Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe?
Abstract
1. Introduction
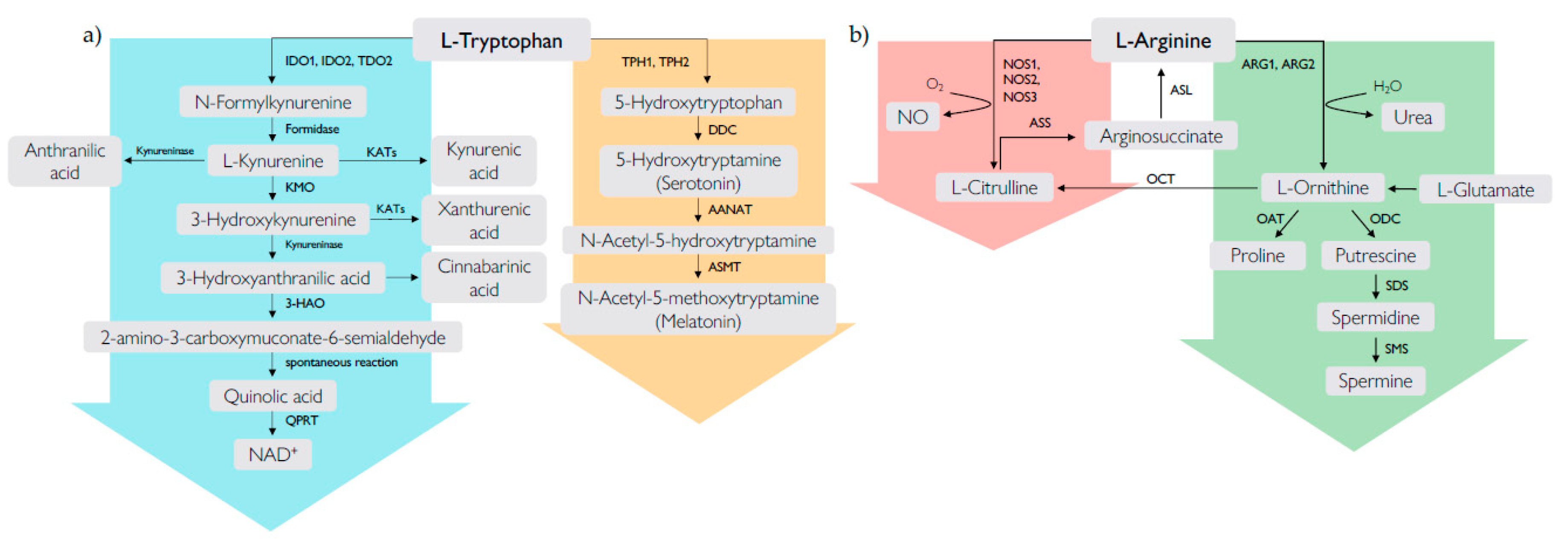
2. Tryptophan Metabolism and RA
2.1. The Kynurenine Pathway
2.1.1. IDO1
2.1.2. IDO2
2.1.3. TDO
2.1.4. Kyn and the Aryl Hydrocarbon Receptor (AhR)
2.2. The Serotonin Pathway
2.2.1. Serotonin
2.2.2. NAS
2.2.3. Melatonin
3. Arginine Metabolism and RA
3.1. ARG1 and ARG2
3.2. ODC and Polyamines
3.3. The NOS Pathway
3.4. Methylated Arg Products
4. Amino Acid Metabolism by Microbiota and RA
4.1. Trp Metabolites
4.1.1. Iald
4.1.2. IAA
4.1.3. ILA
4.2. Arg Metabolites
4.3. Dysbiosis, Amino Acid Metabolism, and RA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karami, J.; Aslani, S.; Jamshidi, A.; Garshasbi, M.; Mahmoudi, M. Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene 2019, 702, 8–16. [Google Scholar] [CrossRef]
- Calabresi, E.; Petrelli, F.; Bonifacio, A.F.; Puxeddu, I.; Alunno, A. One year in review 2018: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 175–184. [Google Scholar]
- Weyand, C.M.; Goronzy, J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 291–301. [Google Scholar] [CrossRef]
- Freitag, J.; Berod, L.; Kamradt, T.; Sparwasser, T. Immunometabolism and autoimmunity. Immunol. Cell Biol. 2016, 94, 925–934. [Google Scholar] [CrossRef]
- Grohmann, U.; Bronte, V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010, 236, 243–264. [Google Scholar] [CrossRef]
- Mondanelli, G.; Iacono, A.; Carvalho, A.; Orabona, C.; Volpi, C.; Pallotta, M.T.; Matino, D.; Esposito, S.; Grohmann, U. Amino acid metabolism as drug target in autoimmune diseases. Autoimmun. Rev. 2019, 18, 334–348. [Google Scholar] [CrossRef]
- Murray, P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol. 2016, 17, 132–139. [Google Scholar] [CrossRef]
- Mondanelli, G.; Ugel, S.; Grohmann, U.; Bronte, V. The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Curr. Opin. Pharmacol. 2017, 35, 30–39. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.C.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Takeda, K. Host–microbiota interactions in rheumatoid arthritis. Exp. Mol. Med. 2019, 51, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Oda, S.-I.; Otsuki, T.; Hino, T.; Yoshida, T.; Shiro, Y. Crystal structure of human indoleamine 2,3-dioxygenase: Catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc. Natl. Acad. Sci. USA 2006, 103, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Curr. Opin. Pharmacol. 2004, 4, 12–17. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Takikawa, O.; Kuroiwa, T.; Yamazaki, F.; Kido, R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J. Biol. Chem. 1988, 263, 2041–2048. [Google Scholar]
- Tykocinski, L.-O.; Lauffer, A.M.; Bohnen, A.; Kaul, N.-C.; Krienke, S.; Tretter, T.; Adam, I.; Mohapatra, S.R.; Saikali, P.; Löhning, M.; et al. Synovial Fibroblasts Selectively Suppress Th1 Cell Responses through IDO1-Mediated Tryptophan Catabolism. J. Immunol. 2017, 198, 3109–3117. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. Ido expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef]
- Grohmann, U.; Fallarino, F.; Puccetti, P. Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 2003, 24, 242–248. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Orabona, C.; Volpi, C.; Vacca, C.; Belladonna, M.L.; Bianchi, R.; Servillo, G.; Brunacci, C.; Calvitti, M.; Bicciato, S.; et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011, 12, 870–878. [Google Scholar] [CrossRef]
- Orabona, C.; Pallotta, M.T.; Grohmann, U. Different Partners, Opposite Outcomes: A New Perspective of the Immunobiology of Indoleamine 2,3-Dioxygenase. Mol. Med. 2012, 18, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Igari, T.; Tsuchizaw, M.; Shimamura, T. Alteration of tryptophan metabolism in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Tohoku J. Exp. Med. 1987, 153, 79–86. [Google Scholar] [CrossRef] [PubMed]
