miR-361-5p Mediates SMAD4 to Promote Porcine Granulosa Cell Apoptosis through VEGFA
Abstract
1. Introduction
2. Materials and Methods
2.1. Follicle Collection
2.2. Cell Culture and Transfection
2.3. Immunohistochemical Assay
2.4. RNA Extraction and qRT-PCR
2.5. Fluorescent In Situ Hybridization (FISH)
2.6. Protein Extraction and Western Immunoblotting Analysis
2.7. Plasmid Construction
2.8. Luciferase Reporter Assays
2.9. Apoptosis Assay
2.10. Statistical Analysis
3. Results
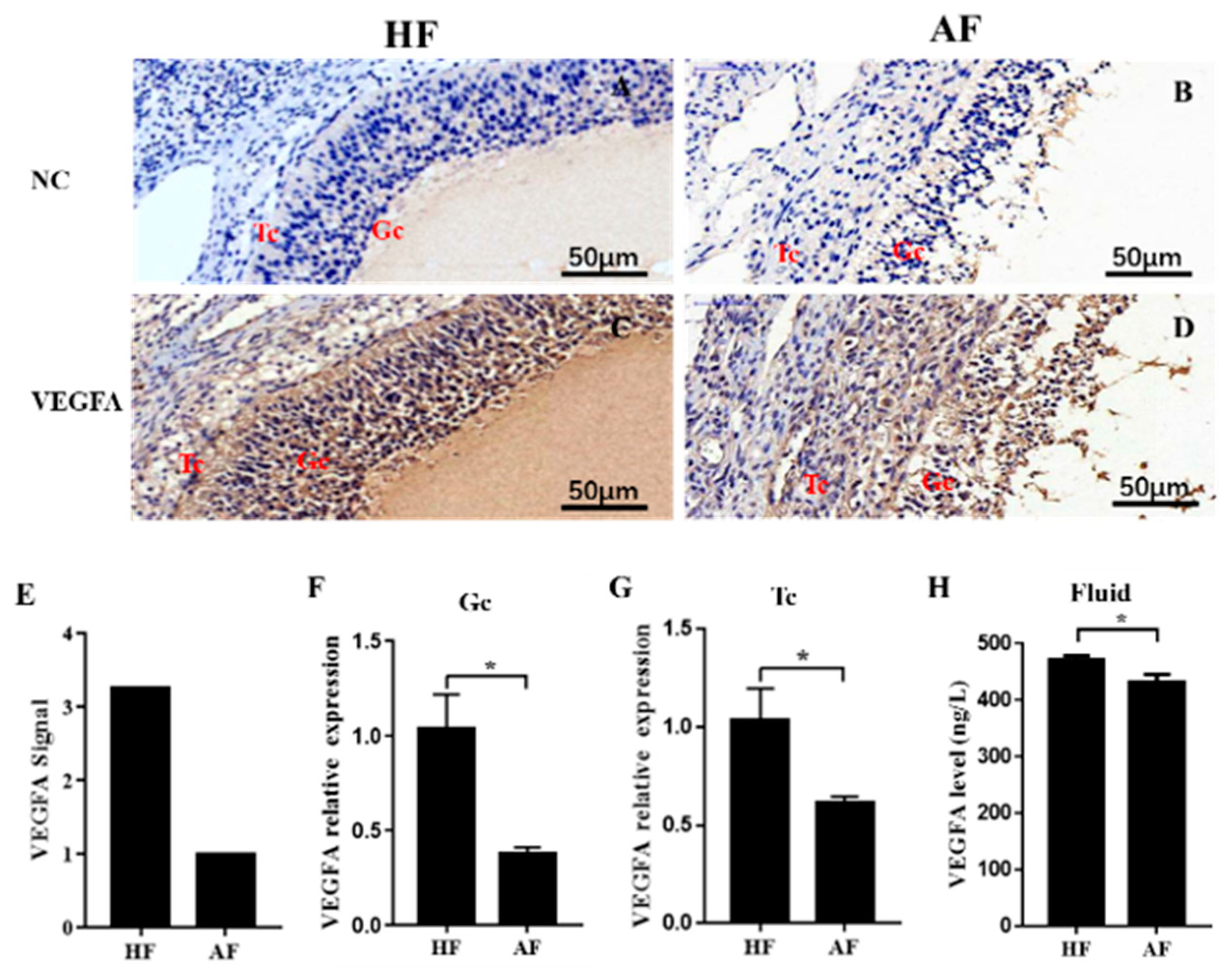
3.1. VEGFA Is Downregulated in Atretic Follicles
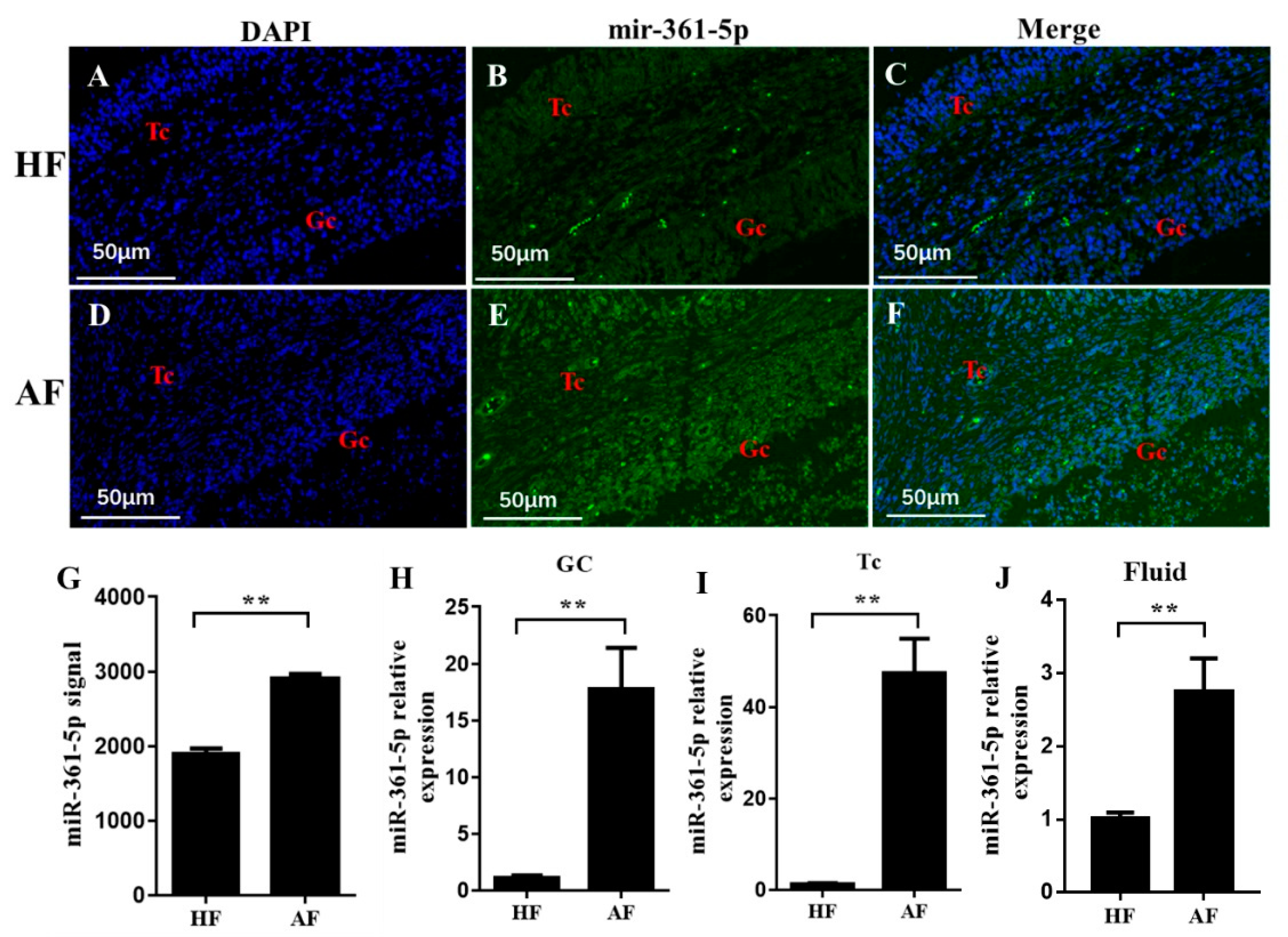
3.2. miR-361-5p Is Upregulated in Atretic Follicles
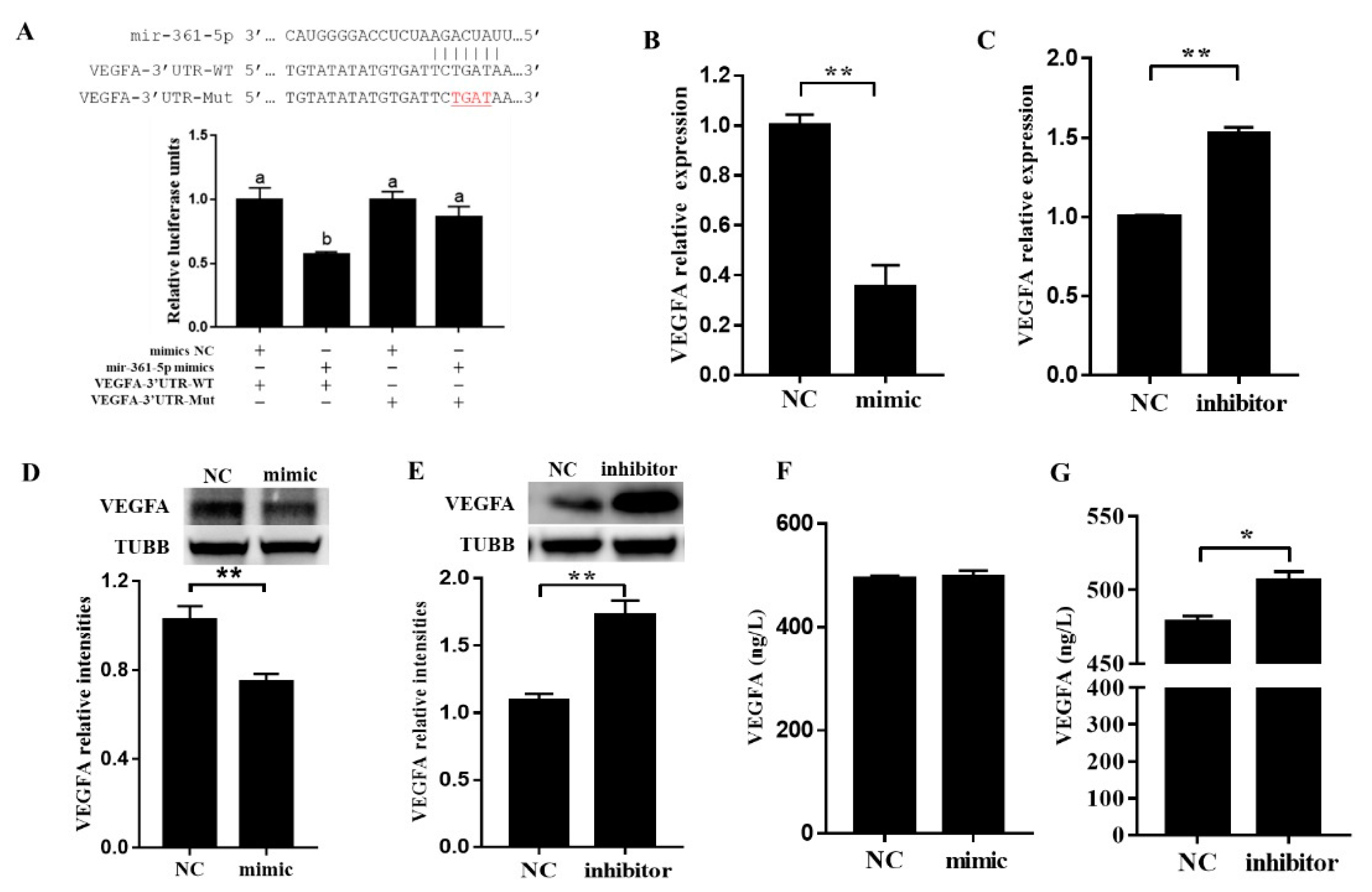
3.3. miR-361-5p Regulates VEGFA by Directly Binding to Its 3′UTR
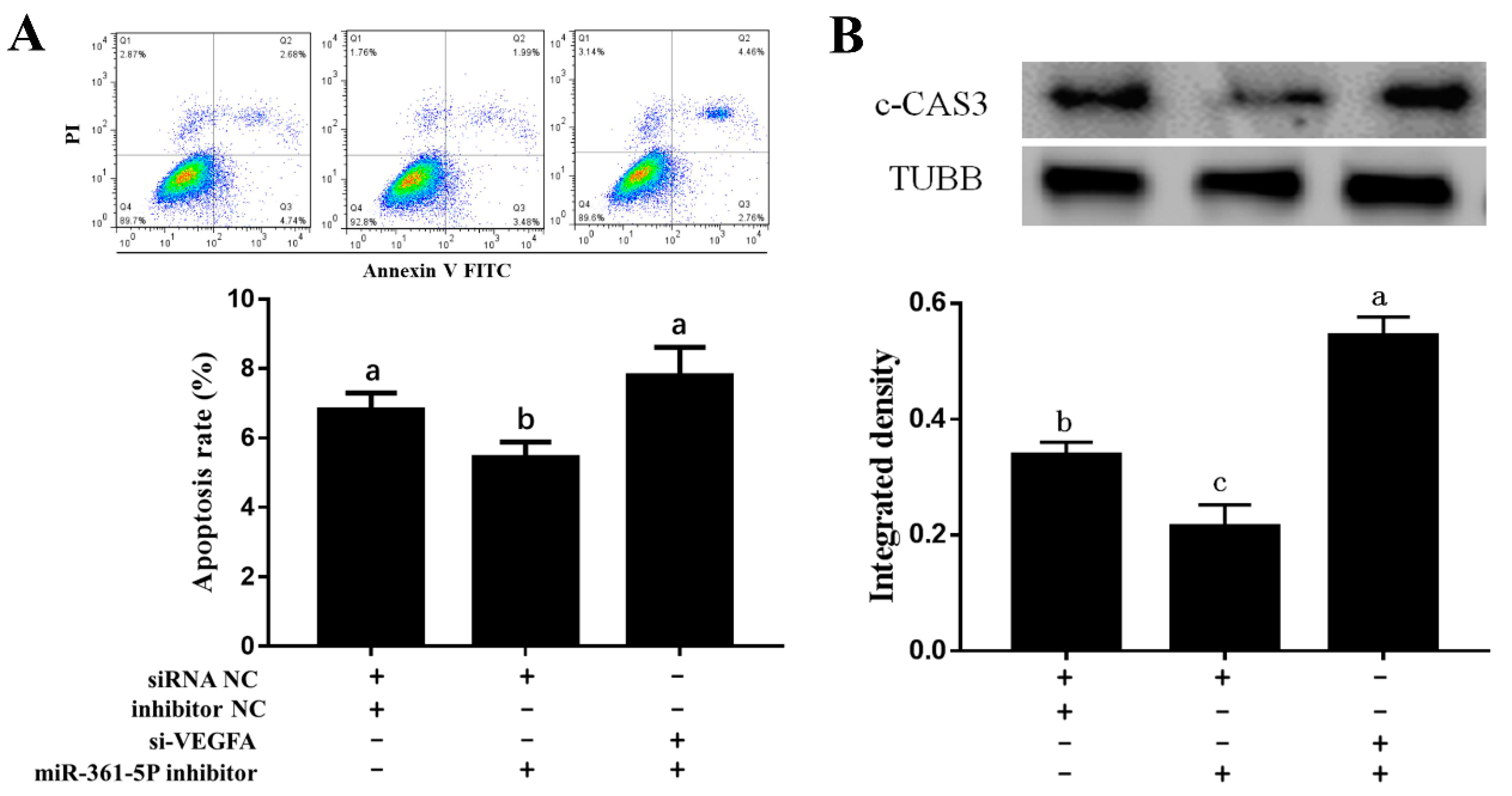
3.4. miR-361-5p Regulates GC Apoptosis through VEGFA
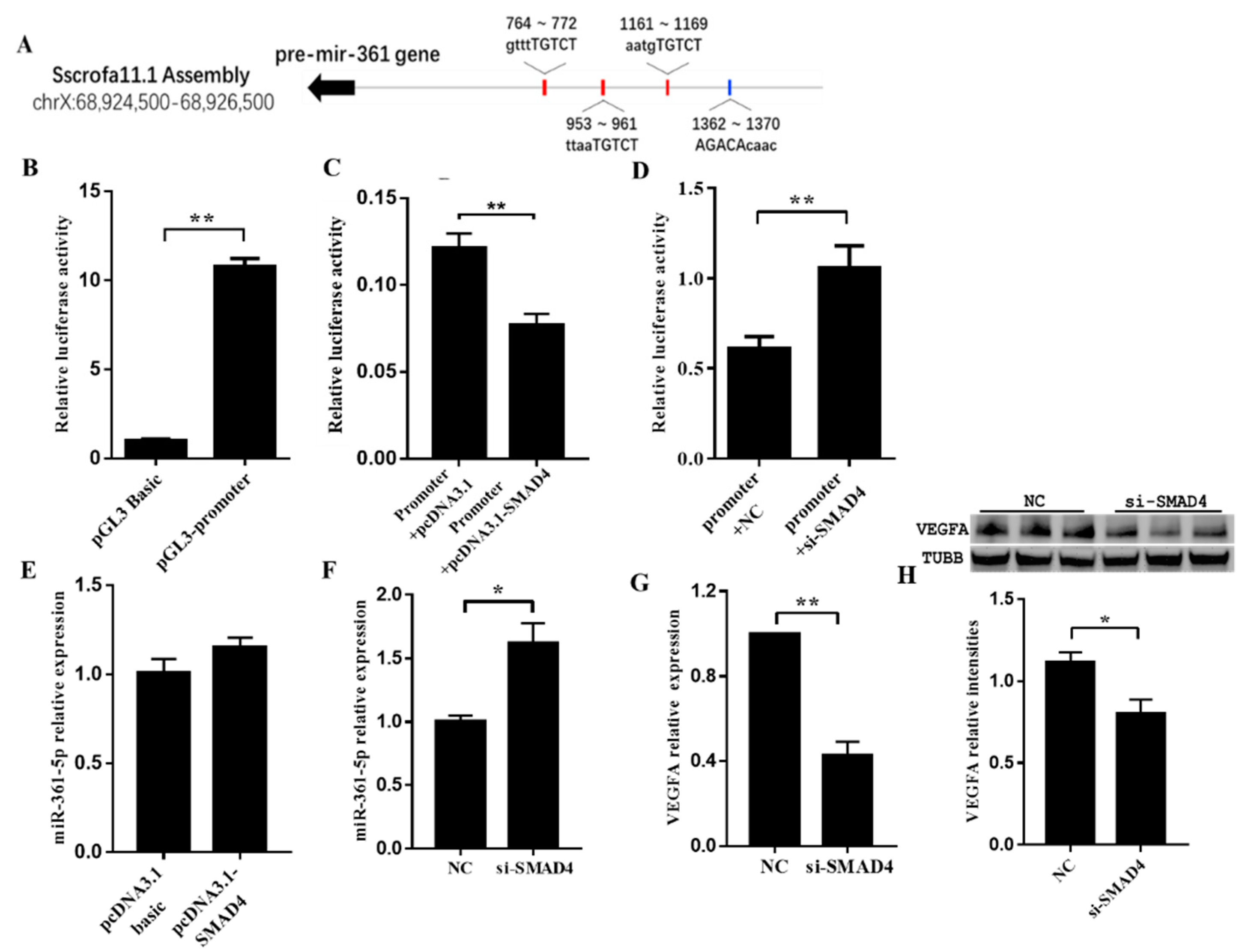
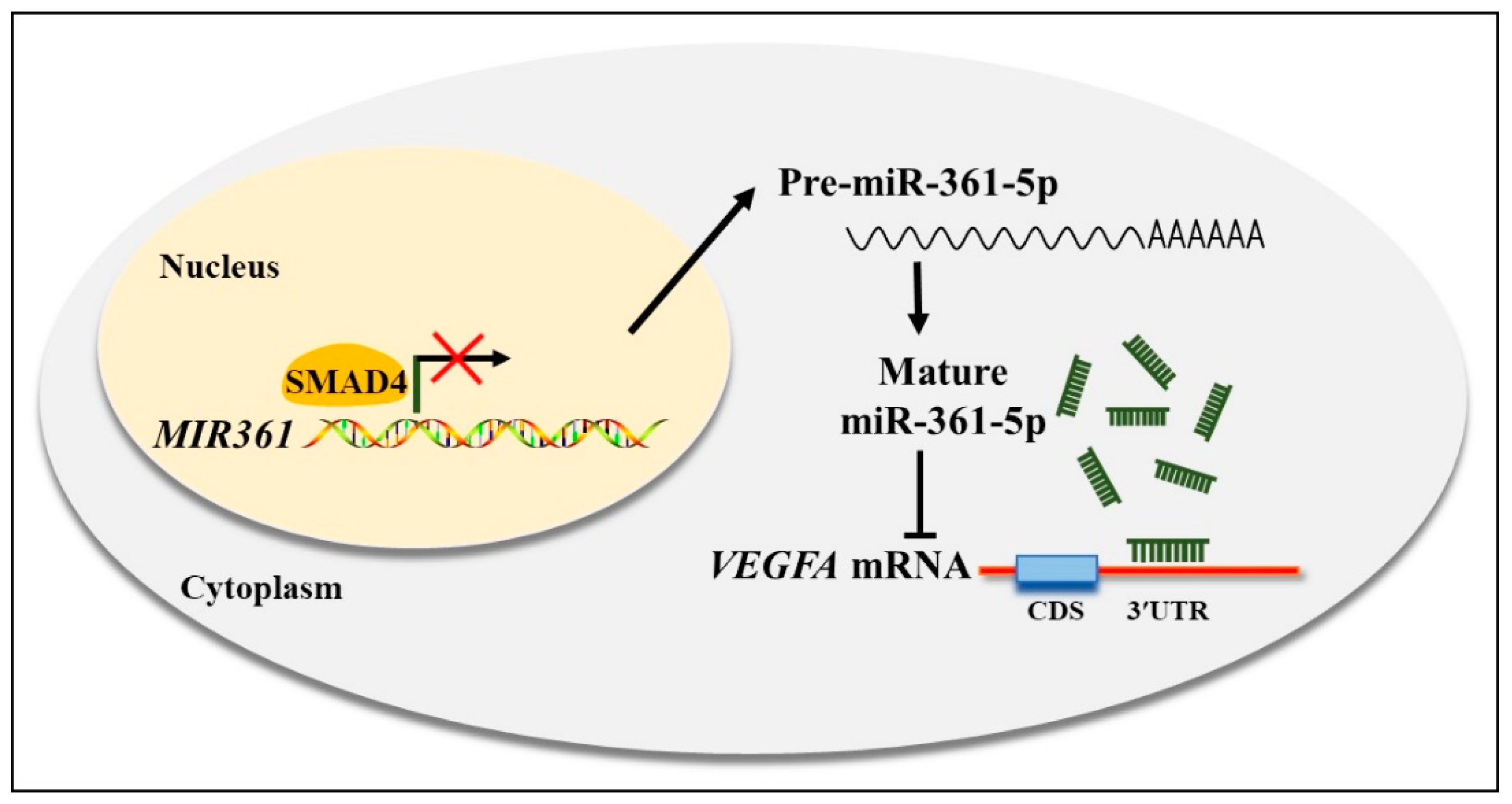
3.5. SMAD4 Involved in miR-361-5p-Mediated VEGFA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marchal, R.; Vigneron, C.; Perreau, C.; Bali-Papp, A.; Mermillod, P. Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology 2002, 57, 1523–1532. [Google Scholar] [CrossRef]
