrossRef]
- Buda, C.; Dey, I.; Balogh, N.; Horvath, L.I.; Maderspach, K.; Juhasz, M.; Yeo, Y.K.; Farkas, T. Structural Order of Membranes and Composition of Phospholipids in Fish Brain Cells during Thermal Acclimatization. Proc. Natl. Acad. Sci. USA 1994, 91, 8234–8238. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, S.; Zhou, Y.; Shi, K.; Pan, Z.; Sun, D.; Gao, Q. Temperature-Dependent Fatty Acid Composition Change of Phospholipid in Steelhead Trout (Oncorhynchus mykiss) Tissues. J. Ocean Univ. China 2019, 18, 519–527. [Google Scholar] [CrossRef]
- Bell, M.V.; Henderson, R.J.; Sargent, J.R. The Role of Polyunsaturated Fatty Acids in Fish. Comp. Biochem. Physiol. B 1986, 83, 711–719. [Google Scholar] [CrossRef]
- Brodte, E.; Graeve, M.; Jacob, U.; Knust, R.; Pörtner, H.-O. Temperature-Dependent Lipid Levels and Components in Polar and Temperate Eelpout (Zoarcidae). Fish Physiol. Biochem. 2008, 34, 261–274. [Google Scholar] [CrossRef]
- Hurst, T.P.; Conover, D.O. Diet and Consumption Rates of Overwintering YOY Striped Bass, Morone saxatilis, in the Hudson River. Fish. Bull. 2001, 99, 545–554. [Google Scholar]
- Eckmayer, W.J.; Margraf, F.J. The Influence of Diet, Consumption and Lipid Use on Recruitment of White Bass. Lakes Reserv. Res. Manage. 2004, 9, 133–141. [Google Scholar] [CrossRef]
- Stone, B.B.; Sidell, B.D. Metabolic Responses of Striped Bass (Morone Saxatilis) to Temperature Acclimation. I. Alterations in Carbon Sources for Hepatic Energy Metabolism. J. Exp. Zool. 1981, 218, 371–379. [Google Scholar] [CrossRef]
- Takeuchi, T.; Watanabe, T.; Satoh, S.; Ida, T.; Yaguchi, M. Development of Less-Polluting Diets for Practical Fish Culture. II. Changes in Proximate and Fatty Acid Compositions of Carp Fed Low Protein-High Energy Diets Due to Starvation during Winter. Nippon Suisan Gakkaishi 1987, 53, 1425–1429. [Google Scholar] [CrossRef]
- Kooka, K.; Yamamura, O.; Ohkubo, N.; Honda, S. Winter Lipid Depletion of Juvenile Walleye Pollock (Theragra chalcogramma) in the Doto Area, Northern Japan. J. Fish Biol. 2009, 75, 186–202. [Google Scholar] [CrossRef]
- White, A.M.; Moore, F.D.; Aldridge, N.A.; Loucks, D.M. The Effects of Natural Winter Stresses on the Mortality of the Eastern Gizzard Shad, Dorosoma cepedianum, in Lake Erie; Cleveland Electrical Illuminating Company: Cleveland, OH, USA, 1987; Report 78. [Google Scholar]
- Fetzer, W.W.; Brooking, T.E.; Jackson, J.R.; Rudstam, L.G. Overwinter Mortality of Gizzard Shad: Evaluation of Starvation and Cold Temperature Stress. Trans. Am. Fish. Soc. 2011, 140, 1460–1471. [Google Scholar] [CrossRef]
- Yamada, K. Conversion of Linolenic Acid to Omega-3 Highly Unsaturated Fatty Acids in Marine Fishes and Rainbow Trout. Nippon Suisan Gakkaishi 1980, 46, 1231. [Google Scholar] [CrossRef][Green Version]
- Hixson, S.M.; Parrish, C.C.; Anderson, D.M. Use of Camelina Oil to Replace Fish Oil in Diets for Farmed Salmonids and Atlantic Cod. Aquaculture 2014, 431, 44–52. [Google Scholar] [CrossRef]
- Watanabe, T. Lipid Nutrition in Fish. Comp. Biochem. Phys. B. 1982, 73, 3–15. [Google Scholar] [CrossRef]