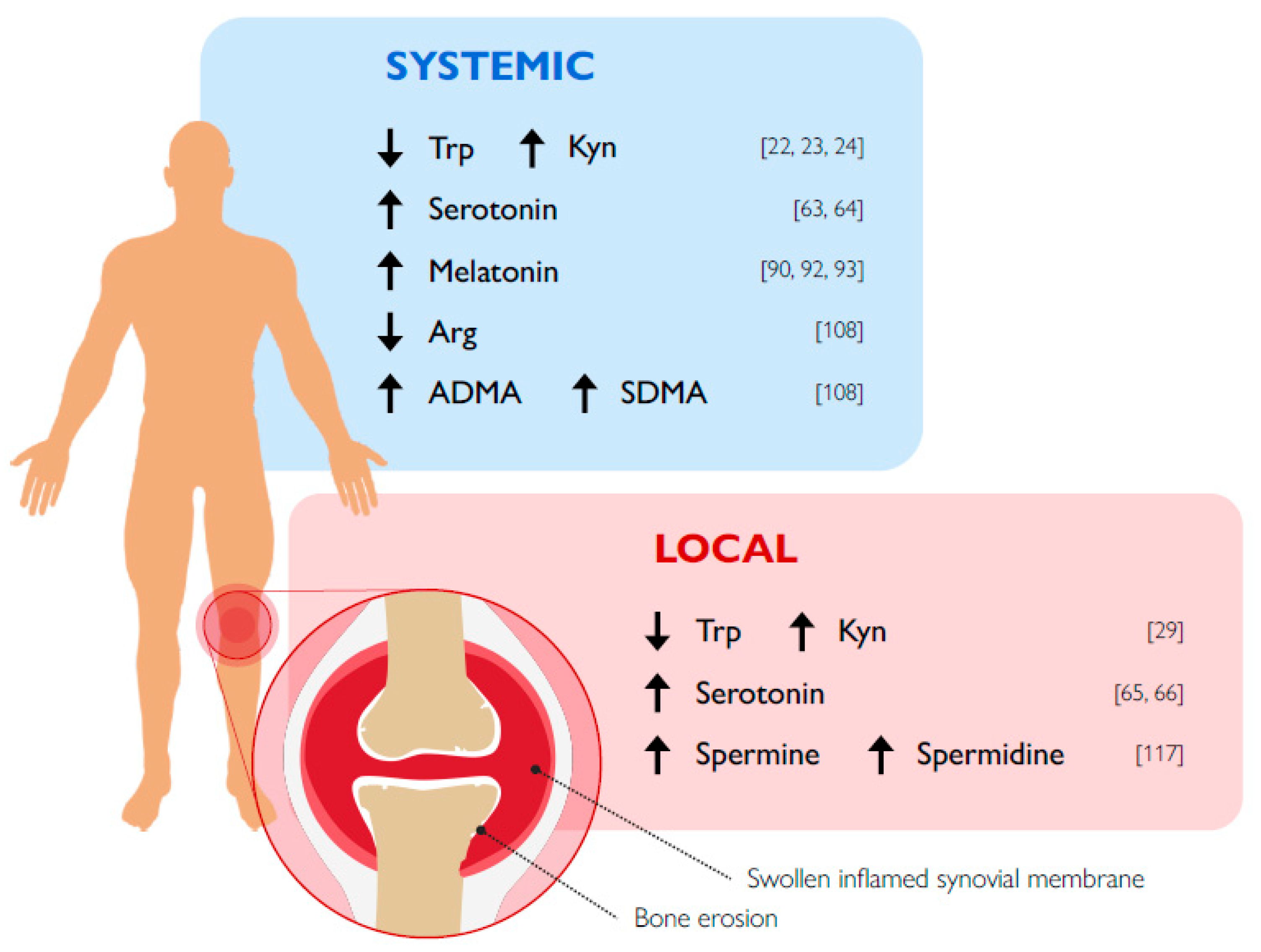
- Schroecksnadel, K.; Winkler, C.; Duftner, C.; Wirleitner, B.; Schirmer, M.; Fuchs, D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin. Rheumatol. 2005, 25, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Kennedy, A.; Stone, T.W.; Stoy, N.; Darlington, L.G. Kynurenine and Neopterin Levels in Patients with Rheumatoid Arthritis and Osteoporosis During Drug Treatment. Adv. Exp. Med. Biol. 2003, 527, 287–295. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suda, T.; Furuhashi, K.; Suzuki, M.; Fujie, M.; Hahimoto, D.; Nakamura, Y.; Inui, N.; Nakamura, H.; Chida, K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010, 67, 361–365. [Google Scholar] [CrossRef]
- Ozkan, Y.; Mete, G.; Sepici-Dincel, A.; Sepici, V.; Simsek, B. Tryptophan degradation and neopterin levels in treated rheumatoid arthritis patients. Clin. Rheumatol. 2011, 31, 29–34. [Google Scholar] [CrossRef]
- Park, M.-K.; Oh, H.-J.; Heo, Y.-M.; Park, E.-M.; Cho, M.-L.; Kim, H.-Y.; Park, S.-H. Myeloid differentiation primary response protein 88 blockade upregulates indoleamine 2,3-dioxygenase expression in rheumatoid synovial fibroblasts. Exp. Mol. Med. 2011, 43, 446–454. [Google Scholar] [CrossRef]
- Parada-Turska, J.; Rzeski, W.; Zgrajka, W.; Majdan, M.; Kandefer-Szerszeń, M.; Turski, W. Kynurenic acid, an endogenous constituent of rheumatoid arthritis synovial fluid, inhibits proliferation of synoviocytes in vitro. Rheumatol. Int. 2006, 26, 422. [Google Scholar] [CrossRef]
- Kang, K.Y.; Lee, S.H.; Jung, S.M.; Park, S.H.; Jung, B.-H.; Ju, J.H. Downregulation of Tryptophan-related Metabolomic Profile in Rheumatoid Arthritis Synovial Fluid. J. Rheumatol. 2015, 42, 2003–2011. [Google Scholar] [CrossRef]
- Cribbs, A.P.; Kennedy, A.; Penn, H.; Read, J.E.; Amjadi, P.; Green, P.; Syed, K.; Manka, S.W.; Brennan, F.M.; Gregory, B.; et al. Treg Cell Function in Rheumatoid Arthritis Is Compromised by CTLA-4 Promoter Methylation Resulting in a Failure to Activate the Indoleamine 2,3-Dioxygenase Pathway. Arthritis Rheumatol. 2014, 66, 2344–2354. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; Khan, M.N.; Kiriakidis, S.; Paleolog, E.M. Hypoxia. The role of hypoxia and HIF-dependent signalling events in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, 201. [Google Scholar] [CrossRef]
- Kaul, N.-C.; Mohapatra, S.R.; Adam, I.; Tucher, C.; Tretter, T.; A Opitz, C.; Lorenz, H.-M.; Tykocinski, L.-O. Hypoxia decreases the T helper cell-suppressive capacity of synovial fibroblasts by downregulating IDO1-mediated tryptophan metabolism. Rheumatology 2019, 59, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Szántó, S.; Koreny, T.; Mikecz, K.; Glant, T.T.; Szekanecz, Z.; Varga, J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res. Ther. 2007, 9, R50. [Google Scholar] [CrossRef] [PubMed]
- Criado, G.; Simelyte, E.; Inglis, J.J.; Essex, D.; Williams, R.O. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009, 60, 1342–1351. [Google Scholar] [CrossRef]
- Kołodziej, Ł.; Paleolog, E.M.; Williams, R.O. Kynurenine metabolism in health and disease. Amino Acids 2010, 41, 1173–1183. [Google Scholar] [CrossRef]
- Kołodziej, Ł. Systemic metabolism of tryptophan and its catabolites, kynurenine and 3-HAA, in mice with inflammatory arthritis. Gene 2013, 512, 23–27. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wu, C.-L.; Lai, M.-D.; Lin, C.-C.; Yo, Y.-T.; Jou, I.-M.; Lee, C.-H.; Weng, C.-T.; Shiau, A.-L.; Wang, C.-R. Amelioration of Rat Collagen-Induced Arthritis Through CD4+ T Cells Apoptosis and Synovial Interleukin-17 Reduction by Indoleamine 2,3-Dioxygenase Gene Therapy. Hum. Gene Ther. 2011, 22, 145–154. [Google Scholar] [CrossRef]