- Manabe, N.; Goto, Y.; Matsuda-Minehata, F.; Inoue, N.; Maeda, A.; Sakamaki, K.; Miyano, T. Regulation Mechanism of Selective Atresia in Porcine Follicles: Regulation of Granulosa Cell Apoptosis during Atresia. J. Reprod. Dev. 2004, 50, 493–514. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Martĺnez-Chequer, J.C.; Molskness, T.A.; Xu, F.; Hazzard, T.M. Regulation and Action of Angiogenic Factors in the Primate Ovary. Arch. Med. Res. 2001, 32, 567–575. [Google Scholar] [CrossRef]
- Albors, O.L.; Olsson, F.; Llinares, A.; Gutiérrez, H.; Latorre, R.; Candanosa, E.; Guillén-Martínez, A.; Izquierdo-Rico, M. Expression of the vascular endothelial growth factor system (VEGF) in the porcine oviduct during the estrous cycle. Theriogenology 2017, 93, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, J.; Connor, K.; Pedersen, H.; Coomber, B.L.; Lamarre, J.; Petrik, J. Vascular Endothelial Growth Factor and Its Receptor, Flk-1/KDR, Are Cytoprotective in the Extravascular Compartment of the Ovarian Follicle. Endocrinology 2004, 145, 2896–2905. [Google Scholar] [CrossRef]
- Yamamoto, S.; Konishi, I.; Tsuruta, Y.; Nanbu, K.; Mandai, M.; Kuroda, H.; Matsushita, K.; Hamid, A.A.; Yura, Y.; Mori, T. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol. Endocrinol. 1997, 11, 371–381. [Google Scholar] [CrossRef]
