AGC2 (Citrin) Deficiency—From Recognition of the Disease till Construction of Therapeutic Procedures
Abstract
1. Introduction
2. Materials and Methods
3. Study Started from Enzymology of Argininosuccinate Synthetase (ASS)
4. Pathogenesis of Adult-Onset Type II Citrullinemia
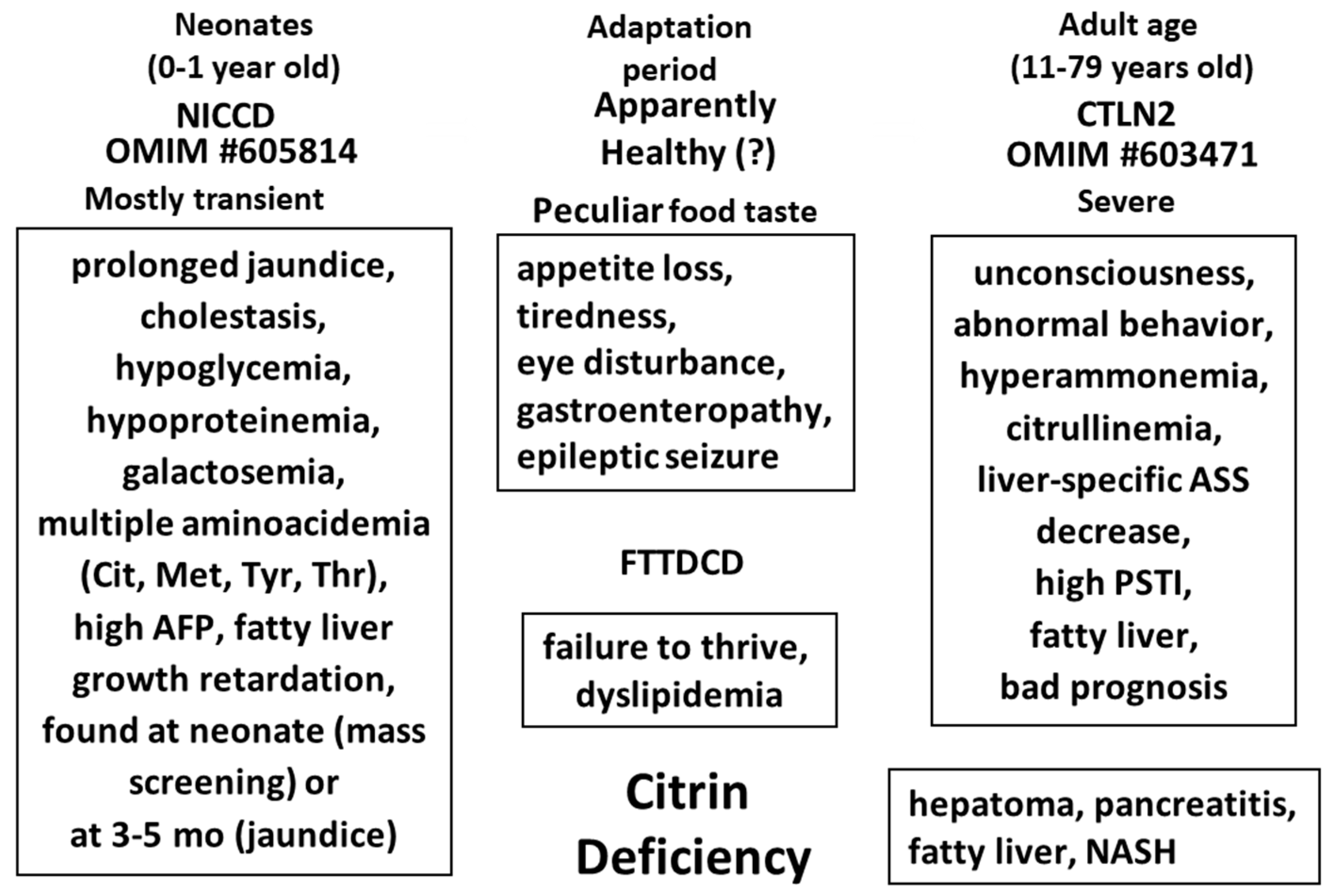
5. Discovery of NICCD (Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency) and Citrin Deficiency Disease Types
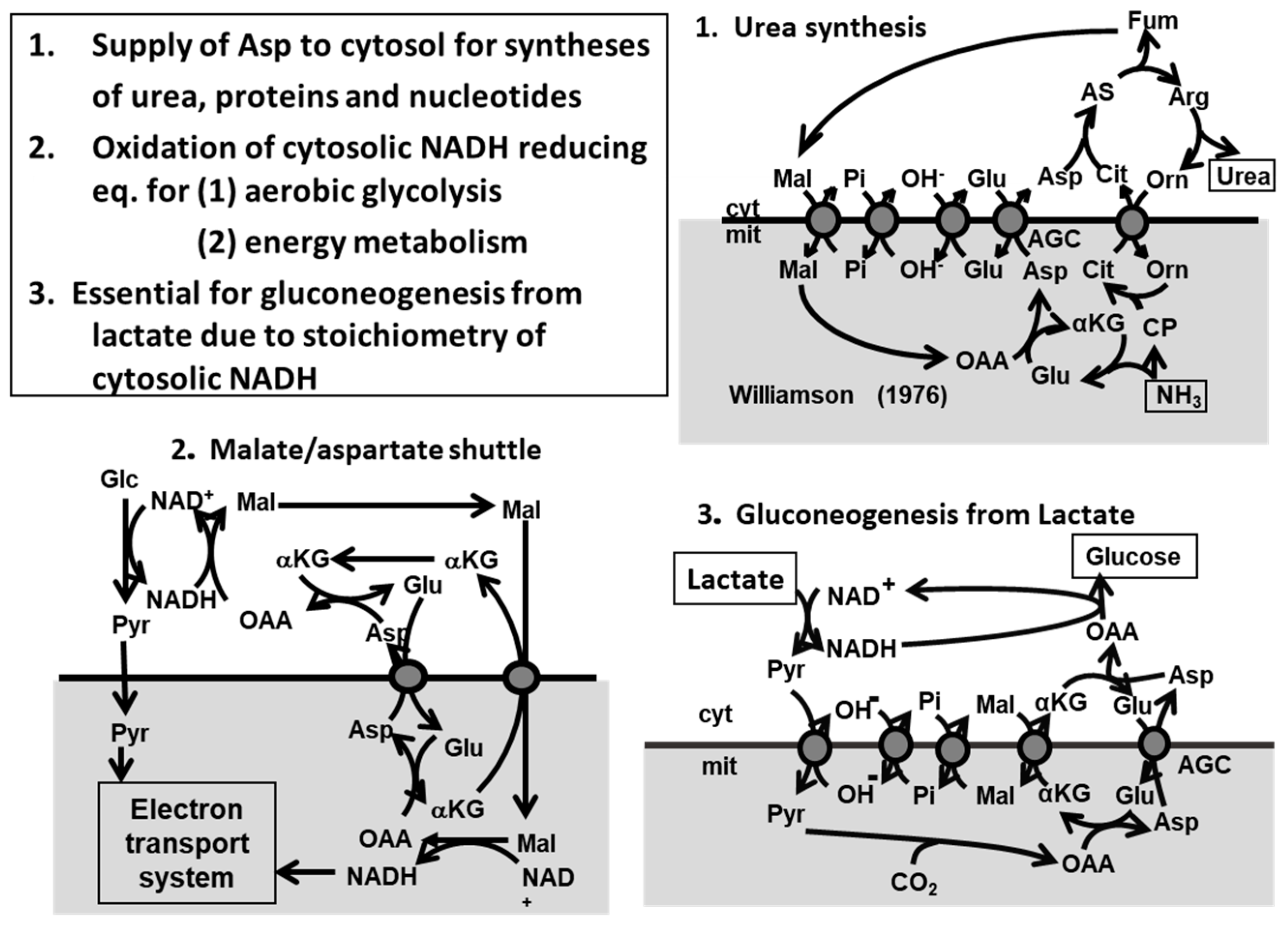
6. The Metabolic Functions of Citrin and Disease Model
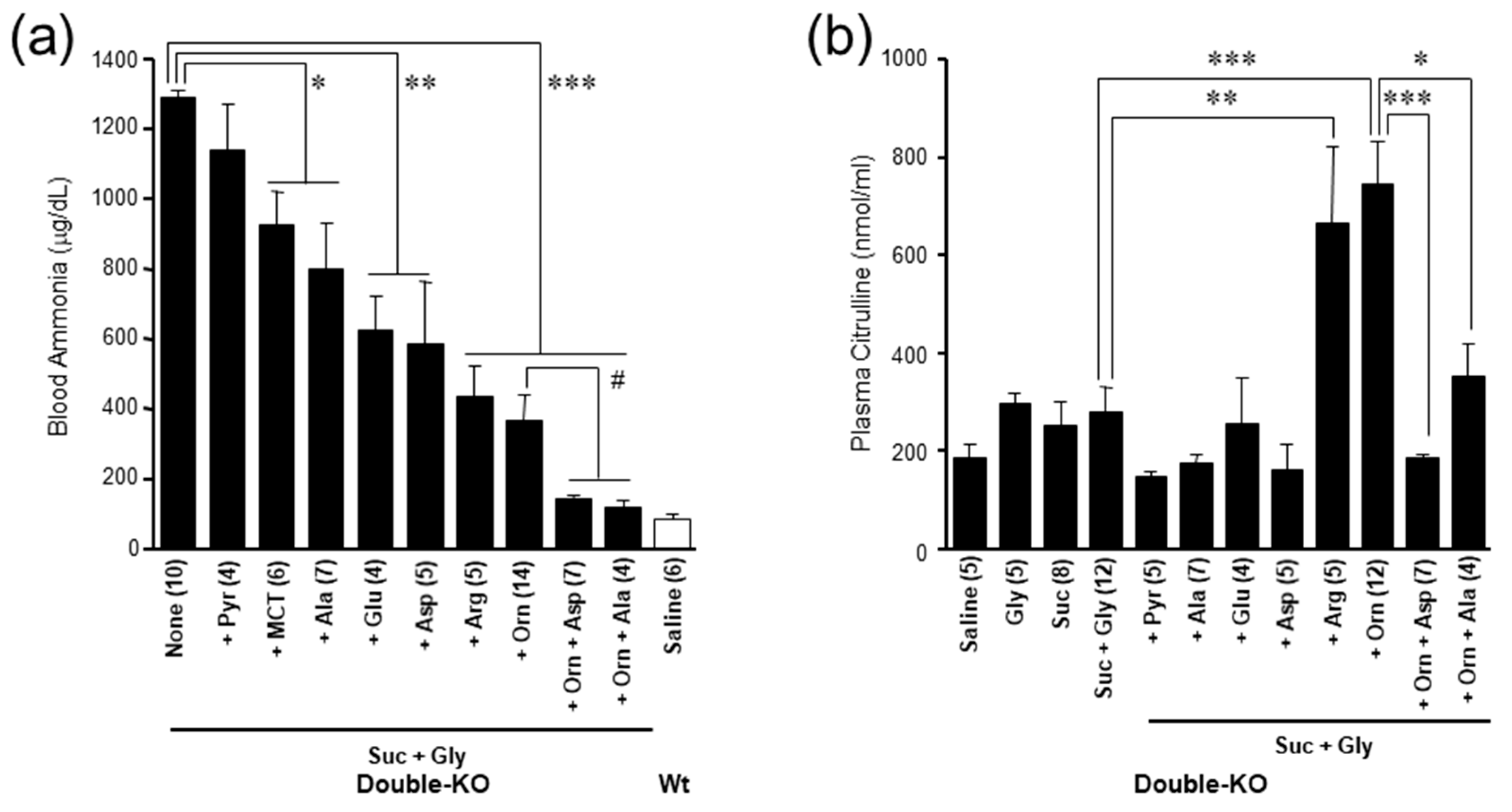
7. Pathophysiology of the Double-KO Mice
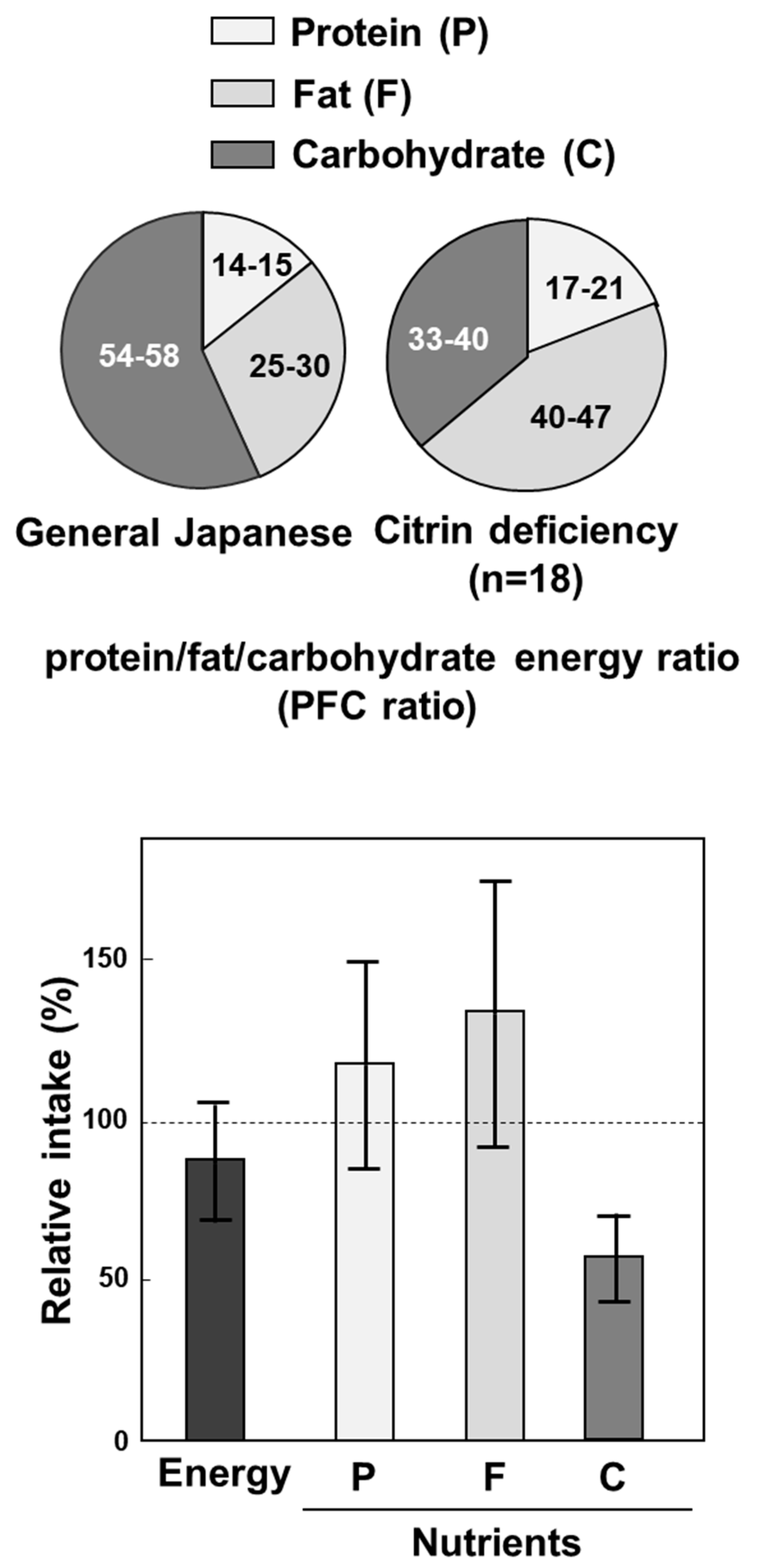
8. Treatment of Citrin Deficiency Based on the Pathophysiology of the Disease
9. Importance of Small Intestine on Metabolism of Amino Acids
10. Drugs or Supplements Used as Therapeutics for Citrin Deficiency at Present
Funding
Acknowledgments
Conflicts of Interest
References
- Saheki, T.; Song, Y.Z. Citrin Deficiency; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Dimmock, D.; Kobayashi, K.; Iijima, M.; Tabata, A.; Wong, L.J.; Saheki, T.; Lee, B.; Scaglia, F. Citrin deficiency: A novel cause of failure to thrive that responds to a high-protein, low-carbohydrate diet. Pediatrics 2007, 119, e773–e777. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Yamaguchi, N.; Kobayashi, K.; Nishi, I.; Horinouchi, H.; Jalil, M.A.; Li, M.X.; Ushikai, M.; Iijima, M.; Kondo, I.; et al. Identification of two novel mutations in the SLC25A13 gene and detection of seven mutations in 102 patients with adult-onset type II citrullinemia. Hum. Genet. 2000, 107, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Kobayashi, K.; Yasuda, T.; Nishi, I.; Iijima, M.; Nakagawa, M.; Osame, M.; Kondo, I.; Saheki, T. Screening of SLC25A13 mutations in early and late onset patients with citrin deficiency and in the Japanese population: Identification of two novel mutations and establishment of multiple DNA diagnosis methods for nine mutations. Hum. Mutat. 