- Chalise, J.P.; Pallotta, M.T.; Narendra, S.C.; Carlsson, B.; Iacono, A.; Namale, J.; Boon, L.; Grohmann, U.; Magnusson, M. IDO1 and TGF-beta Mediate Protective Effects of IFN-alpha in Antigen-Induced Arthritis. J. Immunol. 2016, 197, 3142–3151. [Google Scholar] [CrossRef]
- Royzman, D.; Andreev, D.; Stich, L.; Rauh, M.; Bäuerle, T.; Ellmann, S.; Boon, L.; Kindermann, M.; Peckert, K.; Bozec, A.; et al. Soluble CD83 Triggers Resolution of Arthritis and Sustained Inflammation Control in IDO Dependent Manner. Front. Immunol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Orabona, C.; Mondanelli, G.; Pallotta, M.T.; Carvalho, A.; Albini, E.; Fallarino, F.; Vacca, C.; Volpi, C.; Belladonna, M.L.; Berioli, M.G.; et al. Deficiency of immunoregulatory indoleamine 2,3-dioxygenase 1in juvenile diabetes. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Mondanelli, G.; Coletti, A.; Greco, F.A.; Pallotta, M.T.; Orabona, C.; Iacono, A.; Belladonna, M.L.; Albini, E.; Panfili, E.; Fallarino, F.; et al. Positive allosteric modulation of indoleamine 2,3-dioxygenase 1 restrains neuroinflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 3848–3857. [Google Scholar] [CrossRef] [PubMed]
- Mondanelli, G.; Di Battista, V.; Pellanera, F.; Mammoli, A.; Macchiarulo, A.; Gargaro, M.; Mavridou, E.; Matteucci, C.; Ruggeri, L.; Orabona, C.; et al. A novel mutation of indoleamine 2,3-dioxygenase 1 causes a rapid proteasomal degradation and compromises protein function. J. Autoimmun. 2020, 102509. [Google Scholar] [CrossRef] [PubMed]
- Kouskoff, V.; Korganow, A.-S.; Duchatelle, V.; Degott, C.; Benoist, C.; Mathis, D. Organ-Specific Disease Provoked by Systemic Autoimmunity. Cell 1996, 87, 811–822. [Google Scholar] [CrossRef]
- Merlo, L.M.F.; Pigott, E.; DuHadaway, J.B.; Grabler, S.; Metz, R.; Prendergast, G.C.; Mandik-Nayak, L. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J. Immunol. 2014, 192, 2082–2090. [Google Scholar] [CrossRef]
- Merlo, L.M.F.; DuHadaway, J.B.; Grabler, S.; Prendergast, G.C.; Muller, A.J.; Mandik-Nayak, L. IDO2 Modulates T Cell-Dependent Autoimmune Responses through a B Cell-Intrinsic Mechanism. J. Immunol. 2016, 196, 4487–4497. [Google Scholar] [CrossRef]
- Merlo, L.M.F.; Grabler, S.; DuHadaway, J.B.; Pigott, E.; Manley, K.; Prendergast, G.C.; Laury-Kleintop, L.D.; Mandik-Nayak, L. Therapeutic antibody targeting of indoleamine-2,3-dioxygenase (IDO2) inhibits autoimmune arthritis. Clin. Immunol. 2017, 179, 8–16. [Google Scholar] [CrossRef]
- Merlo, L.M.F.; Mandik-Nayak, L. IDO2: A Pathogenic Mediator of Inflammatory Autoimmunity. Clin. Med. Insights: Pathol. 2016, 9, 21–28. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Haber, R.; Bessette, D.; Durcan, M.J.; Goldman, D.; Hulihan-Giblin, B. Identification of Tryptophan 2,3-Dioxygenase RNA in Rodent Brain. J. Neurochem. 1993, 60, 1159–1162. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 19961–19966. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Puccetti, P. The Coevolution of IDO1 and AhR in the Emergence of Regulatory T-Cells in Mammals. Front. Immunol. 2015, 6, 58. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nakahama, T.; Kishimoto, T. Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin. Immunopathol. 2013, 35, 637–644. [Google Scholar] [CrossRef]
- Kazantseva, M.G.; Highton, J.; Stamp, L.K.; Hessian, P.A. Dendritic cells provide a potential link between smoking and inflammation in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R208. [Google Scholar] [CrossRef]
- O’Donnell, E.F.; Saili, K.S.; Koch, D.C.; Kopparapu, P.R.; Farrer, D.; Bisson, W.H.; Mathew, L.K.; Sengupta, S.; Kerkvliet, N.I.; Tanguay, R.L.; et al. The Anti-Inflammatory Drug Leflunomide Is an Agonist of the Aryl Hydrocarbon Receptor. PLoS ONE 2010, 5, e13128. [Google Scholar] [CrossRef]
