Excretory/Secretory Metabolome of the Zoonotic Roundworm Parasite Toxocara canis
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Toxocara canis ESPs and Sample Preparation
2.2. Cryomill Extraction of ESP Samples for GC-MS Analysis
2.3. Targeted Analysis of Polar Metabolites Using GC-MS
2.4. Targeted Analysis of Medium–Long Chain Fatty Acids (MLCFAs) Using GC-MS
2.5. Targeted Analysis of Short Chain Fatty Acids Using LC-MS
2.6. Metabolite Identification Standards and Data Analysis
2.7. Literature Assessment of the Small Molecules Identified from Toxocara canis ESPs
3. Results
3.1. Polar Metabolites of Toxocara canis ESPs Identified Using GC-MS
3.2. Reported Biological Activities of Polar Metabolites
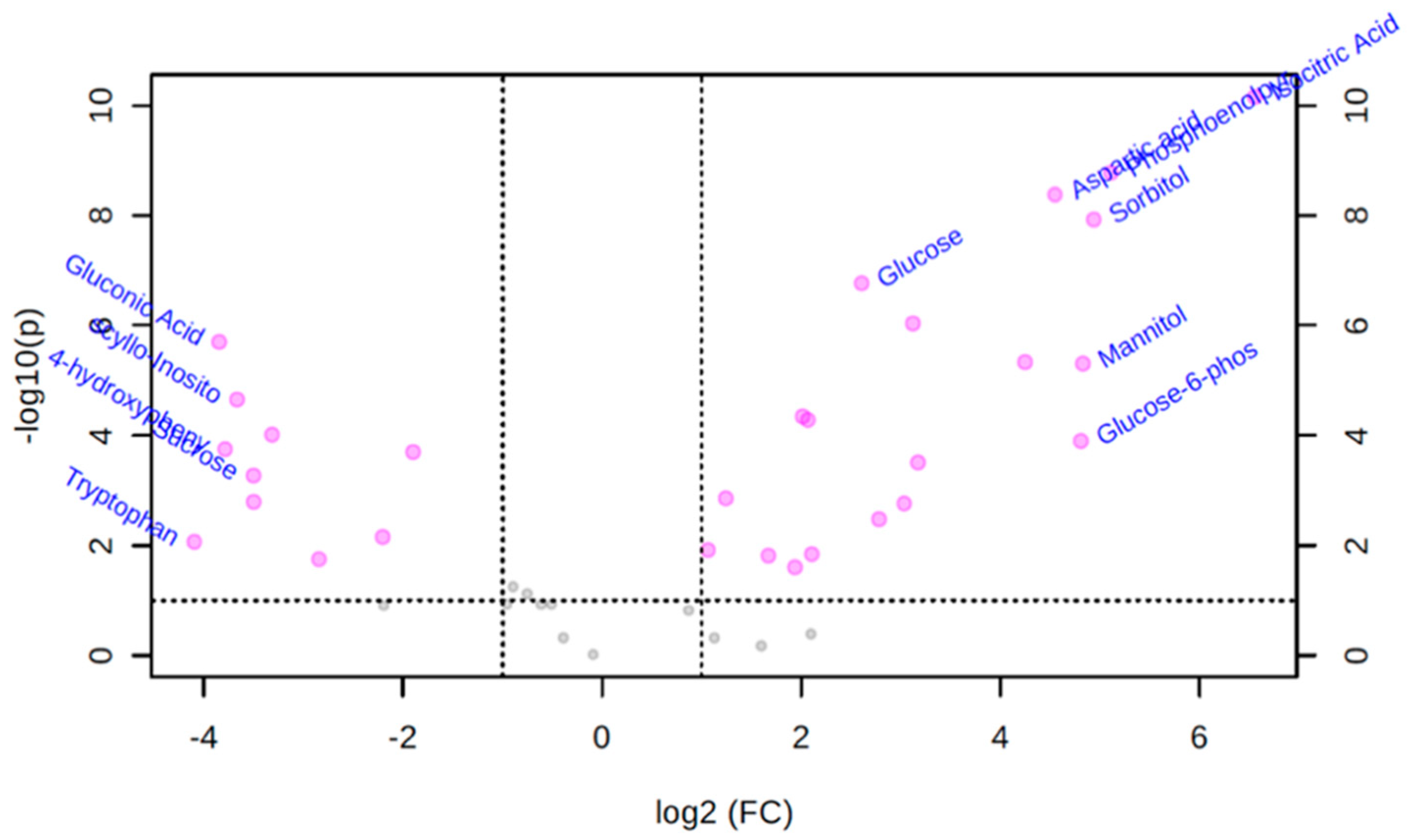
3.3. Univariate Volcano Plot Analysis of Polar Metabolites
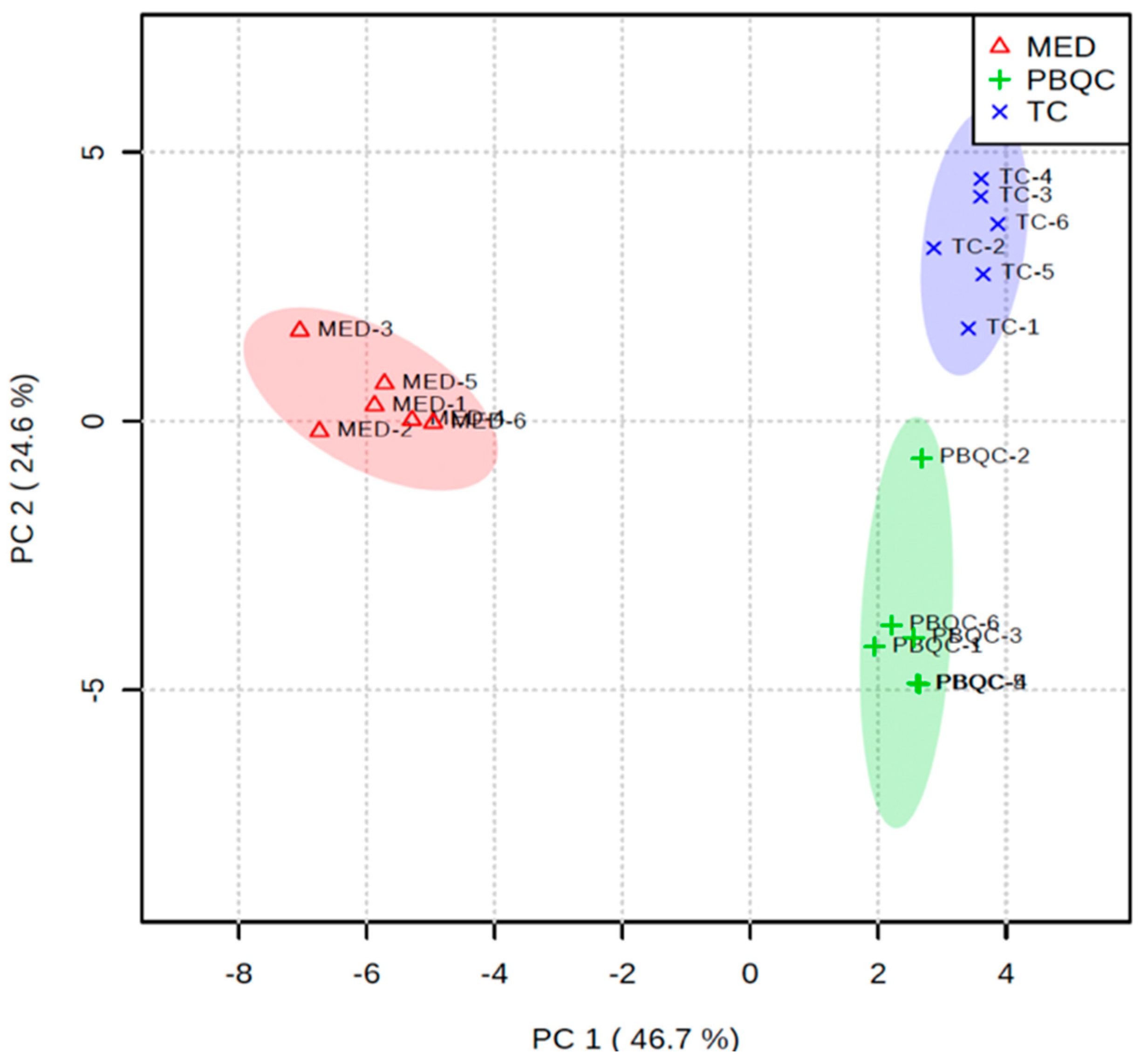
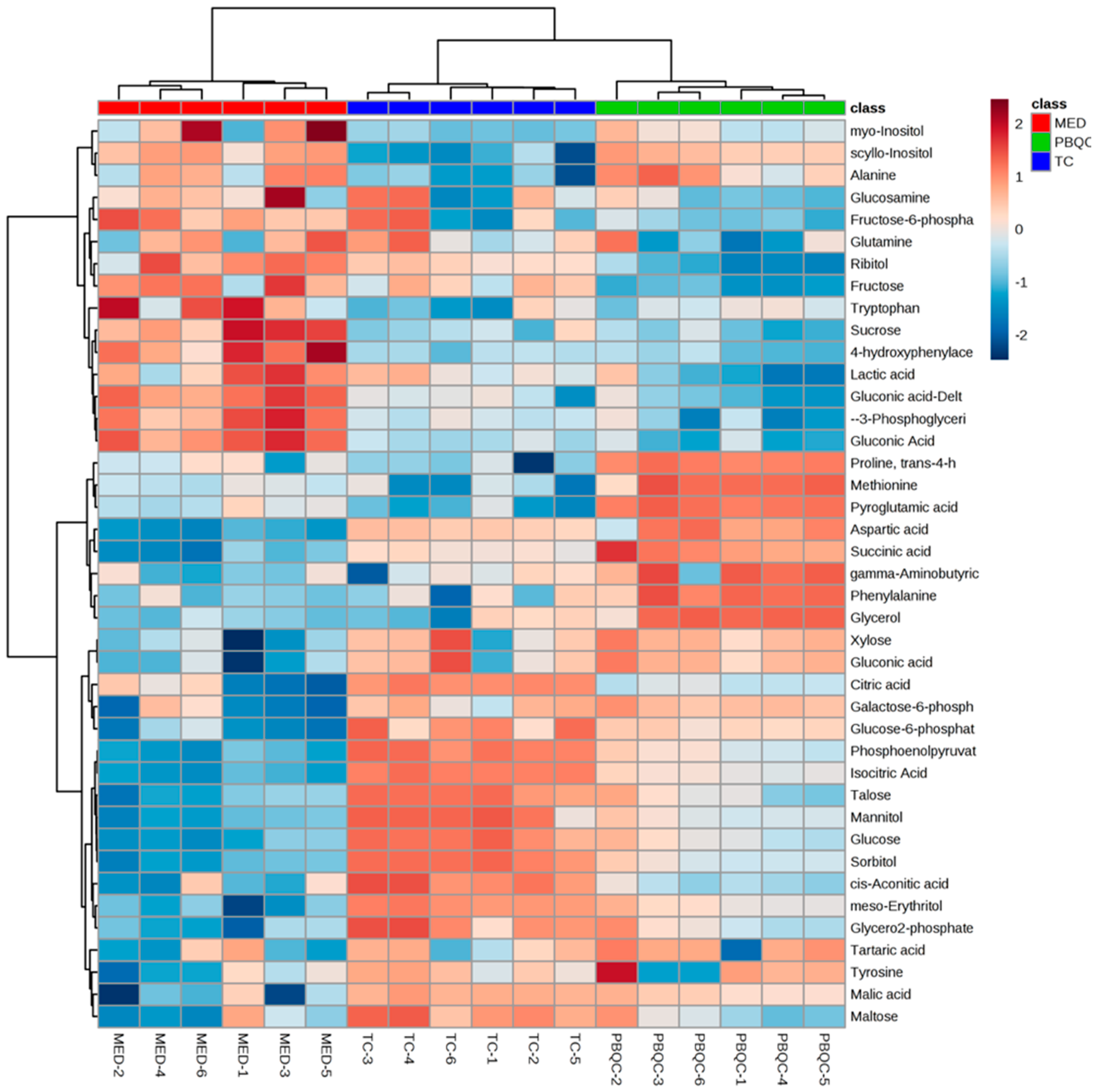
3.4. Multivariate Chemometric Analyses of Polar Metabolites
3.5. Non-Polar Components of T. canis ESPs
3.5.1. Medium–Long Chain Fatty Acids Identified Using GC-MS
3.5.2. Short Chain Fatty Acids Identified Using LC-MS
3.6. Potential Biological Properties of Non-Polar Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wangchuk, P.; Constantinoiu, C.; Eichenberger, R.M.; Field, M.; Loukas, A. Characterization of Tapeworm Metabolites and Their Reported Biological Activities. Molecules 2019, 24, 1480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Q.; Liu, G.-H.; Zheng, W.-B.; Hong, S.-J.; Sugiyama, H.; Zhu, X.-Q.; Elsheikha, H.M. Toxocariasis: A silent threat with a progressive public health impact. Infect. Dis. Poverty 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.K.C.; Yamasaki, H.; Mak, J.W.; Wong, S.F.; Chong, C.W.; Yap, I.K.S.; Ambu, S.; Kumarasamy, V. Field evaluation of a rapid diagnostic test to detect antibodies in human toxocariasis. Acta Trop. 2015, 148, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.Y.; Schantz, P.M.; Kazacos, K.R.; Montgomery, S.P.; Bowman, D.D. Epidemiologic and zoonotic aspects of ascarid infections in dogs and cats. Trends Parasitol. 2010, 26, 155–161. [Google Scholar] [CrossRef]
- Taira, K.; Saeed, I.; Permin, A.; Kapel, C.M.O. Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet. Parasitol. 2004, 121, 115–124. [Google Scholar] [CrossRef]
- Nagakura, K.; Tachibana, H.; Kaneda, Y.; Kato, Y. Toxocariasis Possibly Caused by Ingesting Raw Chicken. J. Infect. Dis. 1989, 160, 735–736. [Google Scholar] [CrossRef]