2002, 19, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hwu, W.L.; Kobayashi, K.; Hu, Y.H.; Yamaguchi, N.; Saheki, T.; Chou, S.P.; Wan, J.H. A Chinese adult onset type II citrullinaemia patient with 851del4/1638ins23 mutations in the SLC25A13 Gene. J. Med. Genet. 2001, 38, E23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ko, J.M.; Kim, G.H.; Kim, J.H.; Kim, J.Y.; Choi, J.H.; Ushikai, M.; Saheki, T.; Kobayashi, K.; Yoo, H.W. Six cases of citrin deficiency in Korea. Int. J. Mol. Med. 2007, 20, 809–815. [Google Scholar] [CrossRef]
- Song, Y.Z.; Li, B.X.; Chen, F.P.; Liu, S.R.; Sheng, J.S.; Ushikai, M.; Zhang, C.H.; Zhang, T.; Wang, Z.N.; Kobayashi, K.; et al. Neonatal intrahepatic cholestasis caused by citrin deficiency: Clinical and laboratory investigation of 13 subjects in mainland of China. Dig. Liver Dis. 2009, 41, 683–689. [Google Scholar] [CrossRef]
- Hutchin, T.; Preece, M.A.; Hendriksz, C.; Chakrapani, A.; McClelland, V.; Okumura, F.; Song, Y.Z.; Iijima, M.; Kobayashi, K.; Saheki, T.; et al. Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency (NICCD) as a Cause of Liver Disease in Infants in the UK. J. Inherit. Metab. Dis. 2009, 32, S151–S155. [Google Scholar] [CrossRef]
- Fiermonte, G.; Parisi, G.; Martinelli, D.; De Leonardis, F.; Torre, G.; Pierri, C.L.; Saccari, A.; Lasorsa, F.M.; Vozza, A.; Palmieri, F.; et al. A new Caucasian case of neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD): A clinical, molecular, and functional study. Mol. Genet. Metab. 2011, 104, 501–506. [Google Scholar] [CrossRef]
- Palmieri, F. The mitochondrial transporter family SLC25; Identification, properties and physiopathology. Mol. Aspects Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Palmieri, F.; Scarcia, P.; Monne, M. Diseases caused by mutations in mitochondrial carrier genes SLC25: A review. Biomolecules 2020, 10, 655. [Google Scholar] [CrossRef]