- Ogando, J.; Tardáguila, M.; Díaz-Alderete, A.; Usategui, A.; Miranda-Ramos, V.; Martínez-Herrera, D.J.; De La Fuente, L.; García-León, M.J.; Moreno, M.C.; Escudero, S.; et al. Notch-regulated miR-223 targets the aryl hydrocarbon receptor pathway and increases cytokine production in macrophages from rheumatoid arthritis patients. Sci. Rep. 2016, 6, 20223. [Google Scholar] [CrossRef]
- Gülçin, I. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J. Enzym. Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef]
- Yoo, J.M.; Lee, B.D.; Sok, D.E.; Ma, J.Y.; Kim, M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-N.; Wang, P.; Mao, Y.-M.; Dan, Y.-L.; Wu, Q.; Li, X.-M.; Wang, D.-G.; Davis, C.; Hu, W.; Pan, H.-F. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Anagnostouli, M. The role of melatonin in Multiple Sclerosis. Neurol. Sci. 2019, 41, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Voog, U.; Alstergren, P.; Eliasson, S.; Leibur, E.; Kallikorm, R.; Kopp, S. Progression of radiographic changes in the temporomandibular joints of patients with rheumatoid arthritis in relation to inflammatory markers and mediators in the blood. Acta Odontol. Scand. 2004, 62, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, M.; Vieira, T.; Lucas, R.; Pereira, J.; Costa, L.; Simões-Ventura, F.; Martins, M.J. Serum serotonin levels and bone in rheumatoid arthritis patients. Rheumatol. Int. 2017, 37, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mitchell, J.; Sharma, M.; Gabriel, A.; Moriyama, K.; Palmer, P.P. Leukotrienes mediate 5-hydroxytryptamine-induced plasm extravasation in the rat knee joint via CysLT-type receptors. Inflamm. Res. 2004, 53, 66–71. [Google Scholar] [CrossRef]
- Seidel, M.F.; Fiebich, B.L.; Ulrich-Merzenich, G.; Candelario-Jalil, E.; Koch, F.-W.; Vetter, H. Serotonin mediates PGE2 overexpression through 5-HT2A and 5-HT3 receptor subtypes in serum-free tissue culture of macrophage-like synovial cells. Rheumatol. Int. 2008, 28, 1017–1022. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Akundi, R.; Lieb, K.; Candelario-Jalil, E.; Gmeiner, D.; Haus, U.; Müller, W.; Stratz, T.; Muñoz, E. Antiinflammatory effects of 5-HT3receptor antagonists in lipopolysaccharide-stimulated primary human monocytes. Scand. J. Rheumatol. 2004, 33, 28–32. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Rahimian, R.; Ghia, J.-E.; Khan, W.I.; Dehpour, A.R. Impact of 5-HT3 receptor antagonists on peripheral and central diseases. Drug Discov. Today 2012, 17, 741–747. [Google Scholar] [CrossRef]
- Chabbi-Achengli, Y.; Coman, T.; Collet, C.; Callebert, J.; Corcelli, M.; Lin, H.; Rignault, R.; Dy, M.; De Vernejoul, M.-C.; Côté, F. Serotonin Is Involved in Autoimmune Arthritis through Th17 Immunity and Bone Resorption. Am. J. Pathol. 2016, 186, 927–937. [Google Scholar] [CrossRef]
- Snir, O.; Hesselberg, E.; Amoudruz, P.; Klareskog, L.; Zarea-Ganji, I.; I Catrina, A.; Padyukov, L.; Malmström, V.; Seddighzadeh, M. Genetic variation in the serotonin receptor gene affects immune responses in rheumatoid arthritis. Genes Immun. 2012, 14, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kling, A.; Seddighzadeh, M.; Ärlestig, L.; Alfredsson, L.; Rantapaa-Dahlqvist, S.; Padyukov, L. Genetic variations in the serotonin 5-HT2A receptor gene (HTR2A) are associated with rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Kling, A.; Rantapää-Dahlqvist, S.; Stenlund, H.; Mjörndal, T. Decreased density of serotonin 5-HT2A receptors in rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Hsu, P.-Y.; Meng, M.; Su, C.-C. Supplement of 5-hydroxytryptophan before induction suppresses inflammation and collagen-induced arthritis. Arthritis Res. Ther. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Brown, E.; Mc Veigh, C.J.; Santos, L.; Gogarty, M.; Muller, H.K.; Elfving, B.; Brayden, D.J.; Haase, J. TNFalpha-dependent anhedonia and upregulation of hippocampal serotonin transporter activity in a mouse model of collagen-induced arthritis. Neuropharmacology 2018, 137, 211–220. [Google Scholar] [CrossRef]
