Heparin Administered to Anopheles in Membrane Feeding Assays Blocks Plasmodium Development in the Mosquito
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sugar Feed
2.3. Membrane Blood Feeding Assay
2.4. Detection of Heparin-Cy5 in Mosquitoes
2.5. Ex Vivo Production of Ookinetes and Flow Cytometry Analysis
2.6. Statistical Analysis
3. Results
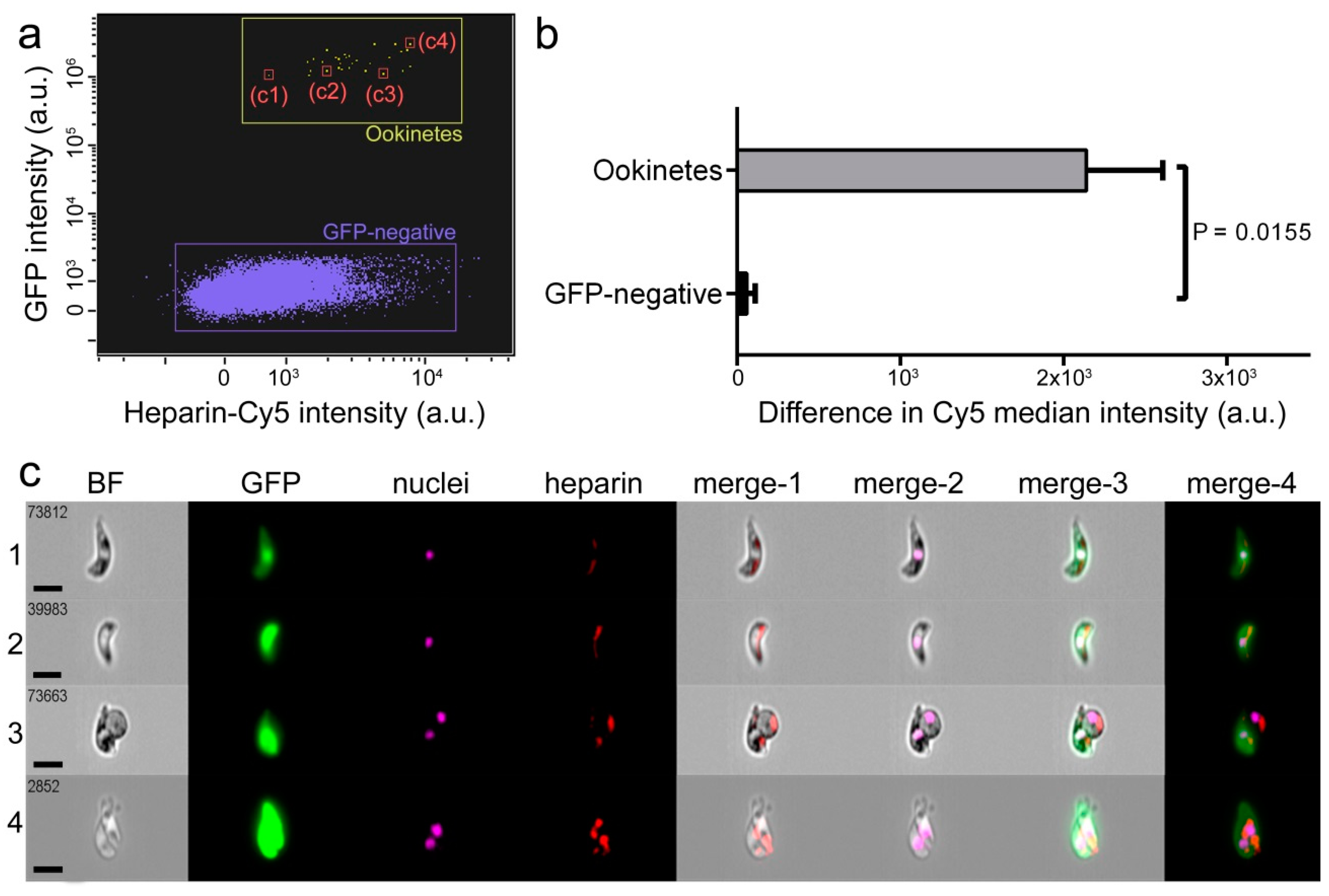
3.1. Characterization of Heparin-Cy5 Binding to Ookinetes
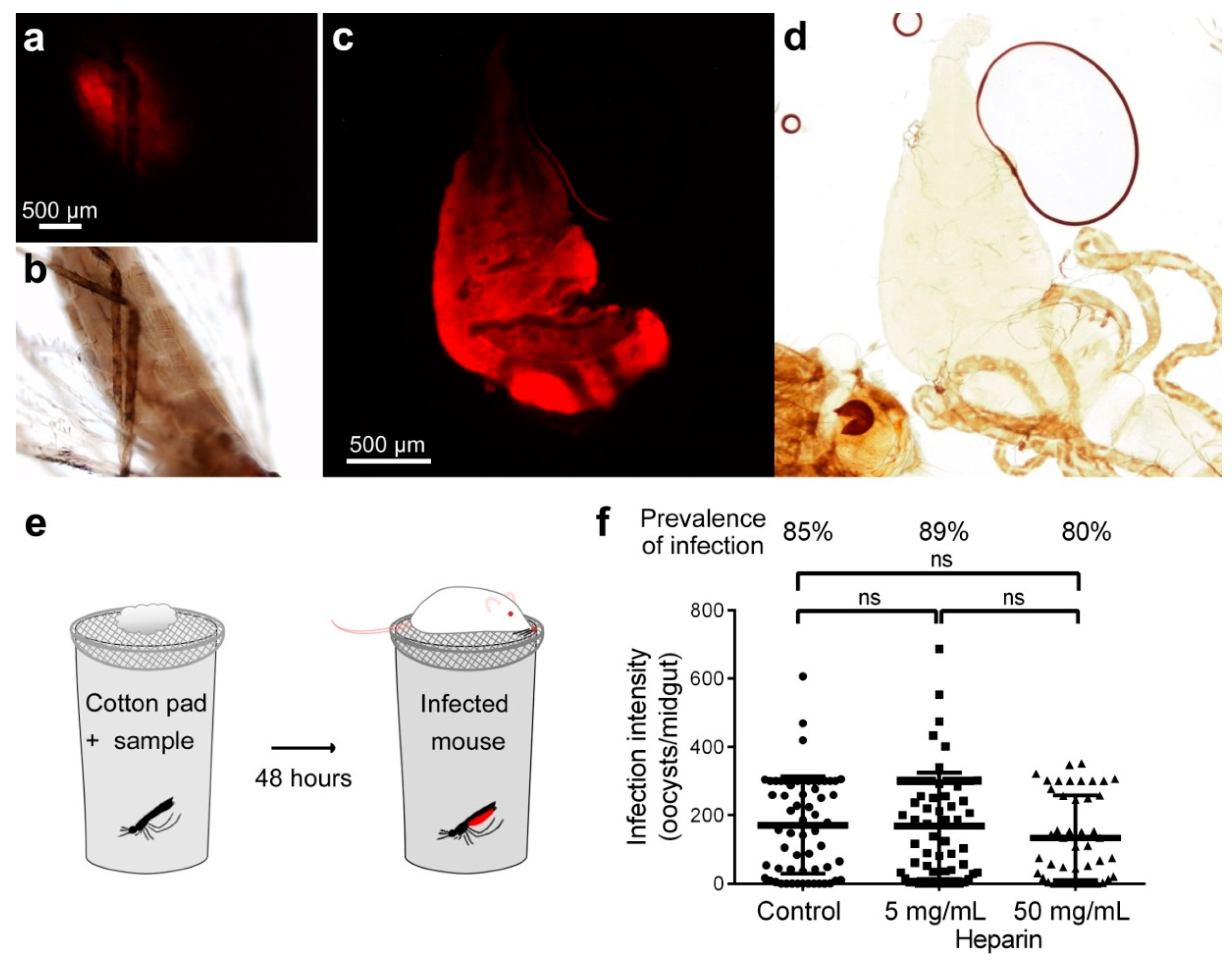
3.2. Effect on Oocyst Development of Heparin Administered to Mosquitoes by Sugar Meal
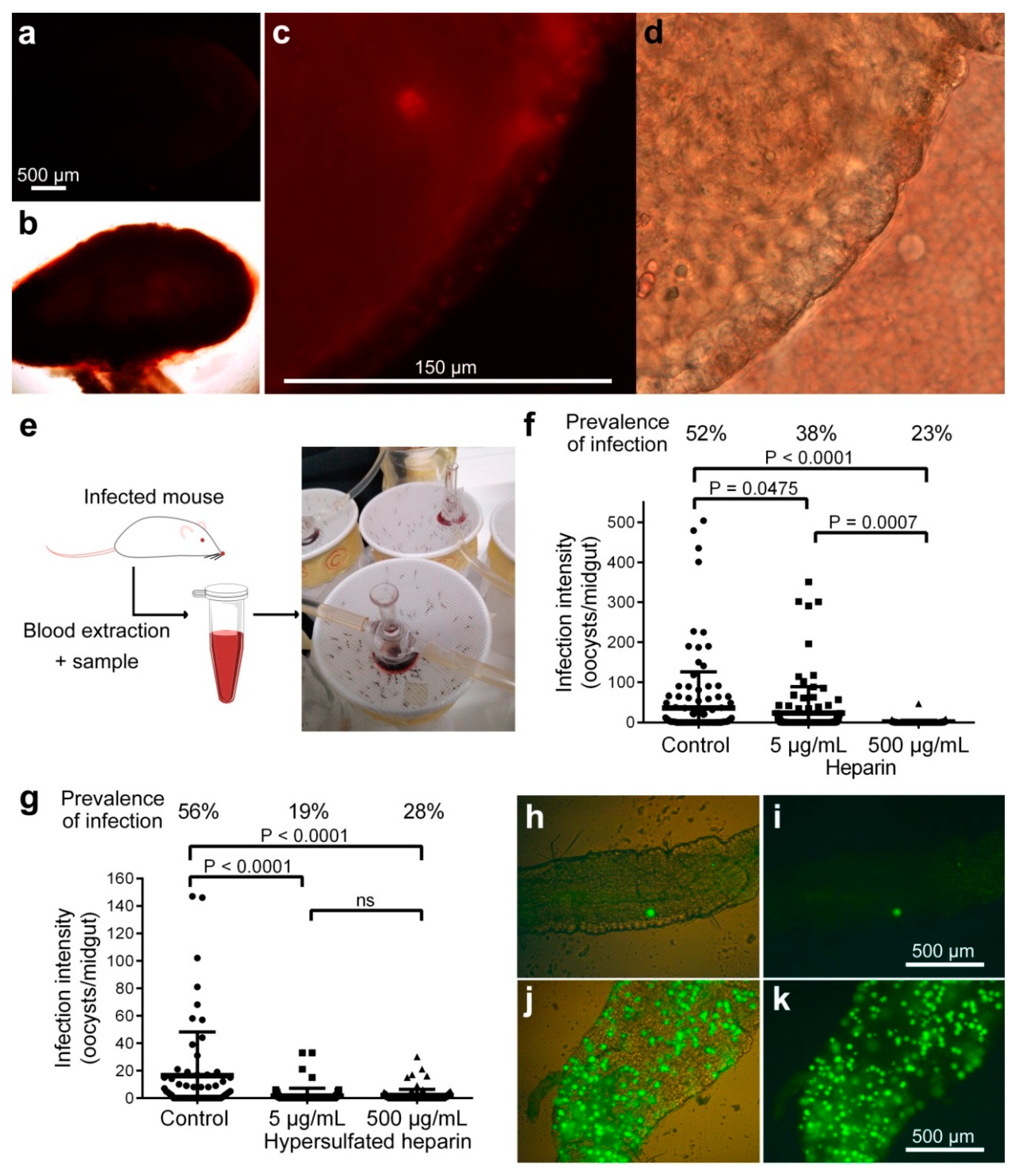
3.3. Effect on Oocyst Development of Heparin Administered to Mosquitoes by Blood Membrane Feeding
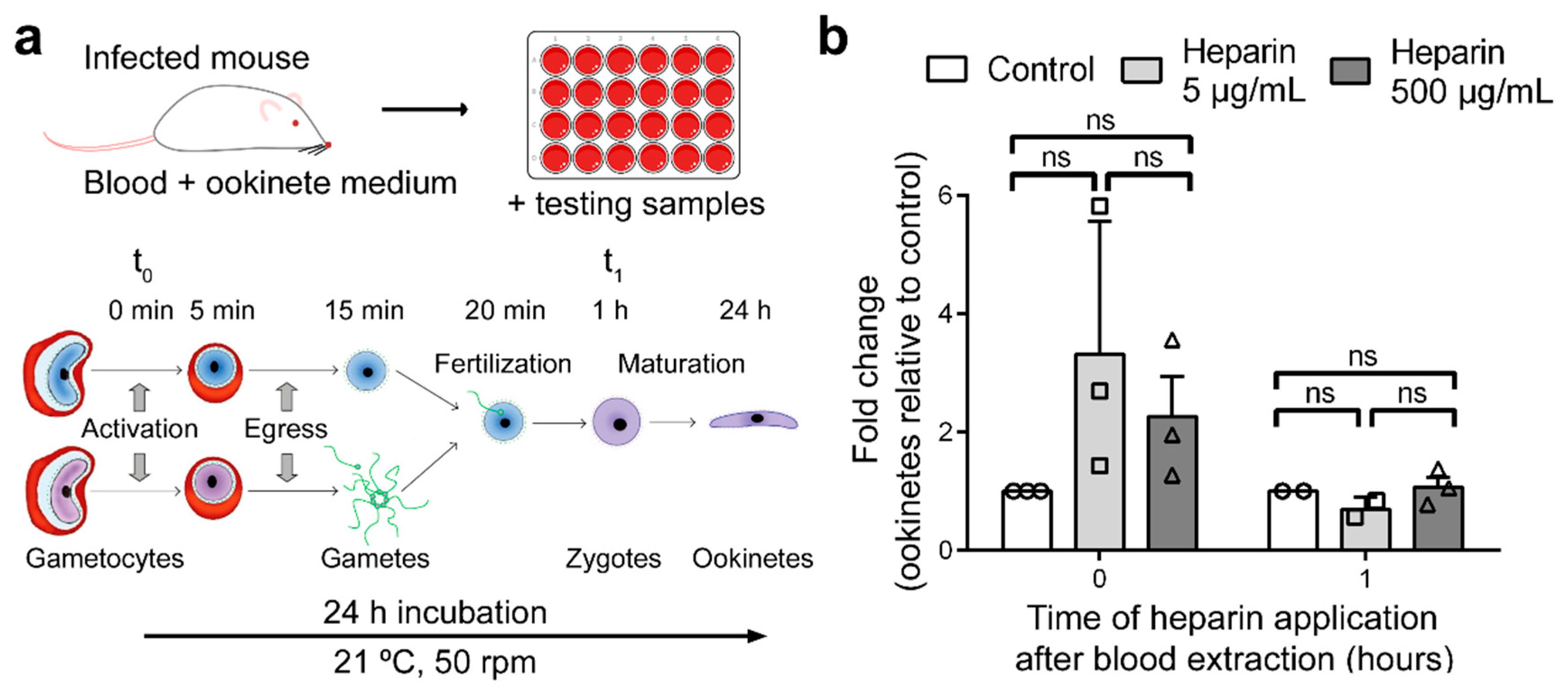
3.4. Effect of Heparin on Fertilization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Sinden, R.; Carter, R.; Drakeley, C.; Leroy, D. The biology of sexual development of Plasmodium: The design and implementation of transmission-blocking strategies. Malar. J. 2012, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.; Sinden, R.E.; Leroy, D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: A comparative study with human and rodent parasites. PLoS Med. 2012, 9, e1001169. [Google Scholar] [CrossRef] [PubMed]
- Kapulu, M.C.; Da, D.F.; Miura, K.; Li, Y.; Blagborough, A.M.; Churcher, T.S.; Nikolaeva, D.; Williams, A.R.; Goodman, A.L.; Sangare, I.; et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci. Rep. 2015, 5, 11193. [Google Scholar] [CrossRef]