- Berisha, B.; Schams, D.; Kosmann, M.; Amselgruber, W.; Einspanier, R. Expression and localisation of vascular endothelial growth factor and basic fibroblast growth factor during the final growth of bovine ovarian follicles. J. Endocrinol. 2000, 167, 371–382. [Google Scholar] [CrossRef]
- Shimizu, T.; Jiang, J.-Y.; Sasada, H.; Sato, E. Changes of messenger RNA expression of angiogenic factors and related receptors during follicular development in gilts. Boil. Reprod. 2002, 67, 1846–1852. [Google Scholar] [CrossRef]
- Bianco, F.; Basini, G.; Grasselli, F. Angiogenic activity of swine granulosa cells: Effects of hypoxia and vascular endothelial growth factor Trap R1R2, a VEGF blocker. Domest. Anim. Endocrinol. 2005, 28, 308–319. [Google Scholar] [CrossRef]
- Bianco, F.; Basini, G.; Santini, S.; Grasselli, F. Angiogenic Activity of Swine Granulosa Cells: Effects of Hypoxia and the Role of VEGF. Vet. Res. Commun. 2005, 29, 157–159. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Yao, W.; Li, Q.; Liu, H.; Pan, Z.-X. Initiation of follicular atresia: Gene networks during early atresia in pig ovaries. Reproduction 2018, 156, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Damert, A.; Ikeda, E.; Risau, W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem. J. 1997, 327, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Buteau-Lozano, H.; Ancelin, M.; Lardeux, B.; Milanini, J.; Perrot-Applanat, M. Transcriptional Regulation of Vascular Endothelial Growth Factor by Estradiol and Tamoxifen in Breast Cancer Cells A Complex. Interplay between Estrogen Receptors α and β. Cancer Res. 2002, 62, 4977. [Google Scholar] [PubMed]
