Recent Advances in Detection, Isolation, and Imaging Techniques for Sulfane Sulfur-Containing Biomolecules
Abstract
:1. Introduction
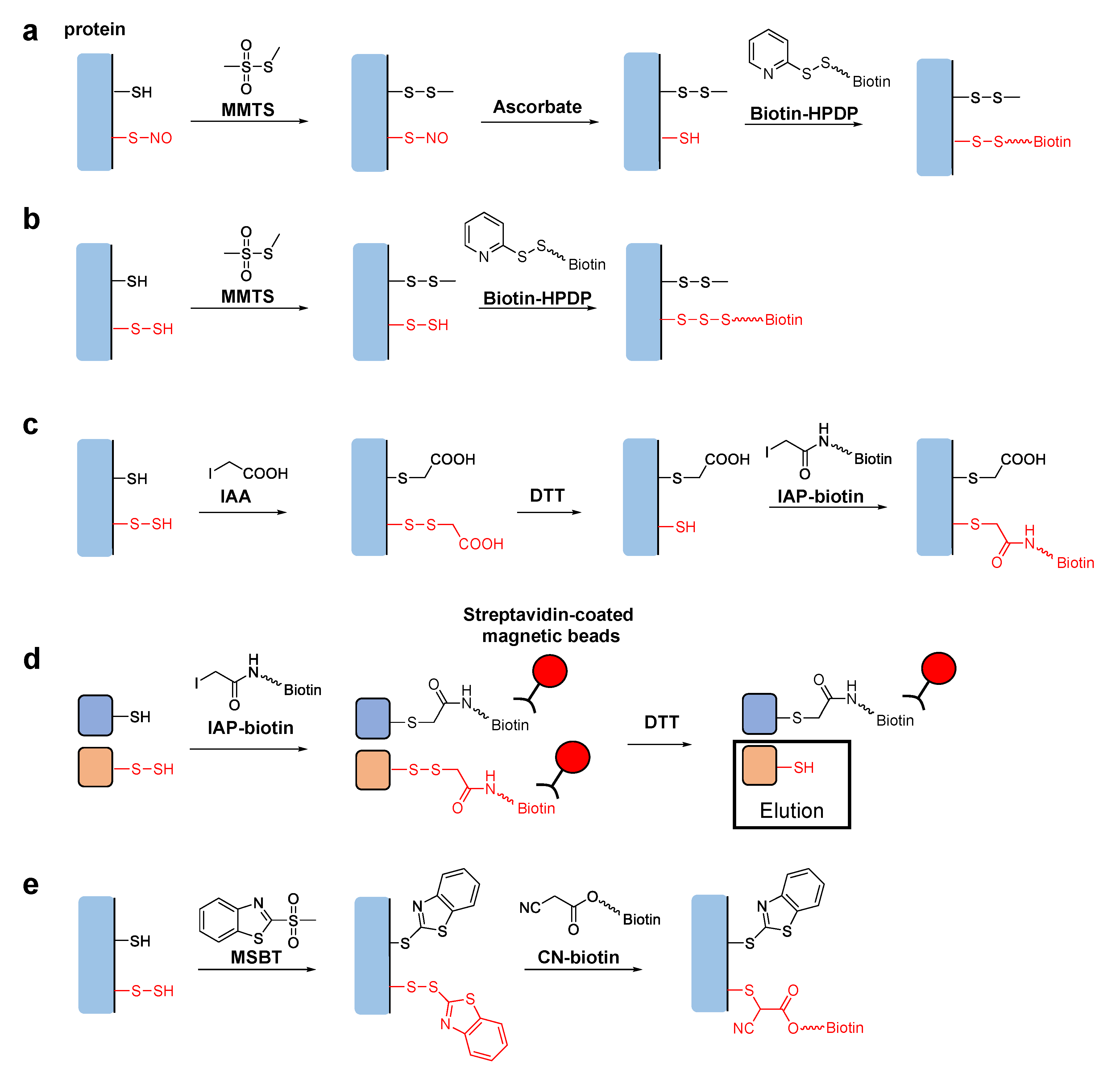
Tag-Switch Techniques
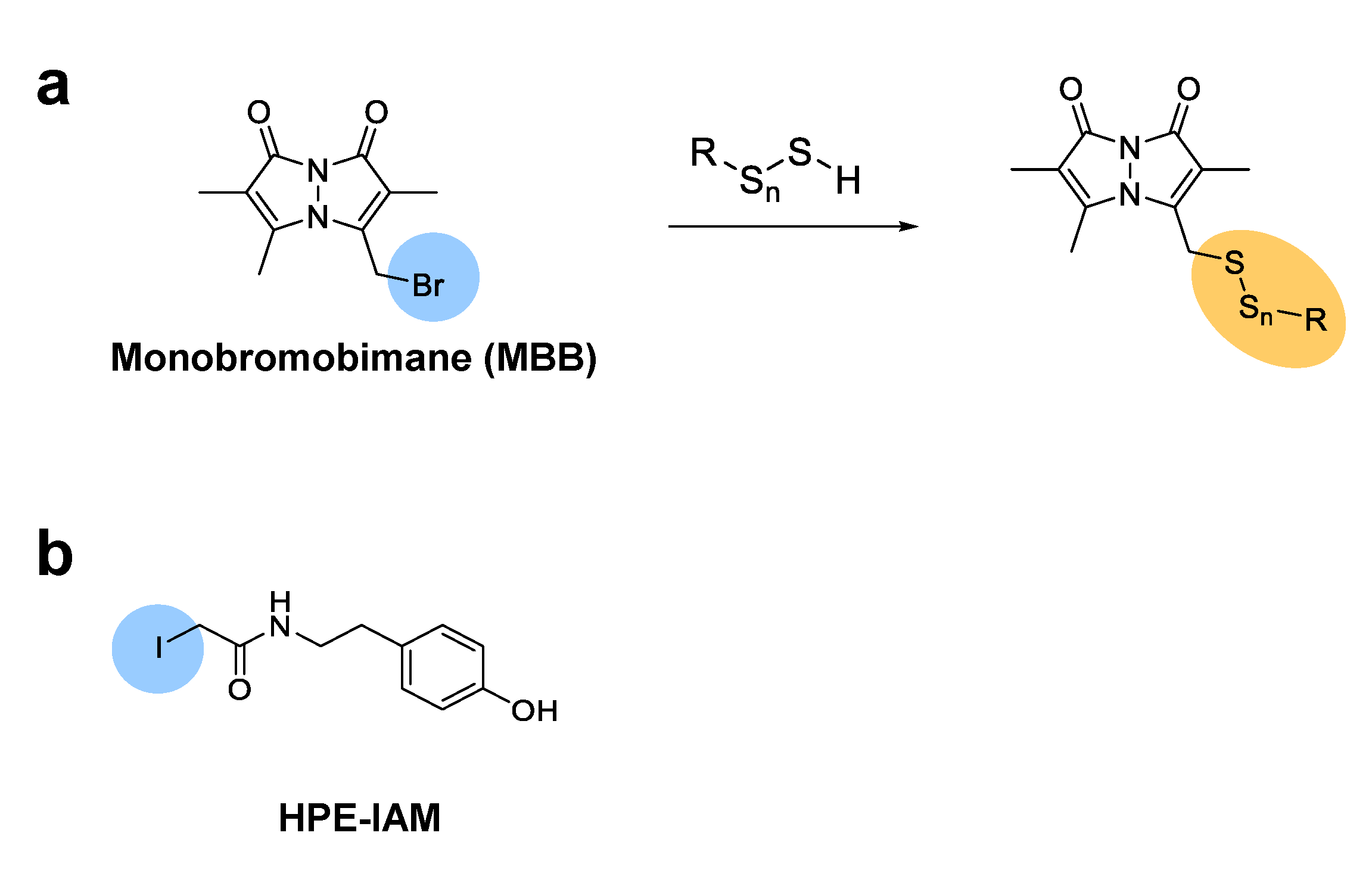
2. LC-MS/MS
3. Raman Imaging
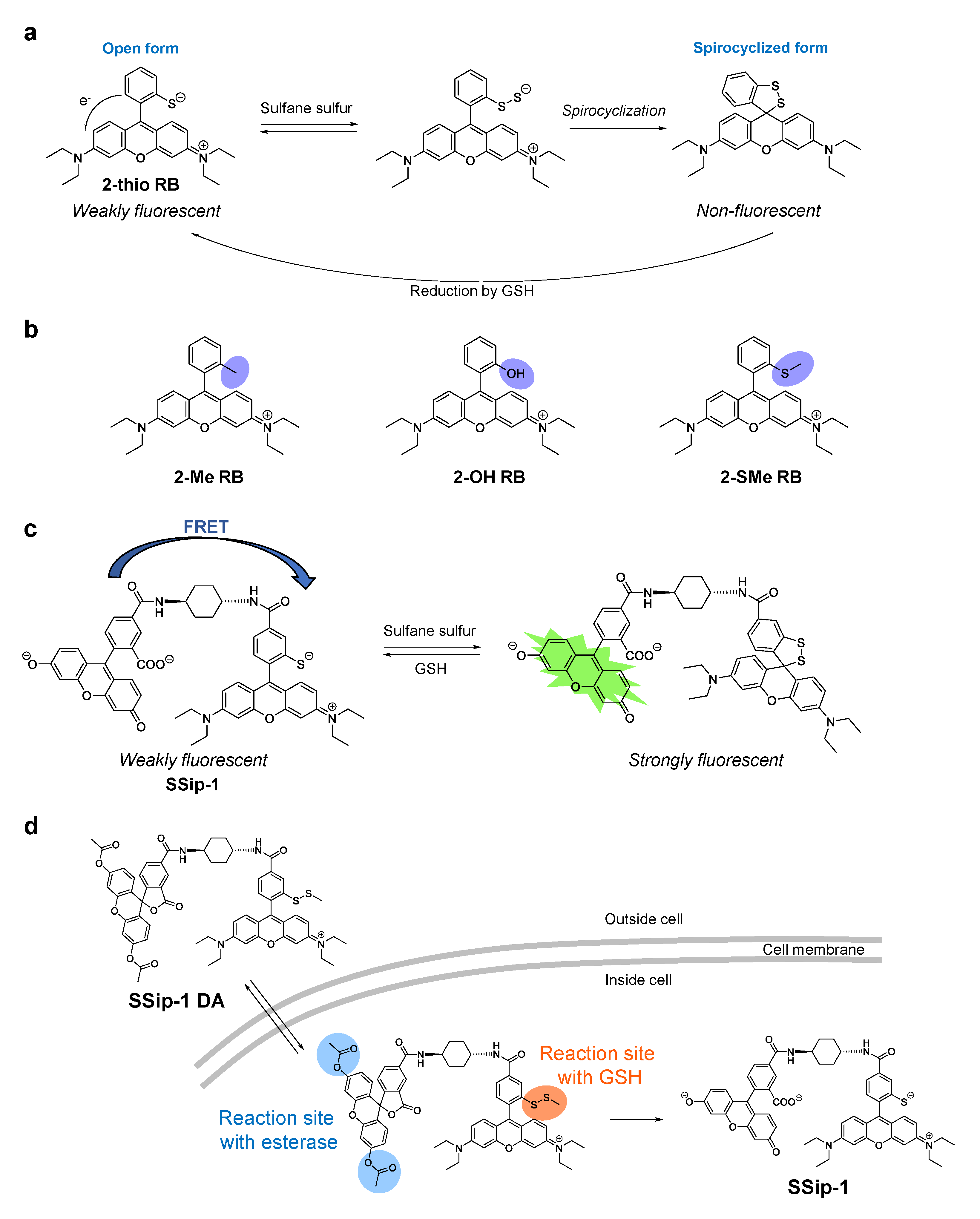
4. Fluorescent Probes
5. Conclusions
Funding
Conflicts of Interest
References
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yu, B.; De La Cruz, L.K.; Roy Choudhury, M.; Anifowose, A.; Wang, B. Toward Hydrogen Sulfide Based Therapeutics: Critical Drug Delivery and Developability Issues. Med. Res. Rev. 2018, 38, 57–100. [Google Scholar] [CrossRef]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, G.I.; Nasim, M.J.; Ali, W.; Jacob, C. The reactive sulfur species concept: 15 years on. Antioxidants 2017, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.L. Sulfane Sulfur. Methods Enzymol. 1987, 143, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Sulphane sulphur in biological systems: A possible regulatory role. Biochem. J. 1989, 264, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toohey, J.I. Sulfur signaling: Is the agent sulfide or sulfane? Anal. Biochem. 2011, 413, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I.; Cooper, A.J.L. Thiosulfoxide (Sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef] [Green Version]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, R.; Koike, S.; Takano, Y.; Shibuya, N.; Kimura, Y.; Hanaoka, K.; Urano, Y.; Ogasawara, Y.; Kimura, H. Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci. Rep. 2017, 7, 45995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, M.; Ohnishi, T.; Toyoshima, M.; Balan, S.; Maekawa, M.; Shimamoto-Mitsuyama, C.; Iwayama, Y.; Ohba, H.; Watanabe, A.; Ishii, T.; et al. Excess hydrogen sulfide and polysulfides production underlies a schizophrenia pathophysiology. EMBO Mol. Med. 2019, 11, e10695. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Weerasinghe, L.; Day, J.J.; Fukuto, J.M.; Xian, M. Persulfides: Current knowledge and challenges in chemistry and chemical biology. Mol. Biosyst. 2015, 11, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef]
- Forrester, M.T.; Foster, M.W.; Benhar, M.; Stamler, J.S. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic. Biol. Med. 2009, 46, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. HS signals through protein S-Sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Carroll, K.S. Persulfide reactivity in the detection of protein S-sulfhydration. ACS Chem. Biol. 2013, 8, 1110–1116. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, N.; Fu, C.; Pappin, D.J.; Tonks, N.K. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011, 4, ra86. [Google Scholar] [CrossRef] [Green Version]
- Dóka, É.; Pader, I.; Bíró, A.; Johansson, K.; Cheng, Q.; Ballagó, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; MacInkovic, I.; Devarie-Baez, N.O.; Pan, J.; Park, C.M.; Carroll, K.S.; Filipovic, M.R.; Xian, M. Detection of protein S-sulfhydration by a tag-switch technique. Angew. Chem. Int. Ed. 2014, 53, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Devarie-Baez, N.O.; Li, Q.; Lancaster, J.R.; Xian, M. Methylsulfonyl benzothiazole (MSBT): A selective protein thiol blocking reagent. Org. Lett. 2012, 14, 3396–3399. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Wedmann, R.; Onderka, C.; Wei, S.; Szijártó, I.A.; Miljkovic, J.L.; Mitrovic, A.; Lange, M.; Savitsky, S.; Yadav, P.K.; Torregrossa, R.; et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem. Sci. 2016, 7, 3414–3426. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Radford, M.N.; Yang, C.; Chen, W.; Xian, M. Inorganic hydrogen polysulfides: Chemistry, chemical biology and detection. Br. J. Pharmacol. 