- Duffy, P.E.; Kaslow, D.C. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 1997, 65, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Kim, H.H.; Moon, S.U.; Lee, S.S.; Shin, E.H.; Oh, C.M.; Kang, Y.J.; Kim, D.K.; Sohn, Y.; Kim, H.; et al. The role of Pvs28 in sporozoite development in Anopheles sinensis and its longevity in BALB/c mice. Exp. Parasitol. 2011, 127, 346–350. [Google Scholar] [CrossRef]
- Ancsin, J.B.; Kisilevsky, R. A binding site for highly sulfated heparan sulfate is identified in the N terminus of the circumsporozoite protein: Significance for malarial sporozoite attachment to hepatocytes. J. Biol. Chem. 2004, 279, 21824–21832. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.J.; Richards, J.S.; Gilson, P.R.; Chai, W.; Beeson, J.G. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood 2010, 115, 4559–4568. [Google Scholar] [CrossRef]
- Mathias, D.K.; Pastrana-Mena, R.; Ranucci, E.; Tao, D.; Ferruti, P.; Ortega, C.; Staples, G.O.; Zaia, J.; Takashima, E.; Tsuboi, T.; et al. A small molecule glycosaminoglycan mimetic blocks Plasmodium invasion of the mosquito midgut. PLoS Pathog. 2013, 9, e1003757. [Google Scholar] [CrossRef]
- Dinglasan, R.R.; Alaganan, A.; Ghosh, A.K.; Saito, A.; van Kuppevelt, T.H.; Jacobs-Lorena, M. Plasmodium falciparum ookinetes require mosquito midgut chondroitin sulfate proteoglycans for cell invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 15882–15887. [Google Scholar] [CrossRef]
- Marques, J.; Valle-Delgado, J.J.; Urbán, P.; Baró, E.; Prohens, R.; Mayor, A.; Cisteró, P.; Delves, M.; Sinden, R.E.; Grandfils, C.; et al. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomed. NBM 2017, 13, 515–525. [Google Scholar] [CrossRef]