- Hanson, J.; Gorman, J.; Reese, J.; Fraizer, G. Regulation of Vascular Endothelial Growth Factor, VEGF, Gene Promoter by the Tumor Suppressor, WT1. Front. Biosci. 2007, 12, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.-T.; Kong, J.; Zhu, X.-D.; Zhang, Y.-Y.; Lu, L.; Zhou, J.-M.; Wang, L.-R.; Zhang, K.-Z.; Zhang, Q.-B.; Ao, J.-Y.; et al. MicroRNA-26a Inhibits Angiogenesis by Down-Regulating VEGFA through the PIK3C2α/Akt/HIF-1α Pathway in Hepatocellular Carcinoma. PLoS ONE 2013, 8, 77957. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.J.; Danesh, F.R. Identification of MicroRNA-93 as a Novel Regulator of Vascular Endothelial Growth Factor in Hyperglycemic Conditions. J. Boil. Chem. 2010, 285, 23457–23465. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, Z.; Xu, J.; Chen, C.; Ge, Q.; Li, P.; Wei, D.; Wu, Z.; Sun, X. Micro RNA -134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR 1 pathway. FEBS J. 2018, 285, 1359–1371. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Zhang, H.-F.; Su, J.-Y. Downregulation of microRNA-195 promotes angiogenesis induced by cerebral infarction via targeting VEGFA. Mol. Med. Rep. 2017, 16, 5434–5440. [Google Scholar] [CrossRef]