2019, 176, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Bogdándi, V.; Ida, T.; Sutton, T.R.; Bianco, C.; Ditrói, T.; Koster, G.; Henthorn, H.A.; Minnion, M.; Toscano, J.P.; van der Vliet, A.; et al. Speciation of reactive sulfur species and their reactions with alkylating agents: Do we have any clue about what is present inside the cell? Br. J. Pharmacol. 2019, 176, 646–670. [Google Scholar] [CrossRef] [Green Version]
- Nagy, P.; Pálinkás, Z.; Nagy, A.; Budai, B.; Tóth, I.; Vasas, A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim. Biophys. Acta 2014, 1840, 876–891. [Google Scholar] [CrossRef] [PubMed]
- Numakura, T.; Sugiura, H.; Akaike, T.; Ida, T.; Fujii, S.; Koarai, A.; Yamada, M.; Onodera, K.; Hashimoto, Y.; Tanaka, R.; et al. Production of reactive persulfide species in chronic obstructive pulmonary disease. Thorax 2017, 72, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sawa, T.; Motohashi, H.; Ihara, H.; Akaike, T. Enzymatic regulation and biological functions of reactive cysteine persulfides and polysulfides. Biomolecules 2020, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, S.; Yamashina, M.; Sei, Y.; Akita, M.; Kuzume, A.; Yamamoto, K.; Yoshizawa, M. Exact mass analysis of sulfur clusters upon encapsulation by a polyaromatic capsular matrix. Nat. Commun. 2017, 8, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Li, L.; Tian, Y. A Single Nanoprobe for Ratiometric Imaging and Biosensing of Hypochlorite and Glutathione in Live Cells Using Surface-Enhanced Raman Scattering. Anal. Chem. 2016, 88, 9518–9523. [Google Scholar] [CrossRef]