- Li, F.; Templeton, T.J.; Popov, V.; Comer, J.E.; Tsuboi, T.; Torii, M.; Vinetz, J.M. Plasmodium ookinete-secreted proteins secreted through a common micronemal pathway are targets of blocking malaria transmission. J. Biol. Chem. 2004, 279, 26635–26644. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Toida, T.; Imanari, T.; Yu, G.; Linhardt, R.J. Conformational changes and anticoagulant activity of chondroitin sulfate following its O-sulfonation. Carbohydr. Res. 1998, 306, 35–43. [Google Scholar] [CrossRef]
- Franke-Fayard, B.; Trueman, H.; Ramesar, J.; Mendoza, J.; van der Keur, M.; van der Linden, R.; Sinden, R.E.; Waters, A.P.; Janse, C.J. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 2004, 137, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, D.; Zimmermann, T.; Cantera, R.; Janse, C.J.; Waters, A.P.; Kafatos, F.C. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell. Microbiol. 2004, 6, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Blagborough, A.M.; Delves, M.J.; Ramakrishnan, C.; Lal, K.; Butcher, G.; Sinden, R.E. Assessing transmission blockade in Plasmodium spp. In Malaria: Methods and Protocols; Ménard, R., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 577–600. [Google Scholar]
- Bansal, A.; Molina-Cruz, A.; Brzostowski, J.; Mu, J.; Miller, L.H. Plasmodium falciparum calcium-dependent protein kinase 2 is critical for male gametocyte exflagellation but not essential for asexual proliferation. mBio 2017, 8. [Google Scholar] [CrossRef]