- Kim, H.; Chen, L.; Lim, G.; Sung, B.; Wang, S.; McCabe, M.F.; Rusanescu, G.; Yang, L.; Tian, Y.; Mao, J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J. Clin. Investig. 2012, 122, 2940–2954. [Google Scholar] [CrossRef]
- Müller, N.; Schwarz, M.J. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol. Psychiatry 2007, 12, 988–1000. [Google Scholar] [CrossRef]
- Nerurkar, L.; Siebert, S.; McInnes, I.B.; Cavanagh, J. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2019, 6, 164–173. [Google Scholar] [CrossRef]
- Krishnadas, R.; Krishnadas, R.; Cavanagh, J. Sustained remission of rheumatoid arthritis with a specific serotonin reuptake inhibitor antidepressant: A case report and review of the literature. J. Med. Case Rep. 2011, 5, 112. [Google Scholar] [CrossRef]
- Sacre, S.; Medghalchi, M.; Gregory, B.; Brennan, F.; Williams, R. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010, 62, 683–693. [Google Scholar] [CrossRef]
- Liou, Y.-S.; Lin, T.-K.; Chen, H.-Y.; Jong, G.-P. Medications associated with fracture risk in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Ye, K.; Iuvone, P.M. N-acetylserotonin: Neuroprotection, neurogenesis, and the sleepy brain. Neuroscientist 2012, 18, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.J.; Huang, C.-C.; Liu, S.-C.; Tang, C. Reconsidering the Role of Melatonin in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 2877. [Google Scholar] [CrossRef] [PubMed]
- Hansson, I.; Holmdahl, R.; Mattsson, R. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. J. Neuroimmunol. 1990, 27, 79–84. [Google Scholar] [CrossRef]
- Hansson, I.; Holmdahl, R.; Mattsson, R. The pineal hormone melatonin exaggerates development of collagen-induced arthritis in mice. J. Neuroimmunol. 1992, 39, 23–30. [Google Scholar] [CrossRef]
- Hansson, I.; Holmdahl, R.; Mattsson, R. Pinealectomy ameliorates collagen II-induced arthritis in mice. Clin. Exp. Immunol. 1993, 92, 432–436. [Google Scholar] [CrossRef]
- Bang, J.; Chang, H.W.; Jung, H.-R.; Cho, C.-H.; Hur, J.-A.; Lee, S.-I.; Choi, T.H.; Kim, S.-H.; Ha, E. Melatonin attenuates clock gene Cryptochrome1, which may aggravate mouse anti-type II collagen antibody-induced arthritis. Rheumatol. Int. 2010, 32, 379–385. [Google Scholar] [CrossRef]
- Jimenez-Caliani, A.J.; Jiménez-Jorge, S.; Molinero, P.; Guerrero, J.M.; Fernández-Santos, J.M.; Lacave, I.M.; Osuna, C. Dual effect of melatonin as proinflammatory and antioxidant in collagen-induced arthritis in rats. J. Pineal Res. 2005, 38, 93–99. [Google Scholar] [CrossRef]
- Huang, C.C.; Chiou, C.H.; Liu, S.C.; Hu, S.L.; Su, C.M.; Tsai, C.H.; Tang, C.H. Melatonin attenuates TNF-alpha and IL-1beta expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal Res. 2019, 66, e12560. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, W. Effects and mechanisms of melatonin on inflammatory and immune responses of adjuvant arthritis rat. Int. Immunopharmacol. 2002, 2, 1443–1449. [Google Scholar] [CrossRef]