- Zhu, X.; Er, K.; Mao, C.; Yan, Q.; Xu, H.; Zhang, Y.; Zhu, J.; Cui, F.; Zhao, W.; Shi, H. miR-203 Suppresses Tumor Growth and Angiogenesis by Targeting VEGFA in Cervical Cancer. Cell. Physiol. Biochem. 2013, 32, 64–73. [Google Scholar] [CrossRef]
- Kanitz, A.; Imig, J.; Dziunycz, P.J.; Primorac, A.; Galgano, A.; Hofbauer, G.F.L.; Gerber, A.P.; Detmar, M. The Expression Levels of MicroRNA-361-5p and Its Target VEGFA Are Inversely Correlated in Human Cutaneous Squamous Cell Carcinoma. PLoS ONE 2012, 7, e49568. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, R.; Si, W.; Li, S.; Xu, Y.; Tu, X.; Wang, Q.K. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013, 333, 159–169. [Google Scholar] [CrossRef]
- Quezada, M.; Wang, J.; Hoang, V.; McGee, E.A. Smad7 is a transforming growth factor-beta–inducible mediator of apoptosis in granulosa cells. Fertil. Steril. 2012, 97, 1452–1459.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wang, W.; He, Y.; Wang, L.; Tian, K.X.; Song, X.G.; Xu, Y.X. Effects of Silencing Smad4 Gene by Small Interfering RNA on Apoptosis of Porcine Granulosa Cells. Chin. J. Cell Biol. 2010, 32, 596–600. [Google Scholar]
- Xing, D.; Zhang, L.; Li, X.; Pan, Z.; Liu, H.; Li, Q. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell Death Dis. 2016, 7, 2476. [Google Scholar]