- Banerjee, H.; Verma, M. Intraoperative brain cancer detection with Raman spectroscopy in humans. Ann. Transl. Med. 2016, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Naya, M.; Yamamoto, T.; Hishiki, T.; Tani, T.; Takahashi, H.; Kubo, A.; Koike, D.; Itoh, M.; Ohmura, M.; et al. Gold-nanofève surface-enhanced Raman spectroscopy visualizes hypotaurine as a robust anti-oxidant consumed in cancer survival. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Hishiki, T.; Yamamoto, S.; Yamamoto, T.; Miura, N.; Kubo, A.; Itoh, M.; Chen, W.Y.; Takano, M.; Yoshikawa, T.; et al. On-tissue polysulfide visualization by surface-enhanced Raman spectroscopy benefits patients with ovarian cancer to predict post-operative chemosensitivity. Redox Biol. 2021, 41, 101926. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.; Zhang, X.; Chen, Z.; Zhao, R.; Hou, N.; Liu, J.; Xun, L.; Liu, H. Developing polysulfide-sensitive gfps for real-time analysis of polysulfides in live cells and subcellular organelles. Anal. Chem. 2019, 91, 3893–3901. [Google Scholar] [CrossRef]
- Chen, W.; Liu, C.; Peng, B.; Zhao, Y.; Pacheco, A.; Xian, M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem. Sci. 2013, 4, 2892–2896. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.K.; Martinov, M.; Vitvitsky, V.; Seravalli, J.; Wedmann, R.; Filipovic, M.R.; Banerjee, R. Biosynthesis and Reactivity of Cysteine Persulfides in Signaling. J. Am. Chem. Soc. 2016, 138, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Toyofuku, Y.; Koike, S.; Shibuya, N.; Nagahara, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Takano, Y.; Hanaoka, K.; Shimamoto, K.; Miyamoto, R.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T.; Urano, Y. Development of a reversible fluorescent probe for reactive sulfur species, sulfane sulfur, and its biological application. Chem. Commun. 2017, 53, 1064–1067. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, W.; Shi, W.; Peng, B.; Zhao, Y.; Ma, H.; Xian, M. Rational design and bioimaging applications of highly selective fluorescence probes for hydrogen polysulfides. J. Am. Chem. Soc. 2014, 136, 7257–7260. [Google Scholar] [CrossRef]