- Lu, Y.B.; Kobayashi, K.; Ushikai, M.; Tabata, A.; Iijima, M.; Li, M.X.; Lei, L.; Kawabe, K.; Taura, S.; Yang, Y.; et al. Frequency and Distribution in East Asia of 12 Mutations Identified in the SLC25A13 Gene of Japanese Patients With Citrin Deficiency. J. Hum. Genet. 2005, 50, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Arai-Ichinoi, N.; Sakamoto, O.; Matsubara, Y.; Saheki, T.; Kobayashi, K.; Ohura, T.; Kure, S. Simple and Rapid Genetic Testing for Citrin Deficiency by Screening 11 Prevalent Mutations in SLC25A13. Mol. Genet. Metab. 2012, 105, 553–558. [Google Scholar] [CrossRef]
- Saheki, T.; Kobayashi, K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD). J. Hum. Genet. 2002, 47, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, Y.; Kobayashi, K.; Ohura, T.; Abukawa, D.; Nishinomiya, F.; Hosoda, Y.; Yamashita, M.; Nagata, I.; Kono, Y.; Yasuda, T.; et al. Infantile cholestatic jaundice associated with adult-onset type II citrullinemia. J. Pediatr. 2001, 138, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Kobayashi, K.; Tazawa, Y.; Nishi, I.; Abukawa, D.; Sakamoto, O.; Iinuma, K.; Saheki, T. Neonatal presentation of adult-onset type II citrullinemia. Hum. Genet. 2001, 108, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Tomomasa, T.; Kobayashi, K.; Kaneko, H.; Shimura, H.; Fukusato, T.; Tabata, M.; Inoue, Y.; Ohwada, S.; Kasahara, M.; Morishita, Y.; et al. Possible clinical and histologic manifestations of adult-onset type II citrullinemia in early infancy. J. Pediatr. 2001, 138, 741–743. [Google Scholar] [CrossRef]
- Song, Y.Z.; Guo, L.; Yang, Y.L.; Han, L.S.; Kobayashi, K.; Saheki, T. Failure to thrive and dyslipidemia caused by citrin deficiency: A novel clinical phenotype. Chin. J. Contemp. Pediatrics 2009, 11, 328–332. [Google Scholar]
- Ikeda, S.; Kawa, S.; Takei, Y.; Yamamoto, K.; Shimojo, H.; Tabata, K.; Kobayashi, K.; Saheki, T. Chronic pancreatitis associated with adult-onset type II citrullinemia: Clinical and pathologic findings. Ann. Intern. Med. 2004, 141, W109–W110. [Google Scholar] [CrossRef]
