Natural Products Are a Promising Source for Anthelmintic Drug Discovery
Abstract
1. Introduction
1.1. Parasitic Infections in Humans
1.2. Parasitic Infections in Animals
2. Anthelminthics
Anthelmintic Resistance
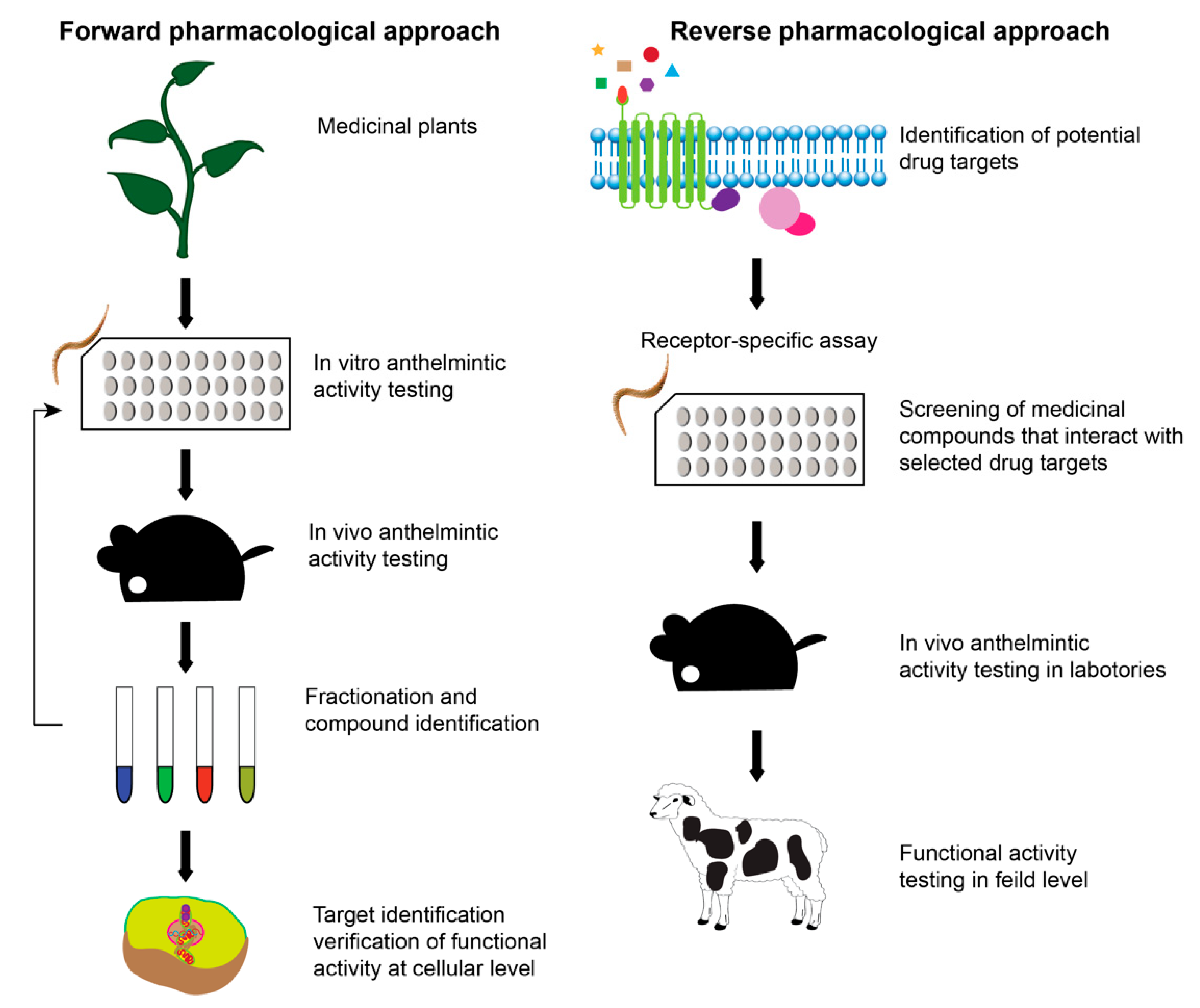
3. Plant Extracts as a Source of New Anthelmintics Compounds
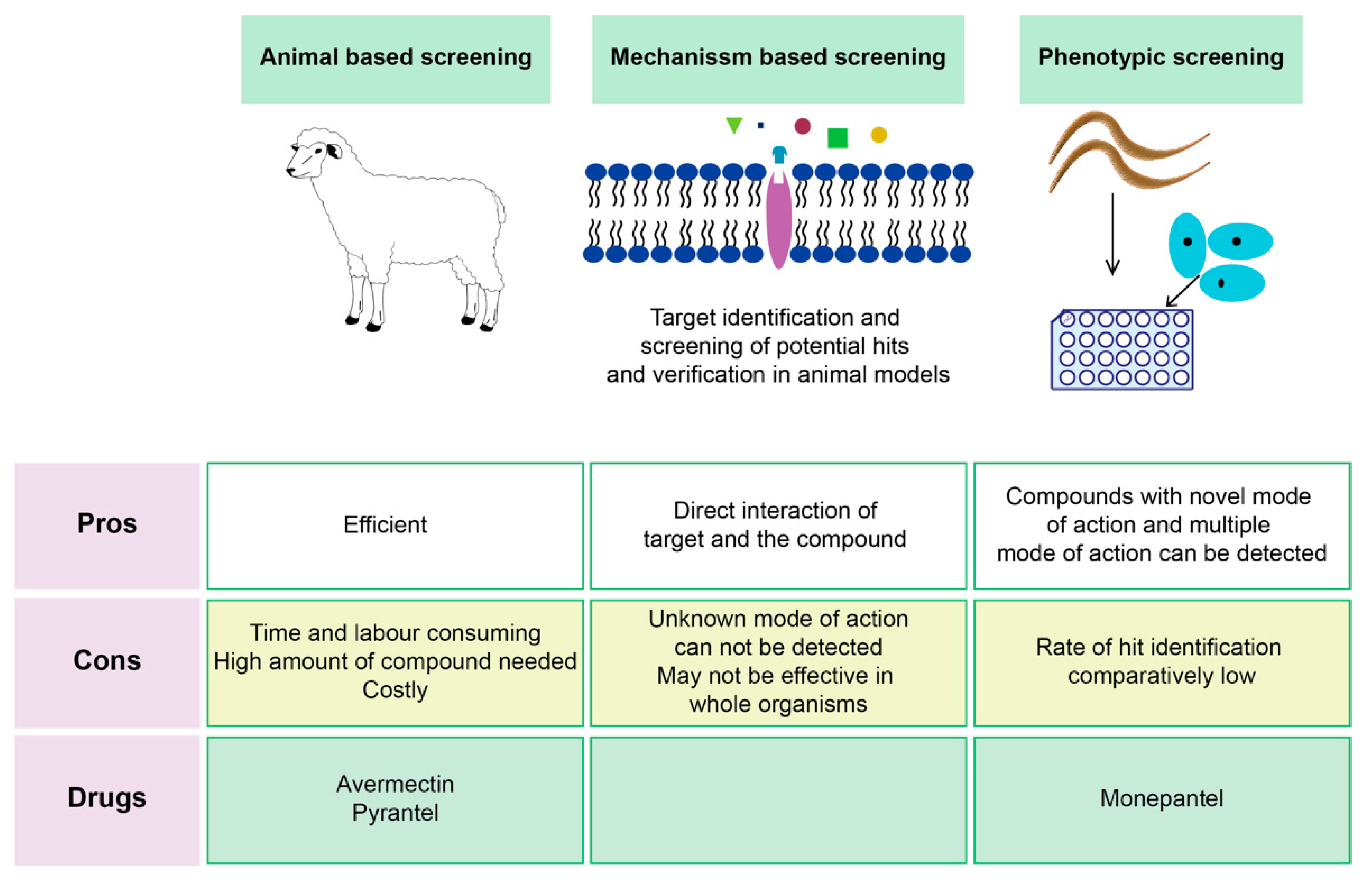
Screening Techniques of Plant-Based Compounds
4. Examples of Anthelmintic Drugs from Plants or Plant Extracts
5. Challenges in Natural Product Based Anthelmintic Drug Discovery
6. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaltz, O.; A Shykoff, J. Local adaptation in host–parasite systems. Heredity 1998, 81, 361–370. [Google Scholar] [CrossRef]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Mukherjee, N.; Mukherjee, S.; Saini, P.; Roy, P.; Babu, S.P.S. Phenolics and Terpenoids; the Promising New Search for Anthelmintics: A Critical Review. Mini-Rev. Med. Chem. 2016, 16, 1415–1441. [Google Scholar] [CrossRef] [PubMed]
- King, C.H. Health metrics for helminth infections. Acta Trop. 2013, 141, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- Coombs, I.; Crompton, D. A Guide to Human Helminths; Taylor & Francis: London, UK, 1991. [Google Scholar]
- Herricks, J.R.; Hotez, P.J.; Wanga, V.; Coffeng, L.E.; Haagsma, J.A.; Basáñez, M.G.; Buckle, G.; Budke, C.M.; Carabin, H.; Fevre, E.M.; et al. The global burden of disease study 2013: What does it mean for the NTDs? PLoS Negl. Trop. Dis. 2017, 11, e0005424. [Google Scholar] [CrossRef]
- Adamson, M.L.; Anderson, R.C. Nematode Parasites of Vertebrates. Their Development and Transmission. J. Parasitol. 1993, 79, 634. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Feasey, N.; Wansbrough-Jones, M.; Mabey, D.C.; Solomon, A.W. Neglected tropical diseases. Br. Med. Bull. 2010, 93, 179–200. [Google Scholar] [CrossRef]
- Wink, M. Medicinal Plants: A Source of Anti-Parasitic Secondary Metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef]
- Chesnais, C.B.; Takougang, I.; Paguélé, M.; Pion, S.D.; Boussinesq, M. Excess mortality associated with loiasis: A retrospective population-based cohort study. Lancet Infect. Dis. 2016, 17, 108–116. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Wendt, S.; Trawinski, H.; Schubert, S.; Rodloff, A.C.; Mössner, J.; Lübbert, C. The Diagnosis and Treatment of Pinworm Infection. Dtsch. Aerzteblatt. Online 2019, 116, 213–219. [Google Scholar] [CrossRef]
- Li, H.-M.; Zhou, C.-H.; Li, Z.-S.; Deng, Z.-H.; Ruan, C.-W.; Zhang, Q.-M.; Zhu, T.-J.; Xu, L.-Q.; Chen, Y.-D. Risk factors for Enterobius vermicularis infection in children in Gaozhou, Guangdong, China. Infect. Dis. Poverty 2015, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, C. Dracunculiasis (guinea worm disease). Can. Med. Assoc. J. 2004, 170, 495–500. [Google Scholar]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.; Loukas, A.; Diemert, D.; Hotez, P. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Ben Beard, C. Forgotten People, Forgotten Diseases: The Neglected Tropical Diseases and Their Impact on Global Health and Development. Emerg. Infect. Dis. 2009, 15, 510–511. [Google Scholar] [CrossRef]
- Mathers, C.D.; Ezzati, M.; Lopez, A.D. Measuring the Burden of Neglected Tropical Diseases: The Global Burden of Disease Framework. PLOS Neglected Trop. Dis. 2007, 1, e114. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J. Forgotten People, Forgotten Diseases; George Washington University; Sabin Vaccine Institute; ASM Press: Washington, DC, USA, 2008. [Google Scholar]