- Salem, G.; Schantz, P. Toxocaral Visceral Larva Migrans After Ingestion of Raw Lamb Liver. Clin. Infect. Dis. 1992, 15, 743–744. [Google Scholar] [CrossRef]
- Stürchler, D.; Weiss, N.; Gassner, M. Transmission of Toxocariasis. J. Infect. Dis. 1990, 162, 571. [Google Scholar] [CrossRef]
- Choi, D.; Lim, J.H.; Choi, D.-C.; Paik, S.W.; Kim, S.-H.; Huh, S. Toxocariasis and Ingestion of Raw Cow Liver in Patients with Eosinophilia. Korean J. Parasitol. 2008, 46, 139–143. [Google Scholar] [CrossRef][Green Version]
- Strube, C.; Heuer, L.; Janecek, E. Toxocara spp. infections in paratenic hosts. Vet. Parasitol. 2013, 193, 375–389. [Google Scholar] [CrossRef]
- Rostami, A.; Ma, G.; Wang, T.; Koehler, A.V.; Hofmann, A.; Chang, B.C.H.; Macpherson, C.N.; Gasser, R.B. Human toxocariasis—A look at a neglected disease through an epidemiological ‘prism’. Infect. Genet. Evol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.L.; Kremer, F.S.; Aires Berne, M.E.; de Costa Avila, L.F.; da Silva Pinto, L.; Monteiro, K.M.; Caumo, K.S.; Ferreira, H.B.; Berne, N.; Borsuk, S. Proteomic analysis of Toxocara canis excretory and secretory (TES) proteins. Mol. Biochem. Parasitol. 2017, 211, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Q.; Korhonen, P.K.; Cai, H.; Young, N.D.; Nejsum, P.; von Samson-Himmelstjerna, G.; Boag, P.R.; Tan, P.; Li, Q.; Min, J.; et al. Genetic blueprint of the zoonotic pathogen Toxocara canis. Nat. Commun. 2015, 6, 6145. [Google Scholar] [CrossRef] [PubMed]
- Preidis, G.A.; Hotez, P.J. The newest “omics”—Metagenomics and metabolomics—Enter the battle against the neglected tropical diseases. PLoS Negl. Trop. Dis. 2015, 9, e0003382. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.Y.; Choi, Y.H.; Verpoorte, R.; Wilson, E.G. Extraction for metabolomics: Access to the metabolome. Phytochem. Anal. 2014, 25, 291–306. [Google Scholar] [CrossRef]
- Maizels, R.M. Toxocara canis: Molecular basis of immune recognition and evasion. Vet. Parasitol. 2013, 193, 365–374. [Google Scholar] [CrossRef]
- Loukas, A.; Hintz, M.; Linder, D.; Mullin, N.P.; Parkinson, J.; Tetteh, K.K.A.; Maizels, R.M. A Family of Secreted Mucins from the Parasitic Nematode Toxocara canis Bears Diverse Mucin Domains but Shares Similar Flanking Six-cysteine Repeat Motifs. J. Biol. Chem. 2000, 275, 39600–39607. [Google Scholar] [CrossRef]
- Khoo, K.-H.; Maizels, R.M.; Page, A.P.; Taylor, G.W.; Rendell, N.B.; Dell, A. Characterization of nematode glycoproteins: The major O-glycans of Toxocara excretory-secretory antigens are O-methylated trisaccharides. Glycobiology 1991, 1, 163–171. [Google Scholar] [CrossRef]