- Komatsu, M.; Yazaki, M.; Tanaka, N.; Sano, K.; Hashimoto, E.; Takei, Y.; Song, Y.Z.; Tanaka, E.; Kiyosawa, K.; Saheki, T.; et al. Citrin deficiency as a cause of chronic liver disorder mimicking non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 810–820. [Google Scholar] [CrossRef]
- Lee, B.H.; Jin, H.Y.; Kim, G.H.; Choi, J.H.; Yoo, H.W. Nonalcoholic fatty liver disease in 2 siblings with adult-onset type II citrullinemia. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 682–685. [Google Scholar] [CrossRef]
- Soeda, J.; Yazaki, M.; Nakata, T.; Miwa, S.; Ikeda, S.; Hosoda, W.; Iijima, M.; Kobayashi, K.; Saheki, T.; Kojiro, M.; et al. Primary liver carcinoma exhibiting dual hepatocellular-biliary epithelial differentiations associated with citrin deficiency: A case report. J. Clin. Gastroenterol. 2008, 42, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, N.; Sekijima, Y.; Takei, Y.; Ikeda, S.; Kawasaki, S.; Kobayashi, K.; Saheki, T. Hepatocellular carcinoma in a case of adult-onset type II citrullinemia. Inter. Med. 2003, 42, 978–982. [Google Scholar] [CrossRef][Green Version]
- Saheki, T.; Sase, M.; Nakano, K.; Azuma, F.; Katsunuma, T. Some properties of argininosuccinate synthetase purified from human liver and a comparison with the rat liver enzyme. J. Biochem. 1983, 93, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Ueda, A.; Hosoya, M.; Kusumi, K.; Takada, S.; Tsuda, M.; Katsunuma, T. Qualitative and quantitative abnormalities of argininosuccinate synthetase in citrullinemia. Clin. Chim. Acta 1981, 109, 325–335. [Google Scholar] [CrossRef]
- Saheki, T.; Ueda, A.; Iizima, K.; Yamada, N.; Kobayashi, K.; Takahashi, K.; Katsunuma, T. Argininosuccinate synthetase activity in cultured skin fibroblasts of citrullinemic patients. Clin. Chim. Acta 1982, 118, 93–97. [Google Scholar] [PubMed]