- Nwaka, S.; Hudson, A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006, 5, 941–955. [Google Scholar] [CrossRef]
- Simon, G. Impacts of neglected tropical disease on incidence and progression of HIV/AIDS, tuberculosis, and malaria: Scientific links. Int. J. Infect. Dis. 2016, 42, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mukherjee, N.; Saini, P.; Gayen, P.; Roy, P.; Babu, S.P.S. Molecular evidence on the occurrence of co-infection with Pichia guilliermondii and Wuchereria bancrofti in two filarial endemic districts of India. Infect. Dis. Poverty 2014, 3, 13. [Google Scholar] [CrossRef]
- Upton, M. The Role of Livestock in Economic Development and Poverty Reduction. Anim. Prod. 2004, 1–66. [Google Scholar]
- Herrero, M.; Havlik, P.; Valin, H.; Notenbaert, A.M.O.; Rufino, M.; Thornton, P.K.; Blümmel, M.; Weiss, F.; Grace, D.; Obersteiner, M. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20888–20893. [Google Scholar] [CrossRef]
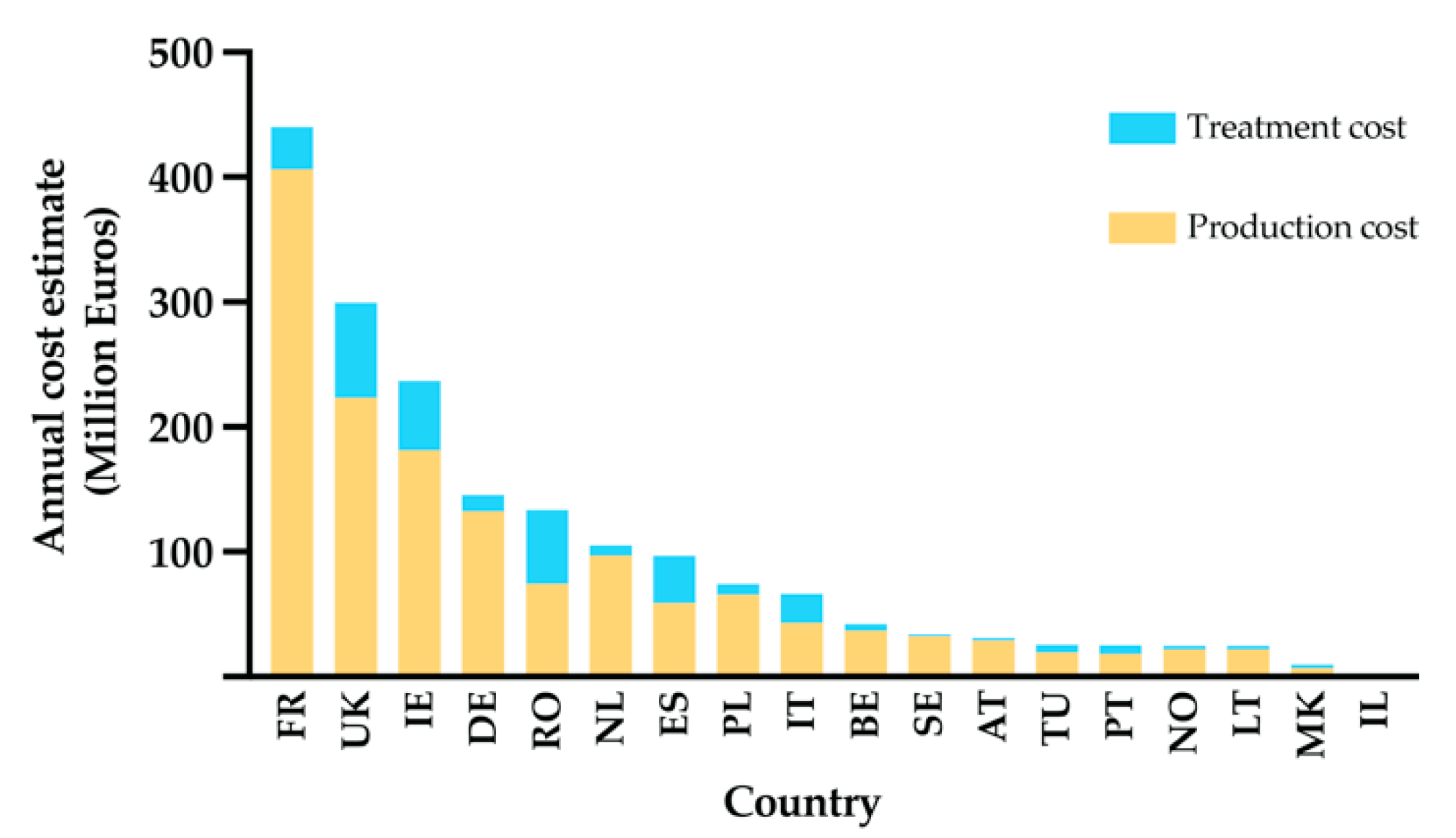
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Veter. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Perry, B.D.; Randolph, T.F. Improving the assessment of the economic impact of parasitic diseases and of their control in produc-tion animals. Vet. Parasitol. 1999, 84, 145–168. [Google Scholar] [CrossRef]
- Grisi, L.; Leite, R.C.; Martins, J.R.D.S.; De Barros, A.T.M.; Andreotti, R.; Cançado, P.H.D.; De León, A.A.P.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Veterinária 2014, 23, 150–156. [Google Scholar] [CrossRef]
- Sackett, D.; Holmes, P.; Abbott, K.; Jephcott, S.; Barber, M. Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers. MLA Rep. AHW 2006, 87. [Google Scholar]
- Sharma, R.; Praveen, P.K. Most prevalent endoparasitic infestation in domestic ruminants and their management in field condition in Indian scenario: A review. Int. J. Environ. Sci. Technol. 2017, 6, 210–216. [Google Scholar]
- Matthews, J.B.; Geldhof, P.; Tzelos, T.; Claerebout, E. Progress in the development of subunit vaccines for gastrointestinal nematodes of ruminants. Parasite Immunol. 2016, 38, 744–753. [Google Scholar] [CrossRef] [PubMed]
- McKellar, Q.A.; Jackson, F. Veterinary anthelmintics: Old and new. Trends Parasitol. 2004, 20, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Swales, W. Tests of Phenothiazine, A Highly Efficient Anthelmintic. Can. J. Comp. Med. 1936, 3, 188–197. [Google Scholar]
- Geary, T.G.; Thompson, D.P. Development of antiparasitic drugs in the 21st century. Veter. Parasitol. 2003, 115, 167–184. [Google Scholar] [CrossRef]
- Ducray, P.; Gauvry, N.; Pautrat, F.; Goebel, T.; Fruechtel, J.; Desaules, Y.; Weber, S.S.; Bouvier, J.; Wagner, T.; Froelich, O.; et al. Discovery of amino-acetonitrile derivatives, a new class of synthetic anthelmintic compounds. Bioorganic Med. Chem. Lett. 2008, 18, 2935–2938. [Google Scholar] [CrossRef] [PubMed]
- Holden-Dye, L. Anthelmintic drugs. WormBook 2007, 44, 1–13. [Google Scholar] [CrossRef]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R. A review on anthelmintic drugs and their future scope. Int. J. Pharm. Pharm. Sci. 2011, 3, 17–21. [Google Scholar]
- Köhler, P. The biochemical basis of anthelmintic action and resistance. Int. J. Parasitol. 2001, 31, 336–345. [Google Scholar] [CrossRef]
- French, K.E. Plant-Based Solutions to Global Livestock Anthelmintic Resistance. Ethnobiol. Lett. 2018, 9, 110–123. [Google Scholar] [CrossRef]