- Grant, D.; Moffat, C.F.; Long, W.F.; Williamson, F.B. Ca2+-heparin interaction investigated polarimetrically. Biochem. Soc. Trans. 1991, 19, 391S. [Google Scholar] [CrossRef]
- Solarte, Y.; Manzano, M.R.; Rocha, L.; Castillo, Z.; James, M.A.; Herrera, S.; Arévalo-Herrera, M. Effects of anticoagulants on Plasmodium vivax oocyst development in Anopheles albimanus mosquitoes. Am. J. Trop. Med. Hyg. 2007, 77, 242–245. [Google Scholar] [CrossRef]
- Kuehn, A.; Pradel, G. The coming-out of malaria gametocytes. J. Biomed. Biotechnol. 2010, 2010, 976827. [Google Scholar] [CrossRef]
- Dessens, J.T.; Beetsma, A.L.; Dimopoulos, G.; Wengelnik, K.; Crisanti, A.; Kafatos, F.C.; Sinden, R.E. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999, 18, 6221–6227. [Google Scholar] [CrossRef]
- Wells, T.N.C.; van Huijsduijnen, R.H.; Van Voorhis, W.C. Malaria medicines: A glass half full? Nat. Rev. Drug Discov. 2015, 14, 424–442. [Google Scholar] [CrossRef]
- Paaijmans, K.; Fernàndez-Busquets, X. Antimalarial drug delivery to the mosquito: An option worth exploring? Future Microbiol. 2014, 9, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Chaccour, C.; Kobylinski, K.; Bassat, Q.; Bousema, T.; Drakeley, C.; Alonso, P.; Foy, B. Ivermectin to reduce malaria transmission: A research agenda for a promising new tool for elimination. Malar. J. 2013, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.H.; Curtis, C.F. Optimal life stage for radiation sterilization of Anopheles males and their fitness for release. Med. Vet. Entomol. 2005, 19, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.G.; Childs, L.M.; Itoe, M.A.; Holmdahl, I.E.; Buckee, C.O.; Catteruccia, F. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 2019, 567, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Engelberg, H. Plasma heparin levels in normal man. Circulation 1961, 23, 578–581. [Google Scholar] [CrossRef]
- Boyle, M.J.; Skidmore, M.; Dickerman, B.; Cooper, L.; Devlin, A.; Yates, E.; Horrocks, P.; Freeman, C.; Chai, W.; Beeson, J.G. Identification of heparin modifications and polysaccharide inhibitors of Plasmodium falciparum merozoite invasion that have potential for novel drug development. Antimicrob. Agents Chemother. 2017, 61, e00709–e00717. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Seydel, K.B.; Monteiro, R.Q. Blood coagulation, inflammation, and malaria. Microcirculation 2008, 15, 81–107. [Google Scholar] [CrossRef]
- Dias, L.D.S.; Bauzer, L.G.S.D.; Lima, J.B.P. Artificial blood feeding for Culicidae colony maintenance in laboratories: Does the blood source condition matter? Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e45. [Google Scholar] [CrossRef]
- Haldar, R.; Gupta, D.; Chitranshi, S.; Singh, M.K.; Sachan, S. Artificial blood: A futuristic dimension of modern day transfusion sciences. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Cardoso, J.C.R.; Felix, R.C.; Power, D.M.; Silveira, H. A blood-free diet to rear anopheline mosquitoes. J. Vis. Exp. 2020, 155, e60144. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Cardoso, J.C.R.; Felix, R.C.; Santana, R.A.G.; Guerra, M.D.G.B.; Power, D.; Silveira, H. Fresh-blood-free diet for rearing malaria mosquito vectors. Sci. Rep. 2018, 8, 17807. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.K.; Hansen, I.A. Artificial diets for mosquitoes. Int. J. Environ. Res. Public Health 2016, 13, 1267. [Google Scholar] [CrossRef]