- Kobayashi, K.; Jackson, M.J.; Tick, D.B.; O’Brien, W.E.; Beaudet, A.L. Heterogeneity of mutations in argininosuccinate synthetase causing human citrullinemia. J. Biol. Chem. 1990, 265, 11361–11367. [Google Scholar] [PubMed]
- Saheki, T.; Kobayashi, K.; Inoue, I.; Matuo, S.; Hagihara, S.; Noda, T. Increased urinary excretion of argininosuccinate in type II citrullinemia. Clin. Chim. Acta 1987, 170, 297–304. [Google Scholar] [CrossRef]
- Kobayashi, K.; Horiuchi, M.; Saheki, T. Pancreatic secretory trypsin inhibitor as a diagnostic marker for adult-onset type II citrullinemia. Hepatology 1997, 25, 1160–1165. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nakata, M.; Terazono, H.; Shinsato, T.; Saheki, T. Pancreatic secretory trypsin inhibitor gene is highly expressed in the liver of adult-onset type II citrullinemia. FEBS Lett. 1995, 372, 69–73. [Google Scholar] [CrossRef]
- Tsuboi, H.; Fijino, Y.; Kobayashi, K.; Saheki, T.; Yamada, T. High serum pancreatic secretory trypsin inhibitor before onset of type II citrullinemia. Neurology 2001, 57, 933. [Google Scholar] [CrossRef]
- Kobayashi, K.; Shaheen, N.; Terazono, H.; Saheki, T. Mutations in argininosuccinate synthetase mRNA of Japanese patients, causing classical citrullinemia. Am. J. Hum. Genet. 1994, 53, 1103–1112. [Google Scholar]
- Kakinoki, H.; Kobayashi, K.; Terazono, H.; Nagata, Y.; Saheki, T. Mutations and DNA diagnoses of classical citrullienemia. Hum. Mutat. 1997, 9, 250–259. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kakinoki, H.; Fukushige, T.; Shaheen, N.; Terazono, H.; Saheki, T. Nature and frequency of mutations in the argininosuccinate synthetase gene that cause classical citrullinemia. Hum. Genet. 1995, 96, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Shaheen, N.; Kumashiro, R.; Tanikawa, K.; O’Brien, W.E.; Beaudet, A.L.; Saheki, T. A search for the primary abnormality in adult-onset type II citrullinemia. Am. J. Hum. Genet. 1993, 53, 1024–1030. [Google Scholar] [PubMed]