- El-Wakkad, A.S.E.-D.; El-Awady, H.M.; Saleh, M.T.; Muhammad, S.I.; Ghaniema, E.M. Serum melatonin in juvenile rheumatoid arthritis: Correlation with disease activity. Pak. J. Biol. Sci. 2007, 10, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-J.; Huang, S.-H.; Chen, S.-J.; Wang, C.-H.; Chang, D.-M.; Sytwu, H.-K. Modulation by Melatonin of the Pathogenesis of Inflammatory Autoimmune Diseases. Int. J. Mol. Sci. 2013, 14, 11742–11766. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Maestroni, G.M.; Villaggio, B.; Hertens, E.; Craviotto, C.; Pizzorni, C.; Briata, M.; Seriolo, B.; Cutolo, M. Melatonin serum levels in rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2002, 966, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Afkhamizadeh, M.; Sahebari, M.; Seyyed-Hoseini, S.-R. Morning melatonin serum values do not correlate with disease activity in rheumatoid arthritis: A cross-sectional study. Rheumatol. Int. 2014, 34, 1145–1151. [Google Scholar] [CrossRef]
- Ha, E.; Choe, B.-K.; Jung, K.H.; Yoon, S.H.; Park, H.J.; Park, H.K.; Yim, S.-V.; Chung, J.-H.; Bae, H.; Nam, M.; et al. Positive relationship between melatonin receptor type 1B polymorphism and rheumatoid factor in rheumatoid arthritis patients in the Korean population. J. Pineal Res. 2005, 39, 201–205. [Google Scholar] [CrossRef]
- Korkmaz, A. Melatonin as an adjuvant therapy in patients with rheumatoid arthritis. Br. J. Clin. Pharmacol. 2008, 66, 316–317. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2006, 109, 1568–1573. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Cardounel, A.J.; Cui, H.; Samouilov, A.; Johnson, W.; Kearns, P.; Tsai, A.-L.; Berka, V.; Zweier, J.L. Evidence for the Pathophysiological Role of Endogenous Methylarginines in Regulation of Endothelial NO Production and Vascular Function. J. Biol. Chem. 2006, 282, 879–887. [Google Scholar] [CrossRef]
- Konya, H.; Miuchi, M.; Satani, K.; Matsutani, S.; Yano, Y.; Tsunoda, T.; Ikawa, T.; Matsuo, T.; Ochi, F.; Kusunoki, Y.; et al. Asymmetric dimethylarginine, a biomarker of cardiovascular complications in diabetes mellitus. World J. Exp. Med. 2015, 5, 110–119. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Rhoads, J.M.; Satterfield, M.C.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2008, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Durante, W. Role of Arginase in Vessel Wall Remodeling. Front. Immunol. 2013, 4, 111. [Google Scholar] [CrossRef]
- Ming, X.-F.; Rajapakse, A.G.; Yepuri, G.; Xiong, Y.; Carvas, J.M.; Ruffieux, J.; Scerri, I.; Wu, Z.; Popp, K.; Li, J.; et al. Arginase II Promotes Macrophage Inflammatory Responses Through Mitochondrial Reactive Oxygen Species, Contributing to Insulin Resistance and Atherogenesis. J. Am. Heart Assoc. 2012, 1, e000992. [Google Scholar] [CrossRef] [PubMed]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Dzik, J.M. Evolutionary Roots of Arginase Expression and Regulation. Front. Immunol. 2014, 5, 544. [Google Scholar] [CrossRef] [PubMed]
- Corraliza, I.; Moncada, S. Increased expression of arginase II in patients with different forms of arthritis. Implications of the regulation of nitric oxide. J. Rheumatol. 2002, 29, 2261–2265. [Google Scholar] [PubMed]
- Chandrasekharan, U.M.; Wang, Z.; Wu, Y.; Tang, W.W.; Hazen, S.L.; Wang, S.; Husni, M.E. Elevated levels of plasma symmetric dimethylarginine and increased arginase activity as potential indicators of cardiovascular comorbidity in rheumatoid arthritis. Arthritis Res. 2018, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Berthelot, A.; Kantelip, B.; Wendling, D.; Demougeot, C. Treatment with the arginase inhibitor Nw-hydroxy-nor-L-arginine restores endothelial function in rat adjuvant-induced arthritis. Arthritis Res. Ther. 2012, 14, R130. [Google Scholar] [CrossRef] [PubMed]
- Bordy, R.; Verhoeven, F.; Tournier-Nappey, M.; Wendling, D.; Demougeot, C.; Totoson, P. Methotrexate did not improve endothelial function in rheumatoid arthritis: A study in rats with adjuvant-induced arthritis. Clin. Exp. Rheumatol. 2018, 37, 81–88. [Google Scholar]