- Schabussova, I.; Amer, H.; van Die, I.; Kosma, P.; Maizels, R.M. O-Methylated glycans from Toxocara are specific targets for antibody binding in human and animal infections. Int. J. Parasitol. 2007, 37, 97–109. [Google Scholar] [CrossRef]
- Carlson, D.A.; Langley, P.A.; Huyton, P. Sex pheromone of the tsetse fly: Isolation, identification, and synthesis of contact aphrodisiacs. Science 1978, 201, 750–753. [Google Scholar] [CrossRef]
- Lima, E.O.; Esteves, C.Z.; Oliveira, D.N.; Guerreiro, T.M.; Melo, C.F.O.R.; Catharino, R.R. Mass Spectrometry and Metabolomics—New Approaches for Helminth Biochemical Studies. In Human Helminthiasis; Rodrigo, L., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Aguero, F.; Al-Lazikani, B.; Aslett, M.; Berriman, M.; Buckner, F.S.; Campbell, R.K.; Carmona, S.; Carruthers, I.M.; Chan, A.W.; Chen, F.; et al. Genomic-scale prioritization of drug targets: The TDR Targets database. Nat. Rev. Drug Discov. 2008, 7, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Bjorn, F.C.; Llinas, M. Eating at the table of another: Metabolomics of host/parasite interactions. Cell Host Microbe 2010, 7, 90–99. [Google Scholar]
- Wangchuk, P.; Kouremenos, K.; Eichenberger, R.M.; Pearson, M.; Susianto, A.; Wishart, D.S.; McConville, M.J.; Loukas, A. Metabolomic profiling of the excretory–secretory products of hookworm and whipworm. Metabolomics 2019, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Shepherd, C.; Constantinoiu, C.; Ryan, R.Y.M.; Kouremenos, K.A.; Becker, L.; Jones, L.; Buitrago, G.; Giacomin, P.; Wilson, D.; et al. Hookworm-derived metabolites suppress pathology in a mouse model of colitis and inhibit secretion of key inflammatory cytokines in primary human leukocytes. Infect. Immun. 2019. [Google Scholar] [CrossRef]
- Overgaard, A.J.; Weir, J.M.; De Souza, D.P.; Tull, D.; Haase, C.; Meikle, P.J.; Pociot, F. Lipidomic and metabolomic characterization of a genetically modified mouse model of the early stages of human type 1 diabetes pathogenesis. Metabolomics 2016, 12, 13. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2017. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Sakoguchi, H.; Yoshihara, A.; Izumori, K.; Sato, M. Screening of biologically active monosaccharides: Growth inhibitory effects of d-allose, d-talose, and l-idose against the nematode Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2016, 80, 1058–1061. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Cavone, L.; Calosi, L.; Cinci, L.; Moroni, F.; Chiarugi, A. Topical mannitol reduces inflammatory edema in a rat model of arthritis. Pharmacology 2012, 89, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, O.; Kosugi, T.; Roncal, C.; Mu, W.; Heinig, M.; Cirillo, P.; Sánchez-Lozada, L.G.; Johnson, R.J.; Nakagawa, T. Fructose Induces the Inflammatory Molecule ICAM-1 in Endothelial Cells. J. Am. Soc. Nephrol. 2008, 19, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric Acid and L-malic Acid on myocardial ischemia/reperfusion injury. Evid. Based Complement. Altern. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef]
- Largo, R.; Alvarez-Soria, M.A.; Dı́ez-Ortego, I.; Calvo, E.; Sánchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1β-induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2003, 11, 290–298. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Han, D.; Kim, H.Y.; Lee, H.J.; Shim, I.; Hahm, D.H. Wound healing activity of gamma-aminobutyric Acid (GABA) in rats. J. Microbiol. Biotechnol. 2007, 17, 1661–1669. [Google Scholar]
- Alamshah, A.; Spreckley, E.; Norton, M.; Kinsey-Jones, J.S.; Amin, A.; Ramgulam, A.; Cao, Y.; Johnson, R.; Saleh, K.; Akalestou, E.; et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int. J. Obes. 2017, 41, 1693–1701. [Google Scholar] [CrossRef]
- Nägeli, M.; Fasshauer, M.; Sommerfeld, J.; Fendel, A.; Brandi, G.; Stover, J.F. Prolonged continuous intravenous infusion of the dipeptide L-alanine- L-glutamine significantly increases plasma glutamine and alanine without elevating brain glutamate in patients with severe traumatic brain injury. Crit. Care 2014, 18, R139. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; Raizel, R.; Hypólito, T.M.; Tirapegui, J. Effects of supplementation with L-glutamine and L-alanine in the body composition of rats submitted to resistance exercise. Rev. Bras. Ciências Esporte 2017, 39, 417–423. [Google Scholar] [CrossRef]
- Raizel, R.; Leite, J.S.; Hypolito, T.M.; Coqueiro, A.Y.; Newsholme, P.; Cruzat, V.F.; Tirapegui, J. Determination of the anti-inflammatory and cytoprotective effects of L-glutamine and L-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br. J. Nutr. 2016, 116, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Szél, E.; Polyánka, H.; Szabó, K.; Hartmann, P.; Degovics, D.; Balázs, B.; Németh, I.B.; Korponyai, C.; Csányi, E.; Kaszaki, J.; et al. Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Zhang, H. Calcium-sensing receptor (CaSR)-mediated anti-inflammatory effects of L-amino acids in intestinal epithelial cells. J. Agric. Food Chem. 2015, 63, 9987–9995. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, Y.; Yang, Y. Effects of dietary L-tryptophan supplementation on intestinal response to chronic unpredictable stress in broilers. Amino Acids 2017, 49, 1227–1236. [Google Scholar] [CrossRef]