- Geary, T.G. Mechanism-Based Screening Strategies for Anthelmintic Discovery. In Parasitic Helminths: Targets, Screens, Drugs and Vaccines; Wiley: Hoboken, NJ, USA, 2012; pp. 121–134. [Google Scholar] [CrossRef]
- Wagil, M.; Białk-Bielińska, A.; Puckowski, A.; Wychodnik, K.; Maszkowska, J.; Mulkiewicz, E.; Kumirska, J.; Stepnowski, P.; Stolte, S. Toxicity of anthelmintic drugs (fenbendazole and flubendazole) to aquatic organisms. Environ. Sci. Pollut. Res. 2014, 22, 2566–2573. [Google Scholar] [CrossRef]
- Hrckova, G.; Velebny, S. Pharmacological Potential of Selected Natural Compounds in the Control of Parasitic Diseases; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Kaminsky, R.; Ducray, P.; Jung, M.; Clover, R.; Rufener, L.; Bouvier, J.; Weber, S.S.; Wenger, A.; Wieland-Berghausen, S.; Goebel, T.; et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature 2008, 452, 176–180. [Google Scholar] [CrossRef]
- Bossche, H.V. How anthelmintics help us to understand helminths. Parasitology 1985, 90, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Knowles, C.O.; Casida, J.E. Mode of Action of Organophosphate Anthelmintics. Cholinesterase Inhibition in Ascaris lumbricoides. J. Agric. Food Chem. 1966, 14, 566–572. [Google Scholar] [CrossRef]
- Sargison, N. Pharmaceutical treatments of gastrointestinal nematode infections of sheep—Future of anthelmintic drugs. Veter. Parasitol. 2012, 189, 79–84. [Google Scholar] [CrossRef]
- Harnett, W. The anthelmintic action of praziquantel. Parasitol. Today 1988, 4, 144–146. [Google Scholar] [CrossRef]
- Martin, R.J.; Robertson, A.P.; Bjorn, H. Target sites of anthelmintics. Parasitology 1997, 114, 111–124. [Google Scholar] [CrossRef]
- Laudisi, F.; Marônek, M.; Di Grazia, A.; Monteleone, G.; Stolfi, C. Repositioning of Anthelmintic Drugs for the Treatment of Cancers of the Digestive System. Int. J. Mol. Sci. 2020, 21, 4957. [Google Scholar] [CrossRef]
- De Clercq, D.; Dorny, P.; Behnke, J.; Sacko, M.; Vercruysse, J.; Gilbert, F. Failure of Mebendazole in Treatment of Human Hookworm Infections in the Southern Region of Mali. Am. J. Trop. Med. Hyg. 1997, 57, 25–30. [Google Scholar] [CrossRef] [PubMed]
- A Reynoldson, J.; Behnke, J.M.; Pallant, L.J.; Macnish, M.G.; Gilbert, F.; Giles, S.; Spargo, R.; Thompson, R.A. Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of North West Australia. Acta Trop. 1997, 68, 301–312. [Google Scholar] [CrossRef]
- Geerts, S.; Gryseels, B. Anthelmintic resistance in human helminths: A review. Trop. Med. Int. Heal. 2001, 6, 915–921. [Google Scholar] [CrossRef]
- Shoop, W. Ivermectin resistance. Parasitol. Today 1993, 9, 154–159. [Google Scholar] [CrossRef]
- Cioli, D. Praziquantel: Is there real resistance and are there alternatives? Curr. Opin. Infect. Dis. 2000, 13, 659–663. [Google Scholar] [CrossRef]
- Liang, Y.S.; Coles, G.C.; Doenhoff, M. Short communication: Detection of praziquantel resistance in schistosomes. Trop. Med. Int. Health 2000, 5, 72. [Google Scholar] [CrossRef]
- Drudge, J.H.; Szanto, J.; Wyant, Z.N.; Elam, G. Field studies on parasite control in sheep: Comparison of thia-bendazole, ruelene, and phenothiazine. Am. J. Vet. Res. 1964, 25, 1512–1518. [Google Scholar] [PubMed]
- Reinemeyer, C. Formulations and Clinical Uses of Pyrimidine Compounds in Domestic Animals. In Pyrantel Parasiticide Therapy in Humans and Domestic Animals; Marchiondo, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 67–107. [Google Scholar]