- Miyoshi, F.; Sato, K.; Mimura, T. Changes in the pattern of cytokine production from peripheral blood mononuclear cells in patients with rheumatoid arthritis treated with infliximab and their relation to plasma arginase activity. Int. J. Rheum. Dis. 2016, 21, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, N.; Cao, S.; Eriksson, D.; Schnelzer, A.; Jordan, J.; Eberhardt, M.; Schleicher, U.; Rech, J.; Ramming, A.; Uebe, S.; et al. Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis. J. Clin. Investig. 2019, 129, 2669–2684. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Potteti, H.R.; Tamatam, C.R.; Elangovan, I.; Reddy, S.P. c-Jun Is Required for Nuclear Factor-κB–Dependent, LPS-Stimulated Fos-Related Antigen-1 Transcription in Alveolar Macrophages. Am. J. Respir. Cell Mol. Biol. 2016, 55, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, F.; Medina, M.Á.; Villalobos-Rueda, L.; Urdiales, J.L. Polyamines in mammalian pathophysiology. Cell. Mol. Life Sci. 2019, 76, 3987–4008. [Google Scholar] [CrossRef]
- Watanabe, S.; Kusama-Eguchi, K.; Kobayashi, H.; Igarashi, K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J. Biol. Chem. 1991, 266, 20803–20809. [Google Scholar]
- Karouzakis, E.; Gay, R.E.; Gay, S.; Neidhart, M. Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2012, 64, 1809–1817. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B.N. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef]
- Iezaki, T.; Hinoi, E.; Yamamoto, T.; Ishiura, R.; Ogawa, S.; Yoneda, Y. Amelioration by the natural polyamine spermine of cartilage and bone destruction in rats with collagen-induced arthritis. J. Pharmacol. Sci. 2012, 119, 107–111. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hinoi, E.; Fujita, H.; Iezaki, T.; Takahata, Y.; Takamori, M.; Yoneda, Y. The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice. Br. J. Pharmacol. 2012, 166, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.-T.; Ryu, B.J.; Choi, S.-W.; Heo, J.-C.; Kim, K.-J.; Son, Y.-J.; Kim, S.H. Natural polyamines inhibit the migration of preosteoclasts by attenuating Ca2+-PYK2-Src-NFATc1 signaling pathways. Amino Acids 2014, 46, 2605–2614. [Google Scholar] [CrossRef]
- Yeon, J.-T.; Choi, S.-W.; Kim, S.H. Arginase 1 is a negative regulator of osteoclast differentiation. Amino Acids 2015, 48, 559–565. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, F.; Sandri, S.; Ferrarini, G.; Pagliarello, I.; Sartoris, S.; Ugel, S.; Marigo, I.; Molon, B.; Bronte, V. The Emerging Immunological Role of Post-Translational Modifications by Reactive Nitrogen Species in Cancer Microenvironment. Front. Immunol. 2014, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Koncz, A.; Telarico, T.; Fernandez, D.; Érsek, B.; Buzás, E.; Perl, A. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. 2010, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- St. Clair, E.W.S.; Wilkinson, W.E.; Lang, T.; Sanders, L.; A Misukonis, M.; Gilkeson, G.S.; Pisetsky, D.S.; I Granger, D.; Weinberg, J.B. Increased expression of blood mononuclear cell nitric oxide synthase type 2 in rheumatoid arthritis patients. J. Exp. Med. 1996, 184, 1173–1178. [Google Scholar] [CrossRef]
- Nilforoushan, D.; Gramoun, A.; Glogauer, M.; Manolson, M.F. Nitric oxide enhances osteoclastogenesis possibly by mediating cell fusion. Nitric Oxide 2009, 21, 27–36. [Google Scholar] [CrossRef]
- Chae, H.-J.; Park, R.; Chung, H.-T.; Kang, J.-S.; Kim, M.-S.; Choi, D.-Y.; Bang, B.-G.; Kim, H.-R. Nitric oxide is a regulator of bone remodelling. J. Pharm. Pharmacol. 1997, 49, 897–902. [Google Scholar] [CrossRef]
- Fulton, M.D.; Brown, T.; Zheng, Y.G. The Biological Axis of Protein Arginine Methylation and Asymmetric Dimethylarginine. Int. J. Mol. Sci. 2019, 20, 3322. [Google Scholar] [CrossRef]
- Brown, E.M.; Kenny, D.; Xavier, R.J. Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu. Rev. Immunol. 2019, 37, 599–624. [Google Scholar] [CrossRef]