- Islam, J.; Sato, S.; Watanabe, K.; Watanabe, T.; Ardiansyah; Hirahara, K.; Aoyama, Y.; Tomita, S.; Aso, H.; Komai, M.; et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J. Nutr. Biochem. 2017, 42, 43–50. [Google Scholar] [CrossRef]
- Jain, P.; Khanna, N.K. Evaluation of anti-inflammatory and analgesic properties of L-glutamine. Agents Actions 1981, 11, 243–249. [Google Scholar] [CrossRef]
- Unnikrishnan, M.K.; Rao, M.N. Antiinflammatory activity of methionine, methionine sulfoxide and methionine sulfone. Agents Actions 1990, 31, 110–112. [Google Scholar] [CrossRef]
- Derakhshanfar, A.; Bidadkosh, A.; Hashempour Sadeghian, M. L-methionine attenuates gentamicin nephrotoxicity in male Wistar rat: Pathological and biochemical findings. Iran. J. Vet. Res. 2009, 10, 323–328. [Google Scholar]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Baba, W.N.; Gani, A.; Wani, T.A.; Gani, A.; Masoodi, F.A. Effect of extraction time on antioxidants and bioactive volatile components of green tea (Camellia sinensis), using GC/MS. Cogent Food Sci. Agric. 2015, 1, 1106387. [Google Scholar] [CrossRef]
- Fava, N.M.N.; Cury, M.C.; Santos, H.A.; Takeuchi-Storm, N.; Strube, C.; Zhu, X.-Q.; Taira, K.; Odoevskaya, I.; Panovag, O.; Mateus, T.L.; et al. Phylogenetic relationships among Toxocara spp. and Toxascaris sp. from different regions of the world. Vet. Parasitol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pöltl, G.; Kerner, D.; Paschinger, K.; Wilson, I.B.H. N-glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues. FEBS J. 2007, 274, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Lok, J.B. Nucleic acid transfection and transgenesis in parasitic nematodes. Parasitology 2012, 139, 574–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kahl, J.; Brattig, N.; Liebau, E. The Untapped Pharmacopeic Potential of Helminths. Trends Parasitol. 2018, 34, 828–842. [Google Scholar] [CrossRef]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef]
- Learmonth, M.P.; Euerby, M.R.; Jacobs, D.E.; Gibbons, W.A. Metabolite mapping of Toxocara canis using one- and two-dimensional proton magnetic resonance spectroscopy. Mol. Biochem. Parasitol. 1987, 25, 293–298. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; van Grinsven, K.W.A.; Henze, K.; van Hellemond, J.J.; Martin, W. Acetate formation in the energy metabolism of parasitic helminths and protists. Int. J. Parasitol. 2010, 40, 387–397. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Rapin, A.; Lebon, L.; Dubey, L.K.; Mosconi, I.; Sarter, K.; Piersigilli, A.; Menin, L.; Walker, A.W.; Rougemont, J.; et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity 2015, 43, 998–1010. [Google Scholar] [CrossRef]