- Romano, D.; Stefanini, C.; Canale, A.; Benelli, G. Artificial blood feeders for mosquito and ticks-Where from, where to? Acta Trop. 2018, 183, 43–56. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lantero, E.; Fernandes, J.; Aláez-Versón, C.R.; Gomes, J.; Silveira, H.; Nogueira, F.; Fernàndez-Busquets, X. Heparin Administered to Anopheles in Membrane Feeding Assays Blocks Plasmodium Development in the Mosquito. Biomolecules 2020, 10, 1136. https://doi.org/10.3390/biom10081136
Lantero E, Fernandes J, Aláez-Versón CR, Gomes J, Silveira H, Nogueira F, Fernàndez-Busquets X. Heparin Administered to Anopheles in Membrane Feeding Assays Blocks Plasmodium Development in the Mosquito. Biomolecules. 2020; 10(8):1136. https://doi.org/10.3390/biom10081136
Chicago/Turabian StyleLantero, Elena, Jessica Fernandes, Carlos Raúl Aláez-Versón, Joana Gomes, Henrique Silveira, Fatima Nogueira, and Xavier Fernàndez-Busquets. 2020. "Heparin Administered to Anopheles in Membrane Feeding Assays Blocks Plasmodium Development in the Mosquito" Biomolecules 10, no. 8: 1136. https://doi.org/10.3390/biom10081136
APA StyleLantero, E., Fernandes, J., Aláez-Versón, C. R., Gomes, J., Silveira, H., Nogueira, F., & Fernàndez-Busquets, X. (2020). Heparin Administered to Anopheles in Membrane Feeding Assays Blocks Plasmodium Development in the Mosquito. Biomolecules, 10(8), 1136. https://doi.org/10.3390/biom10081136