- Wu, H.-J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Mathis, D.; Mathis, D. Gut-Residing Segmented Filamentous Bacteria Drive Autoimmune Arthritis via T Helper 17 Cells. Immunology 2010, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Marion, T.; Schett, G.; Luo, Y.; Liu, Y. Microbiota and metabolites in rheumatic diseases. Autoimmun. Rev. 2020, 102530. [Google Scholar] [CrossRef] [PubMed]
- Rogier, R.; Ederveen, T.H.A.; Boekhorst, J.; Wopereis, H.; Scher, J.U.; Manasson, J.; Frambach, S.J.C.M.; Knol, J.; Garssen, J.; Van Der Kraan, P.M.; et al. Aberrant intestinal microbiota due to IL-1 receptor antagonist deficiency promotes IL-17- and TLR4-dependent arthritis. Microbiome 2017, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, J. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic Phenotyping in Health and Disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.-M.E.; Fish, K.; Fu, Y.-X.; Zhou, L. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunology 2012, 36, 92–104. [Google Scholar] [CrossRef]
- Miani, M.; Le Naour, J.; Waeckel-Enee, E.; Verma, S.C.; Straube, M.; Emond, P.; Ryffel, B.; van Endert, P.; Sokol, H.; Diana, J. Gut Microbiota-Stimulated Innate Lymphoid Cells Support beta-Defensin 14 Expression in Pancreatic Endocrine Cells, Preventing Autoimmune Diabetes. Cell Metab. 2018, 28, 557–572 e6. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.B.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Xu, K.; Zhang, G.; Liu, Y.; Gao, J.; Tian, M.; Wei, C.; Li, J.; Zhang, L. Immunomodulatory effect of human umbilical cord mesenchymal stem cells on T lymphocytes in rheumatoid arthritis. Int. Immunopharmacol. 2019, 74, 105687. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, C.; Fan, D.; Lu, X.; Xia, Y.; Zhao, H.; Xu, H.; Zhu, Y.; Li, J.; Liu, H.; et al. Human Umbilical Mesenchymal Stem Cells Display Therapeutic Potential in Rheumatoid Arthritis by Regulating Interactions Between Immunity and Gut Microbiota via the Aryl Hydrocarbon Receptor. Front. Cell Dev. Biol. 2020, 8, 131. [Google Scholar] [CrossRef]
- Sakurai, T.; Odamaki, T.; Xiao, J.-Z. Production of Indole-3-Lactic Acid by Bifidobacterium Strains Isolated fromHuman Infants. Microorganisms 2019, 7, 340. [Google Scholar] [CrossRef]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2017, 102, 415–425. [Google Scholar] [CrossRef]
- Asquith, M.; Davin, S.; Stauffer, P.; Michell, C.; Janowitz, C.; Lin, P.; Ensign-Lewis, J.; Kinchen, J.M.; Koop, D.R.; Rosenbaum, J.T. Intestinal Metabolites Are Profoundly Altered in the Context of HLA-B27 Expression and Functionally Modulate Disease in a Rat Model of Spondyloarthritis. Arthritis Rheumatol. 2017, 69, 1984–1995. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, N.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Piper, C.J.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851.e10. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panfili, E.; Gerli, R.; Grohmann, U.; Pallotta, M.T. Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe? Biomolecules 2020, 10, 1280. https://doi.org/10.3390/biom10091280
Panfili E, Gerli R, Grohmann U, Pallotta MT. Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe? Biomolecules. 2020; 10(9):1280. https://doi.org/10.3390/biom10091280
Chicago/Turabian StylePanfili, Eleonora, Roberto Gerli, Ursula Grohmann, and Maria Teresa Pallotta. 2020. "Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe?" Biomolecules 10, no. 9: 1280. https://doi.org/10.3390/biom10091280
APA StylePanfili, E., Gerli, R., Grohmann, U., & Pallotta, M. T. (2020). Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe? Biomolecules, 10(9), 1280. https://doi.org/10.3390/biom10091280