- Lall, S.P. Nutrition and health of fish. In Avances en Nutrición Acuícola; Cruz-Suárez, L.E., Ricque-Marie, D., Tapia-Salazar, M., Olvera-Novoa, M.A., y Civera-Cerecedo, R., Eds.; Memorias del V Simposium Internacional de Nutrición Acuícola: Mérida, Mexico, 2000; pp. 19–22. [Google Scholar]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Webster, C.D.; Lovell, R.T. Response of Striped Bass Larvae Fed Brine Shrimp from Different Sources Containing Different Fatty Acid Compositions. Aquaculture 1990, 90, 49–61. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty Acid Requirements in Ontogeny of Marine and Freshwater Fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Clawson, J.A.; Lovell, R. Improvement of Nutritional Value of Artemia for Hybrid Striped Bass/White Bass (Morone saxatilis × M. chrysops) Larvae by n-3 HUFA Enrichment of Nauplii with Menhaden Oil. Aquaculture 1992, 108, 125–134. [Google Scholar] [CrossRef]
- Nematipour, G.R.; Gatlin, D.M. Effects of Different Lipid Sources on Growth and Fatty Acid Composition of Juvenile Sunshine Bass (Morone chrysops × M. saxatilis). Aquaculture 1993, 114, 141–154. [Google Scholar] [CrossRef]
- Nematipour, G.R.; Gatlin, D.M. Requirement of Hybrid Striped Bass for Dietary (n-3) Highly Unsaturated Fatty Acids. J. Nutr. 1993, 123, 744–753. [Google Scholar] [CrossRef]
- Barry, K. Re-evaluating Essential Fatty Acid Requirements in Hybrid Striped Bass Morone chrysops × M. saxatilis and Rainbow Trout Oncorhynchus mykiss. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2017. [Google Scholar]
- Barry, K.J.; Trushenski, J.T. Reevaluating Polyunsaturated Fatty Acid Essentiality in Hybrid Striped Bass. N. Am. J. Aquac. 2020, 82, 307–320. [Google Scholar] [CrossRef]
| Ingredient Name | g Per kg |
|---|---|
| Fish Meal | 500 |
| Empyreal | 125 |
| Blood Meal | 111.5 |
| Ground Wheat | 100 |
| Fat | 80 |
| Wheat Gluten | 50 |
| Dicalcium Phosphate | 20.5 |
| Lysine HCI | 5 |
| Choline Chloride | 3 |
| Vitamin/Mineral Premix 1 | 2.5 |
| Special Premix 2 | 2.5 |
| Total | 1000 |
| Sample | November 2016 | March 2017 (Lower Pond) | March 2017 (Middle Pond) |
|---|---|---|---|
| Total Lipid-Liver | 236 ± 9 a | 201 ± 33 a | 213 ± 24 a |
| Total Lipid-Muscle | 16 ± 2 a | 51 ± 9 b | 39 ± 9 b |
| Liver TAG | 182 ± 9 a | 191 ± 35 a | 202 ± 16 a |
| Muscle TAG | 4 ± 1 a | 21 ± 6 b | 14 ± 5 b |
| Muscle PE | 2 ± 0.4 a | 4 ± 0.3 b | 3 ± 0.7 b |
| Muscle PC | 1 ± 1 a | 2 ± 0.2 a | 1 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonning, K.A.; Budge, S.M.; Tyedmers, P. Overwinter Changes in the Lipid Profile of Young-of-the-Year Striped Bass (Morone saxatilis) in Freshwater Ponds. Biomolecules 2021, 11, 1678. https://doi.org/10.3390/biom11111678
Tonning KA, Budge SM, Tyedmers P. Overwinter Changes in the Lipid Profile of Young-of-the-Year Striped Bass (Morone saxatilis) in Freshwater Ponds. Biomolecules. 2021; 11(11):1678. https://doi.org/10.3390/biom11111678
Chicago/Turabian StyleTonning, Kare A., Suzanne M. Budge, and Peter Tyedmers. 2021. "Overwinter Changes in the Lipid Profile of Young-of-the-Year Striped Bass (Morone saxatilis) in Freshwater Ponds" Biomolecules 11, no. 11: 1678. https://doi.org/10.3390/biom11111678
APA StyleTonning, K. A., Budge, S. M., & Tyedmers, P. (2021). Overwinter Changes in the Lipid Profile of Young-of-the-Year Striped Bass (Morone saxatilis) in Freshwater Ponds. Biomolecules, 11(11), 1678. https://doi.org/10.3390/biom11111678