- Gao, X.; Zhang, J.; Pan, Z.; Li, Q.; Liu, H. The distribution and expression of vascular endothelial growth factor A (VEGFA) during follicular development and atresia in the pig. Reprod. Fertil. Dev. 2020, 32, 259. [Google Scholar] [CrossRef]
- Du, X.; Pan, Z.; Li, Q.; Liu, H.; Li, Q. SMAD4 feedback regulates the canonical TGF-β signaling pathway to control granulosa cell apoptosis. Cell Death Dis. 2018, 9, 151. [Google Scholar] [CrossRef]
- Lin, F.; Li, R.; Pan, Z.X.; Zhou, B.; Yu, D.B.; Wang, X.G.; Ma, X.S.; Han, J.; Shen, M.; Liu, H.L. miR-26b Promotes Granulosa Cell Apoptosis by Targeting ATM during Follicular Atresia in Porcine Ovary. PLoS ONE 2012, 7, e38640. [Google Scholar] [CrossRef]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. BBA-Bioenergetics 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 Mediates RNA Cleavage Targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.-X.; Liu, H.; Pan, Z.-X. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Boil. Endocrinol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, L.; Ma, L.; Zhang, Y.; Zhang, J.; Guo, B. MiR-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J. Exp. Clin. Cancer Res. 2017, 36, 158. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Song, H.; Guo, B.; Zhang, Y.; Zheng, Y.; Lin, C.; Wu, Y.; Guan, G.; Sha, R.; Zhou, Q.; et al. MiR-361-5p inhibits colorectal and gastric cancer growth and metastasis by targeting staphylococcal nuclease domain containing-1. Oncotarget 2015, 6, 17404–17416. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, B.; Wang, X.; Jiang, H.; Kuang, W. MiR-361-5p inhibits cell proliferation and induces cell apoptosis in retinoblastoma by negatively regulating CLDN8. Childs Nerv. Syst. 2019, 35, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Guo, L.-W.; Seedial, S.M.; Si, Y.; Wang, B.; Takayama, T.; Suwanabol, P.A.; Ghosh, S.; DiRenzo, D.; Liu, B.; et al. TGF-β/Smad3 inhibit vascular smooth muscle cell apoptosis through an autocrine signaling mechanism involving VEGF-A. Cell Death Dis. 2014, 5, e1317. [Google Scholar] [CrossRef] [PubMed]
- Papoutsoglou, P.; Louis, C.; Coulouarn, C. Transforming Growth Factor-Beta (TGFβ) Signaling Pathway in Cholangiocarcinoma. Cells 2019, 8, 960. [Google Scholar] [CrossRef]
- Zhang, L.; Du, X.; Wei, S.; Li, N.; Li, Q. A comprehensive transcriptomic view on the role of SMAD4 gene by RNAi-mediated knockdown in porcine follicular granulosa cells. Reproduction 2016, 152, 81–89. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Li, X.; Wang, L.; Zhang, H.; He, Y.; Wang, J.; Zhao, Y.; Zhang, B.; Xu, Y.-X. Interference RNA-based silencing of endogenous SMAD4 in porcine granulosa cells resulted in decreased FSH-mediated granulosa cells proliferation and steroidogenesis. Reproduction 2011, 141, 643–651. [Google Scholar] [CrossRef]