- Sase, M.; Kobayashi, K.; Imamura, Y.; Saheki, T.; Nakano, K.; Miura, S.; Mori, M. Levels of translatable messenger RNA coding for argininosuccinate synthetase in the liver of the patients with quantitative-type citrullinemia. Hum. Genet. 1985, 69, 130–134. [Google Scholar] [CrossRef]
- Kobayashi, K.; Saheki, T.; Imamura, Y.; Noda, T.; Inoue, I.; Matuo, S.; Hagihara, S.; Nomiyama, H.; Jinno, Y.; Shimada, K. Messenger RNA coding for argininosuccinate synthetase in citrullinemia. Am. J. Hum. Genet. 1986, 38, 667–680. [Google Scholar]
- Kobayashi, K.; Sinasac, D.S.; Iijima, M.; Boright, A.P.; Begum, L.; Lee, J.R.; Yasuda, T.; Ikeda, S.; Hirano, R.; Terazono, H.; et al. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat. Genet. 1999, 22, 159–163. [Google Scholar] [CrossRef]
- del Arco, A.; Satrústegui, J. Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J. Biol. Chem. 1998, 273, 23327–23334. [Google Scholar] [CrossRef]
- Palmieri, L.; Pardo, B.; Lasorsa, F.M.; del Arco, A.; Kobayashi, K.; Iijima, M.; Runswick, M.J.; Walker, J.E.; Saheki, T.; Satrústegui, J.; et al. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001, 20, 5060–5069. [Google Scholar] [CrossRef]
- Lasorsa, F.M.; Pinton, P.; Palmieri, L.; Fiermonte, G.; Rizzuto, R.; Palmieri, F. Recombinant expression of the Ca(2+)-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chines hamster ovary cells. J. Biol. Chem. 2003, 378, 38685–38692. [Google Scholar]
- Begum, L.; Jalil, M.A.; Kobayashi, K.; Iijima, M.; Li, M.X.; Yasuda, T.; Horiuchi, M.; del Arco, A.; Satrústegui, J.; Saheki, T. Expression of three mitochondrial solute carriers, citrin, aralar1 and ornithine transporter, in relation to urea cycle in mice. Biochim. Biophys. Acta 2002, 1574, 283–292. [Google Scholar] [CrossRef]
- Amoedo, N.D.; Punzi, G.; Obre, E.; Lacombe, D.; De Grassi, A.; Pierri, C.L.; Rossignol, R. AGC1/2, the mitochondrial aspartate-glutamate carriers. Biochim. Biophys. Acta 2016, 1863, 2394–2412. [Google Scholar] [CrossRef] [PubMed]