- Lagrue, C.; Poulin, R. Intra- and interspecific competition among helminth parasites: Effects on Coitocaecum parvum life history strategy, size and fecundity. Int. J. Parasitol. 2008, 38, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560s–569s. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.B.; O’Keefe, S.J. Microbial induction of immunity, inflammation, and cancer. Front. Physiol. 2011, 1, 168. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlaqain, B.; Piironen, V.; Knol, J.C.; de Vos, W.M. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. mBio 2017, 8, e00770-17. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaoud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain faty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Paller, V.G.V.; de Chavez, E.R.C. Toxocara (Nematoda: Ascaridida) and Other Soil-Transmitted Helminth Eggs Contaminating Soils in Selected Urban and Rural Areas in the Philippines. Sci. World J. 2014, 2014, 386232. [Google Scholar] [CrossRef]
| Compound Name | Rt (min) | Mass (m/z, M+) | KEGG ID | Peak Area | Immunomodulatory Properties |
|---|---|---|---|---|---|
| Talose | 10.82 | 180 | C06467 | 6,433,882 | Sakogochi et al. [31] showed that talose inhibited the growth of Caenorhabditis elegans. |
| Glucose | 10.85 | 180 | C00031 | 5,706,822 | N/A |
| Sorbitol | 10.99 | 182 | C00794 | 4,350,846 | Used as a laxative and irrigating solution (surgeries) [32]. |
| Mannitol | 10.95 | 182 | C00392 | 3,751,129 | Anti-edema and a diuretic agent [33]. |
| Lactic acid | 6.73 | 90 | C00186 | 1,332,469 | Immunosuppressant and anti-inflammatory [34]. |
| Succinic acid | 8.11 | 118 | C00042 | 963,948 | N/A |
| Fructose | 10.73 | 180 | C02336 | 120,152 | Pro-inflammatory [35] and are associated with hypertension and liver disease [36]. |
| Maltose | 13.61 | 342 | C00208 | 114,953 | N/A |
| Xylose | 9.86 | 150 | C00181 | 90,585 | N/A |
| Gluconic acid | 11.26 | 196 | C00257 | 90,577 | N/A |
| Tyrosine | 10.95 | 181 | C00082 | 53,189 | Prevents stress-induced depletion of norepinephrine and can cure biochemical depression [36]. |
| Malic acid | 9.01 | 134 | C03668 | 46,865 | Anti-aggregant and anti-inflammatory [37]. |