- Li, Q.; Du, X.; Pan, Z.; Zhang, L.; Li, Q. The transcription factor SMAD4 and miR-10b contribute to E2 release and cell apoptosis in ovarian granulosa cells by targeting CYP19A1. Mol. Cell. Endocrinol. 2018, 476, 84–95. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Zhang, J.; Gao, X.; Yao, W.; Li, Q.; Pan, Z. miR-361-5p Mediates SMAD4 to Promote Porcine Granulosa Cell Apoptosis through VEGFA. Biomolecules 2020, 10, 1281. https://doi.org/10.3390/biom10091281
Ma M, Zhang J, Gao X, Yao W, Li Q, Pan Z. miR-361-5p Mediates SMAD4 to Promote Porcine Granulosa Cell Apoptosis through VEGFA. Biomolecules. 2020; 10(9):1281. https://doi.org/10.3390/biom10091281
Chicago/Turabian StyleMa, Mengnan, Jinbi Zhang, Xiaomeng Gao, Wang Yao, Qifa Li, and Zengxiang Pan. 2020. "miR-361-5p Mediates SMAD4 to Promote Porcine Granulosa Cell Apoptosis through VEGFA" Biomolecules 10, no. 9: 1281. https://doi.org/10.3390/biom10091281
APA StyleMa, M., Zhang, J., Gao, X., Yao, W., Li, Q., & Pan, Z. (2020). miR-361-5p Mediates SMAD4 to Promote Porcine Granulosa Cell Apoptosis through VEGFA. Biomolecules, 10(9), 1281. https://doi.org/10.3390/biom10091281