- Wibom, R.; Lasorsa, F.M.; Töhönen, V.; Barbaro, M.; Sterky, F.H.; Kucinski, T.; Naess, K.; Jonsson, M.; Pierri, C.L.; Palmieri, F.; et al. AGC1 deficiency associated with global cerebral hypomyelination. N. Engl. J. Med. 2009, 361, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.J.; Li, D.; Gai, X.; McCormick, E.; Place, E.; Lasorsa, F.M.; Otieno, F.G.; Hou, C.; Kim, C.E.; Abdel-Magid, N.; et al. AGC1 deficiency causes infantile epilepsy, abnormal myelination, and reduced N-acetylaspartate. JIMD Rep. 2014, 14, 77–85. [Google Scholar] [PubMed]
- Ohura, T.; Kobayashi, K.; Tazawa, Y.; Abukawa, D.; Sakamoto, O.; Tsuchiya, S.; Saheki, T. Clinical pictures of 75 patients with neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD). J. Inherit. Metab. Dis. 2007, 30, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Lu, Y.B.; Li, M.X.; Nishi, I.; Hsiao, K.j.; Choeh, K.; Yang, Y.L.; Hwu, W.L.; Reinhardt, J.K.V.; Palmieri, F.; et al. Screening of nine SLC25A13 mutations: Their frequency in patients with citrin deficiency and high carrier rates in Asian populations. Mol. Genet. Metab. 2003, 80, 356–359. [Google Scholar] [CrossRef]
- Ben-Shalom, E.; Kobayashi, K.; Shaag, A.; Yasuda, T.; Gao, H.Z.; Saheki, T.; Bachmann, C.; Elpeleg, O. Infantile citrullinemia caused by citrin deficiency with increased dibasic amino acids. Mol. Genet. Metab. 2002, 77, 202–208. [Google Scholar] [CrossRef]
- Naito, E.; Ito, M.; Matsuura, S.; Yokoto, I.; Saijo, T.; Ogura, Y.; Kitamura, S.; Kobayashi, K.; Saheki, T.; Nishimura, Y.; et al. Type II citrullinemia (citrin deficiency) in a neonate with hypergalactosemia detected by mass screening. J. Inherit. Meta. Dis. 2002, 25, 71–76. [Google Scholar] [CrossRef]
- Ohura, T.; Kobayashi, K.; Abukawa, D.; Tazawa, Y.; Akikawa, J.; Sakamoto, O.; Saheki, T.; Iinuma, K. A novel inborn error of metabolism detected by elevated methionine and/or galactose in newborn screening: Neonatal intrahepatic cholestasis caused by citrin deficiency. Eur. J. Pediatr. 2003, 162, 317–322. [Google Scholar] [CrossRef]