- Sangster, N.C.; Whitlock, H.V.; Russ, I.G.; Gunawan, M.; Griffin, D.L.; Kelly, J.D. Trichostrongylus colubriformis and Ostertagia circumcincta resistant to levamisole, morantel tartrate and thiabendazole: Occurrence of field strains. Res. Veter. Sci. 1979, 27, 106–110. [Google Scholar]
- van Wyk, J.; Malan, F. Resistance of field strains of Haemonchus contortus to ivermectin, closantel, rafoxanide and the benzimidazoles in South Africa. Veter. Rec. 1988, 123, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.; Dobson, R.; Obendorf, D.; Gillham, R. Resistance of Trichostrongylus colubriformis to levamisole and morantel: Differences in relation to selection history. Veter. Parasitol. 1986, 21, 255–263. [Google Scholar] [CrossRef]
- Sangster, N. Anthelmintic resistance: Past, present and future. Int. J. Parasitol. 1999, 29, 115–124. [Google Scholar] [CrossRef]
- Flávia da Silva, F.; Bezerra, H.M.F.F.; Feitosa, T.F.; Vilela, V.L.R. Nematode resistance to five anthelmintic classes in naturally infected sheep herds in Northeastern Brazil. Rev. Bras. Parasitol. Veterinária 2018, 27, 423–429. [Google Scholar] [CrossRef]
- Sales, N.; Love, S. Resistance of Haemonchus sp. to monepantel and reduced efficacy of a derquantel/abamectin combination confirmed in sheep in NSW, Australia. Vet. Parasitol. 2016, 228, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.J.; Hosking, B.C.; Jacobson, C.L.; Cotter, J.L.; Besier, R.B.; Stein, P.A.; Reid, S.A. Monepantel resistance reported on Dutch sheep farms. Vet. Rec. 2014, 175, 418. [Google Scholar]
- Bagnall, N.H.; Ruffell, A.; Raza, A.; Elliott, T.P.; Lamb, J.; Hunt, P.W.; Kotze, A.C. Mutations in the Hco-mptl-1 gene in a field-derived monepantel-resistant isolate of Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 236–240. [Google Scholar] [CrossRef]
- Chevalier, F.D.; Le Clec’H, W.; Eng, N.; Rugel, A.R.; de Assis, R.R.; Oliveira, G.; Holloway, S.P.; Cao, X.; Hart, P.J.; LoVerde, P.T.; et al. Independent origins of loss-of-function mutations conferring oxamniquine resistance in a Brazilian schistosome population. Int. J. Parasitol. 2016, 46, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Cioli, D.; Pica-Mattoccia, L.; Archer, S. Resistance of schistosomes to hycanthone and oxamniquine. Mem. do Inst. Oswaldo Cruz 1989, 84, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Botros, S.S.; Bennett, J.L. Praziquantel resistance. Expert Opin. drug Discov. 2007, 2, S35–S40. [Google Scholar] [CrossRef]
- Xu, M.; Molento, M.; Blackhall, W.; Ribeiro, P.; Beech, R.; Prichard, R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 1998, 91, 327–335. [Google Scholar] [CrossRef]
- Le Jambre, L.F.; Gill, J.H.; Lenane, I.J.; Baker, P. Inheritance of avermectin resistance in Haemonchus contortus. Int. J. Parasitol. 2000, 30, 105–111. [Google Scholar] [CrossRef]
- Nega, D.; Seyum, Z. A Review on Anthelmintic Resistance and Potential Risk Factors in Domestic Ruminants. Acta Parasitol. Glob. 2017, 8, 58–67. [Google Scholar]
- Sangster, N.; Riley, F.; Collins, G. Investigation of the mechanism of levamisole resistance in trichostrongylid nematodes of sheep. Int. J. Parasitol. 1988, 18, 813–818. [Google Scholar] [CrossRef]
- Kotze, A.; Prichard, R. Anthelmintic resistance in Haemonchus contortus: History, mechanisms and diagnosis. Adv. Parasitol. 2016, 93, 397–428. [Google Scholar]
- Dutra, R.C.; Campos, M.; Santos, A.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef]