- Chen, W.; Rosser, E.W.; Matsunaga, T.; Pacheco, A.; Akaike, T.; Xian, M. The Development of Fluorescent Probes for Visualizing Intracellular Hydrogen Polysulfides. Angew. Chem. Int. Ed. 2015, 54, 13961–13965. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Urano, Y.; Kamiya, M.; Kanda, K.; Ueno, T.; Hirose, K.; Nagano, T. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J. Am. Chem. Soc. 2005, 127, 4888–4894. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Sasakura, K.; Suwanai, Y.; Toma-Fukai, S.; Shimamoto, K.; Takano, Y.; Shibuya, N.; Terai, T.; Komatsu, T.; Ueno, T.; et al. Discovery and Mechanistic Characterization of Selective Inhibitors of H2S-producing Enzyme: 3-Mercaptopyruvate Sulfurtransferase (3MST) Targeting Active-site Cysteine Persulfide. Sci. Rep. 2017, 7, 40227. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echizen, H.; Sasaki, E.; Hanaoka, K. Recent Advances in Detection, Isolation, and Imaging Techniques for Sulfane Sulfur-Containing Biomolecules. Biomolecules 2021, 11, 1553. https://doi.org/10.3390/biom11111553
Echizen H, Sasaki E, Hanaoka K. Recent Advances in Detection, Isolation, and Imaging Techniques for Sulfane Sulfur-Containing Biomolecules. Biomolecules. 2021; 11(11):1553. https://doi.org/10.3390/biom11111553
Chicago/Turabian StyleEchizen, Honami, Eita Sasaki, and Kenjiro Hanaoka. 2021. "Recent Advances in Detection, Isolation, and Imaging Techniques for Sulfane Sulfur-Containing Biomolecules" Biomolecules 11, no. 11: 1553. https://doi.org/10.3390/biom11111553
APA StyleEchizen, H., Sasaki, E., & Hanaoka, K. (2021). Recent Advances in Detection, Isolation, and Imaging Techniques for Sulfane Sulfur-Containing Biomolecules. Biomolecules, 11(11), 1553. https://doi.org/10.3390/biom11111553