| Glycerol-2-phosphate | 10.11 | 172 | C02979 | 46,569 | N/A |
| meso-Erythritol | 9.05 | 122 | C00503 | 31,069 | N/A |
| Citric acid | 10.54 | 192 | C00158 | 26,859 | Antimicrobial, a natural preservative and cardioprotective [37]. |
| Glucose-6-phosphate | 12.33 | 260 | n/a | 22,966 | N/A |
| Ribitol | 10.09 | 152 | C00474 | 22,726 | N/A |
| Glucosamine | 10.94 | 179 | C00329 | 16,987 | Anti-inflammatory [38] and used for the treatment of osteoarthritis [36]. |
| Isocitric Acid | 10.54 | 192 | C00311 | 16,661 | N/A |
| Gluconic acid-δ -lactone | 10.84 | 178 | C00198 | 16,341 | N/A |
| Pyroglutamic acid | 9.26 | 129 | C01879 | 12,048 | Sold as a “smart drug” for improving blood circulation in the brain [36]. |
| myo-Inositol | 11.65 | 180 | C00137 | 11,665 | Used as a treatment for polycystic ovary syndrome [39]. |
| Sucrose | 13.43 | 342 | C00089 | 10,047 | N/A |
| cis-Aconitic acid | 10.25 | 174 | C00417 | 6546 | N/A |
| Galactose-6-phosphate | 12.32 | 260 | n/a | 6029 | N/A |
| Phosphoenolpyruvate | 9.55 | 168 | C00074 | 6003 | N/A |
| Fructose-6-phosphate | 12.38 | 260 | n/a | 4637 | N/A |
| Aspartic acid | 9.19 | 133 | C00402 | 4042 | Excitatory neurotransmitter [39]. |
| (-)-3-Phosphoglyceric acid | 10.48 | 186 | C00197 | 3590 | N/A |
| γ-Aminobutyric acid (GABA) | 9.28 | 103 | D00058 | 2096 | Controls blood pressure, reduces blood sugar in diabetics [39], and it is a wound healing agent [40]. |
| Tartaric acid | 9.71 | 150 | C02107 | 1927 | N/A |
| Phenylalanine | 9.42 | 165 | C00079 | 1532 | Anti-diabetic [41]. |
| Alanine | 6.93 | 89 | C00041 | 1414 | Anti-inflammatory [42,43,44] and alanine therapy has helped dissolve kidney stones in experimental animals [32]. |
| Glycerol | 10.12 | 92 | C00116 | 1183 | Anti-inflammatory [45]. |
| Tryptophan | 12.20 | 204 | C00806 | 1179 | Anti-inflammatory [46,47,48]. |
| trans-4-Hydroxyproline | 9.22 | 131 | C05147 | 962 | N/A |
| Glutamine | 11.22 | 146 | C00064 | 784 | Anti-inflammatory [49]. Maintains gut barrier function and reduces septic morbidity and the symptoms of Irritable Bowel Syndrome [32]. |
| 4-Hydroxyphenylacetic acid | 11.42 | 152 | C00642 | 539 | N/A |
| Gluconic acid | 11.26 | 196 | C00257 | 221 | N/A |
| Methionine | 9.22 | 149 | C00073 | 81 | Anti-inflammatory [50] and antioxidant [51]. Linked to infants’ intellectual disability, delays in motor skills, sluggishness, muscle weakness, and liver problems [36]. |
| scyllo-Inositol | 11.37 | 180 | C06153 | 24 | Transgenic mice fed with this compound was found to reverse memory deficits, reduce the amount of Aβ plaque in the brains of the mice and reversed other symptoms associated with it [36]. |