- World Health Organization. Traditional and Alternative Medicine, Fact Sheet; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Romero-Benavides, J.C.; Ruano, A.L.; Silva-Rivas, R.; Veintimilla, P.C.; Jaramillo, S.V.; Bailon-Moscoso, N.; Rivas, R.S. Medicinal plants used as anthelmintics: Ethnomedical, pharmacological, and phytochemical studies. Eur. J. Med. Chem. 2017, 129, 209–217. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Kingston, D.G.I. Natural Products as Pharmaceuticals and Sources for Lead Structures. Pract. Med. Chem. 2008, 159–186. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Kumarasingha, R.; Karpe, A.; Preston, S.; Yeo, T.-C.; Lim, D.S.; Tu, C.-L.; Luu, J.; Simpson, K.; Shaw, J.M.; Gasser, R.B.; et al. Metabolic profiling and in vitro assessment of anthelmintic fractions of Picria fel-terrae Lour. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 171–178. [Google Scholar] [CrossRef]
- Williams, A.R.; Ropiak, H.M.; Fryganas, C.; Desrues, O.; Mueller-Harvey, I.; Thamsborg, S.M. Assessment of the anthelmintic activity of medicinal plant extracts and purified condensed tannins against free-living and parasitic stages of Oesophagostomum dentatum. Parasites Vectors 2014, 7, 1–12. [Google Scholar]
- Badar, S.N.; Iqbal, Z.; Sajid, M.S.; Rizwan, H.M.; Shareef, M.; Malik, M.A.; Khan, M.N. Comparative anthelmintic efficacy of Arundo donax, Areca catechu, and Ferula assa-foetida against Haemonchus contortus. Rev. Bras. Parasitol. Veterinária 2021, 30, e001221. [Google Scholar] [CrossRef]
- Soren, A.D.; Chen, R.P.; Yadav, A.K. In vitro and in vivo anthelmintic study of Sesbania sesban var. bicolor, a traditionally used medicinal plant of Santhal tribe in Assam, India. J. Parasit. Dis. 2020, 45, 1–9. [Google Scholar] [CrossRef]
- Castagna, F.; Britti, D.; Oliverio, M.; Bosco, A.; Bonacci, S.; Iriti, G.; Ragusa, M.; Musolino, V.; Rinaldi, L.; Palma, E.; et al. In Vitro Anthelminthic Efficacy of Aqueous Pomegranate (Punica granatum L.) Extracts against Gastrointestinal Nematodes of Sheep. Pathogens 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Ndlela, S.Z.; Mkwanazi, M.V.; Chimonyo, M. In vitro efficacy of plant extracts against gastrointestinal nematodes in goats. Trop. Anim. Health Prod. 2021, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasites Vectors 2019, 12, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.G.; Sakanari, J.A.; Caffrey, C.R. Anthelmintic drug discovery: Into the future. J. Parasitol. 2015, 101, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kotze, A. Target-based and whole-worm screening approaches to anthelmintic discovery. Veter. Parasitol. 2012, 186, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H. The evolution of drug discovery: From phenotypes to targets, and back. Med. Chem. Comm. 2016, 7, 788–798. [Google Scholar] [CrossRef]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and Challenges in Antiparasitic Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Panic, G.; Duthaler, U.; Speich, B.; Keiser, J. Repurposing drugs for the treatment and control of helminth infections. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 185–200. [Google Scholar] [CrossRef]
- Padhy, B.; Gupta, Y.; M, P.B.; K, G.Y. Drug repositioning: Re-investigating existing drugs for new therapeutic indications. J. Postgrad. Med. 2011, 57, 153–160. [Google Scholar] [CrossRef]
- Geary, T.G.; Thompson, D.P.; Klein, R.D. Mechanism-based screening: Discovery of the next generation of anthelmintics depends upon more basic research. Int. J. Parasitol. 1999, 29, 105–112. [Google Scholar] [CrossRef]
- Witty, M.J. Current strategies in the search for novel antiparasitic agents. Int. J. Parasitol. 1999, 29, 95–103. [Google Scholar] [CrossRef]
- Burns, A.R.; Luciani, G.; Musso, G.; Bagg, R.; Yeo, M.; Zhang, Y.; Rajendran, L.; Glavin, J.; Hunter, R.; Redman, E.; et al. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015, 6, 7485. [Google Scholar] [CrossRef]
- Preston, S.; Jabbar, A.; Nowell, C.; Joachim, A.; Ruttkowski, B.; Baell, J.; Cardno, T.; Korhonen, P.; Piedrafita, D.; Ansell, B.; et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015, 45, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Iqbal, Z.; Khan, M.; Lateef, M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo–Pakistan subcontinent. Small Rumin. Res. 2000, 38, 99–107. [Google Scholar] [CrossRef]
- Githiori, J.B.; Höglund, J.; Waller, P.J. Ethnoveterinary plant preparations as livestock dewormers: Practices, popular beliefs, pitfalls and prospects for the future. Anim. Health Res. Rev. 2005, 6, 91–103. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Githiori, J.; Kyriazakis, I. Medicinal plants for helminth parasite control: Facts and fiction. Animal 2007, 1, 1392–1400. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Yu, Y.; Yan, L.-L.; Li, C.; Han, J.-Y.; Qin, Z.-F.; Dai, Y.; Yao, Z.-H.; Zhou, H.; Yao, X.-S. Discovery of cardio-protective constituents of Gualou Xiebai Decoction, a classical traditional Chinese medicinal formula. Phytomedicine 2018, 54, 318–327. [Google Scholar] [CrossRef]
- Wu, S.; Yang, L.; Gao, Y.; Liu, X.; Liu, F. Multi-channel counter-current chromatography for high-throughput fractionation of natural products for drug discovery. J. Chromatogr. A 2008, 1180, 99–107. [Google Scholar] [CrossRef]
- Robinette, S.L.; Brüschweiler, R.; Schroeder, F.C.; Edison, A.S. NMR in Metabolomics and Natural Products Research: Two Sides of the Same Coin. Accounts Chem. Res. 2011, 45, 288–297. [Google Scholar] [CrossRef]
- Simmler, C.; Napolitano, J.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Universal quantitative NMR analysis of complex natural samples. Curr. Opin. Biotechnol. 2013, 25, 51–59. [Google Scholar] [CrossRef]
- Teinkela, J.E.M.; Noundou, X.S.; Nguemfo, E.L.; Meyer, F.; Wintjens, R.; Isaacs, M.; Mpondo, A.E.M.; Hoppe, H.C.; Krause, R.W.M.; Azebaze, A.G.B. Biological activities of plant extracts from Ficus elastica and Selaginella vogelli: An antimalarial, antitrypa-nosomal and cytotoxity evaluation. Saudi J. Biol. Sci. 2018, 25, 117–122. [Google Scholar] [CrossRef]
- Moore, N.; Hamza, N.; Berke, B.; Umar, A. News from Tartary: An ethnopharmacological approach to drug and therapeutic discovery. Br. J. Clin. Pharmacol. 2016, 83, 33–37. [Google Scholar] [CrossRef]
- Rana, A.K.; Misra-Bhattacharya, S. Current drug targets for helminthic diseases. Parasitol. Res. 2013, 112, 1819–1831. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- Ramirez, B.; Bickle, Q.; Yousif, F.; Fakorede, F.; Mouries, M.-A.; Nwaka, S. Schistosomes: Challenges in compound screening. Expert Opin. Drug Discov. 2007, 2, S53–S61. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Sherer, T.B.; Zhang, N.; Taylor, G.; Na, H.M.; Greenamyre, J.T.; Casida, J.E. Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chem. Res. Toxicol. 2004, 17, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.V.; Lodeyro, A.F.; Malheiros, A.; Zacchino, S.A.; Roveri, O.A. Inhibition of the mitochondrial ATP synthesis by polygodial, a naturally occurring dialdehyde unsaturated sesquiterpene. Biochem. Pharmacol. 