| Fatty Acid Name | Rt (min) | Mass (m/z, M+) | KEGG ID | Peak Area | Biological Activities |
|---|---|---|---|---|---|
| Capric acid (C10:0) | 7.68 | 172 | C01571 | 9155 | N/A |
| Undecylic acid C11:0 | 7.36 | 186 | C17715 | 12,033 | N/A |
| Lauric acid (C12:0) | 9.21 | 200 | C02679 | 11,585 | Anti-inflammatory [24]. |
| Tridecylic acid (C13:0) | 9.01 | 214 | N/A | 13,614 | N/A |
| Myristic acid (C14:0) | 11.27 | 228 | C06424 | 24,866 | N/A |
| Pentadecylic acid (C15:0) | 12.52 | 242 | C16537 | 8505 | N/A |
| Palmitic acid (C16:0) | 13.83 | 256 | C00249 | 358,443 | Anti-inflammatory [52]. |
| Margaric acid (C17:0) | 15.26 | 270 | N/A | 6712 | N/A |
| Stearic acid (C18:0) | 15.25 | 284 | C01530 | 565,394 | Anti-inflammatory [53]. |
| Oleic acid (C18:1, 9Z- cis) | 17.15 | 282 | C00712 | 1771 | Anti-inflammatory and wound healing [24], insecticide and fungicide [36]. |
| Linoleic acid (C18:2) | 18.31 | 280 | C01595 | 7507 | Anti-inflammatory and wound healing [24]. |
| Arachidic acid (C20:0) | 19.61 | 312 | C06425 | 11,388 | N/A |
| Arachidonic acid (C20:4) | 18.16 | 304 | C00219 | 1035 | Eicosanoids including prostaglandins, thromboxanes, and leukotrienes mediates inflammation [36]. It regulates platelet aggregation, blood clotting, smooth muscle contraction, leukocyte chemotaxis, inflammatory cytokine production and immune function [36]. |
| Docosahexaenoic acid (C22:6) | 26.83 | 328 | C06429 | 3825 | Anti-inflammatory [24]. It can reduce the level of blood triglycerides in humans, which may reduce the risk of heart disease [32]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangchuk, P.; Lavers, O.; Wishart, D.S.; Loukas, A. Excretory/Secretory Metabolome of the Zoonotic Roundworm Parasite Toxocara canis. Biomolecules 2020, 10, 1157. https://doi.org/10.3390/biom10081157
Wangchuk P, Lavers O, Wishart DS, Loukas A. Excretory/Secretory Metabolome of the Zoonotic Roundworm Parasite Toxocara canis. Biomolecules. 2020; 10(8):1157. https://doi.org/10.3390/biom10081157
Chicago/Turabian StyleWangchuk, Phurpa, Owen Lavers, David S. Wishart, and Alex Loukas. 2020. "Excretory/Secretory Metabolome of the Zoonotic Roundworm Parasite Toxocara canis" Biomolecules 10, no. 8: 1157. https://doi.org/10.3390/biom10081157
APA StyleWangchuk, P., Lavers, O., Wishart, D. S., & Loukas, A. (2020). Excretory/Secretory Metabolome of the Zoonotic Roundworm Parasite Toxocara canis. Biomolecules, 10(8), 1157. https://doi.org/10.3390/biom10081157