2005, 70, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Tritten, L.; Braissant, O.; Keiser, J. Comparison of novel and existing tools for studying drug sensitivity against the hookworm Ancylostoma ceylanicum in vitro. Parasitology 2012, 139, 348–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brunet, S.; Jackson, F.; Hoste, H. Effects of sainfoin (Onobrychis viciifolia) extract and monomers of condensed tannins on the association of abomasal nematode larvae with fundic explants. Int. J. Parasitol. 2008, 38, 783–790. [Google Scholar] [CrossRef]
- Molan, A.L.; Duncan, A.J.; Barry, T.N.; McNabb, W. Effects of condensed tannins and crude sesquiterpene lactones extracted from chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. Parasitol. Int. 2003, 52, 209–218. [Google Scholar] [CrossRef]
- Novobilský, A.; Mueller-Harvey, I.; Thamsborg, S.M. Condensed tannins act against cattle nematodes. Veter. Parasitol. 2011, 182, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Paolini, V.; Fouraste, I.; Hoste, H. In vitro effects of three woody plant and sainfoin extracts on 3rd-stage larvae and adult worms of three gastrointestinal nematodes. Parasitology 2004, 129, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Lam, K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef]
- Singh, S.B.; Barrett, J.F. Empirical antibacterial drug discovery—Foundation in natural products. Biochem. Pharmacol. 2006, 71, 1006–1015. [Google Scholar] [CrossRef]
- MacKenzie, C.D.; Geary, T.G. Addressing the current challenges to finding new anthelminthic drugs. Expert Rev. Anti-Infect. Ther. 2013, 11, 539–541. [Google Scholar] [CrossRef]
- Eggert, U.S. The why and how of phenotypic small-molecule screens. Nat. Chem. Biol. 2013, 9, 206–209. [Google Scholar] [CrossRef]
- Cock, I. Problems of Reproducibility and Efficacy of Bioassays Using Crude Extracts, with reference to Aloe vera. Pharmacogn. Commun. 2011, 1, 52–62. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed]



| Disease | Helminth Species | Estimated Prevalence | Reported Deaths | Geographic Distribution | Refs. |
|---|---|---|---|---|---|
| Ascariasis | Ascaris lumbricoides | 761.9 million | 2700 | Worldwide | [2,9] |
| Trichuriasis | Trichuris trichiura | 1 billion | Not known | Worldwide | [3] |
| Hookworm | Necator americanus Anchylostoma sp. | 428.2 million | Not known | Sub tropics, tropics, and Coastal regions | [2,9] |
| Lymphatic filariasis | Wuchereria bancrofti, Brugia malayi, Brugia timori | 119 million | 4000 | Tropical Africa, Asia, and America | [10,11] |
| Strongyloidiasis | Strongyloides tercoralis | 30–100 million | Not known | Mainly tropical and sub-tropical regions | [3] |
| Loiasis | Loa loa | 29.6 million | 915 | Central Africa | [12] |
| Onchocerciasis | Onchocerca volvulus | 20.9 million | Not known | Tropical Africa America | [13] |
| Trichinosis | Trichinella spiralis | 50 million | Not known | Worldwide | [13] |
| Enterobiosis | Enterobius vermicularis | >1 billion | Not known | Worldwide | [14,15] |
| Dracunculiasis | Dracunculiasis medinensis | >3 million | Not known | Africa and Asia | [3,16] |
| Class of Anthelmintics | Available Drugs | Target Site | Mode of Action | Refs. |
|---|---|---|---|---|
| Imidothiazoles | Levamisole | Nicotinic receptor antagonist in body wall muscle | Cause spastic muscle paralysis through prolonged activation of the excitatory nicotinic acetylcholine receptors | [37,39] |
| Tetrahydro-pyrimidines | Pyrantel, Morantel | Nicotinic receptor antagonist in body wall muscle | Cause spastic muscle paralysis through prolonged activation of the excitatory nicotinic acetylcholine receptors | [37] |
| Macrocyclic lactones (ML) | Moxidectin, Ivermectin, Avermectin, Milbemycin | Allosteric modulator of GABA—gated chloride ion channels | Inhibit pharyngeal pumping, motility and egg laying | [37,44] |
| Benzimidazoles | Albendazole, Mebendazole, Febendazole | β-tubulin protein in cytoskeleton | Affect the locomotion and reproduction | [39] |
| Amino-acetonitrile derivatives (AAD) | Monepantel | Nicotinic acetylcholine receptor subunit | Induce paralysis | [36,45] |
| Salicylanilides | Closantel, Disophenol | Proton ionophores Bioenergetics | Uncoupling of oxidative phosphorylation | [46] |
| Organophosphates | Haloxon dichlorvos, Coumaphos Naphthalophos | Acetylcholinesterase | Lead to spastic paralysis | [47] |
| Spiroindoles | Derquantel | B-subtype nicotinic acetylcholine receptor | B-subtype nicotinic acetylcholine receptor | [47,48] |
| Clorsulon | Inhibition of phosphoglycerate kinase and mutase | [46] | ||
| Praziquantel | Ca ion channels | Increase in membrane permeability towards calcium results in increase calcium influx leading to muscular contracture | [49] | |
| Diamphenethide | Inhibition of malate metabolism | [50] | ||
| Piperazine | Piperazine | GABA receptors | Binding to muscle membrane GABA receptors causing hyperpolarization of nerve endings | [51] |
| Anthelmintic Drugs | Helminth Species | Reported Mechanism of Drug Resistance | Refs |
|---|---|---|---|
| Oxamniquine | Schistosomes | 1. Deficiency in drug-activating enzyme—sulfotransferase 2. Loss of function in drug activating enzyme sulfotransferase | [68,69] |
| Praziquantel | Schistosomes | 1. Deficiency in drug-activating enzyme—sulfotransferase | [57,70] |
| Macrocyclic lactones (Ivermectin and Avermectin) | Haemonchus contortus | 1. Alter the structure of GluCl channel subunits or GABA receptor 2. Overexpression of P-glycoproteins | [71,72,73] |
| Imidazothiazoles (Levamisole) | Trichostrongylids | 1. Reduction in the number of levamisole receptors in resistant trichostrongylids 2. Reduction in the affinity of levamisole receptors for levamisole | [74] |
| Closantel | Fasciola spp. Haemonchus contortus | 1. Reduction of feeding by resistant worms 2. Reduction of dissociation of the drug-albumin complex in the worm gut 2. Overexpress the drug target (P-glycoprotein mediated increased drug efflux) | [75] |
| Amino-acetonitrile derivatives (Monepantel) | Haemonchus contortus | 1. Mutations in the drug target gene (Hco-mptl-1) | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayawardene, K.L.T.D.; Palombo, E.A.; Boag, P.R. Natural Products Are a Promising Source for Anthelmintic Drug Discovery. Biomolecules 2021, 11, 1457. https://doi.org/10.3390/biom11101457
Jayawardene KLTD, Palombo EA, Boag PR. Natural Products Are a Promising Source for Anthelmintic Drug Discovery. Biomolecules. 2021; 11(10):1457. https://doi.org/10.3390/biom11101457
Chicago/Turabian StyleJayawardene, K. L. T. Dilrukshi, Enzo A. Palombo, and Peter R. Boag. 2021. "Natural Products Are a Promising Source for Anthelmintic Drug Discovery" Biomolecules 11, no. 10: 1457. https://doi.org/10.3390/biom11101457
APA StyleJayawardene, K. L. T. D., Palombo, E. A., & Boag, P. R. (2021). Natural Products Are a Promising Source for Anthelmintic Drug Discovery. Biomolecules, 11(10), 1457. https://doi.org/10.3390/biom11101457