- Tamamori, A.; Okano, Y.; Ozaki, H.; Fujimoto, A.; Kajiwara, M.; Fukuda, K.; Kobayashi, K.; Saheki, T.; Tagami, Y.; Yamano, T. Neonatal intrahepatic cholestasis caused by citrin deficiency: Severe hepatic dysfunction in an infant requiring liver transplantation. Eur. J. Pediatr. 2002, 161, 609–613. [Google Scholar] [CrossRef]
- Okano, Y.; Kobayashi, K.; Ihara, K.; Ito, T.; Yoshino, M.; Watanabe, Y.; Kaji, S.; Ohura, T.; Nagao, M.; Noguchi, A.; et al. Fatigue and quality of life in citrin deficiency during adaptation and compensation stage. Mol. Genet. Metab. 2013, 109, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Kobayashi, K.; Terashi, M.; Ohura, T.; Yanagawa, Y.; Okano, Y.; Hattori, T.; Fujimoto, H.; Mutoh, K.; Kizaki, Z.; et al. Reduced carbohydrate intake in citrin-deficient subjects. J. Inherit. Metab. Dis. 2008, 31, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yazaki, M.; Kobayashi, Y.; Fukushima, K.; Ikeda, S.; Kobayashi, K.; Saheki, T.; Nakaya, Y. The characteristics of food intake in patients with type II citrullinemia. J. Nutr. Sci. Vitaminol. 2011, 57, 239–245. [Google Scholar] [CrossRef]
- Saheki, T.; Kobayashi, K. Physiological role of citrin, a liver-type mitochondrial aspartate-glutamate carrier, and pathophysiology of citrin deficiency. Recent Res. Devel. Life Sci. 2005, 3, 59–73. [Google Scholar]
- Saheki, T.; Kobayashi, K.; Iijima, M.; Horiuchi, M.; Begum, L.; Jalil, M.A.; Li, M.X.; Lu, Y.B.; Ushikai, M.; Tabata, A.; et al. Adult-onset type II citrullinemia and idiopathic neonatal hepatitis caused by citrin deficiency: Involvement of the aspartate glutamate carrier for urea synthesis and maintenance of the urea cycle. Mol. Genet. Metab. 2004, 81, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T. What is the function of citrin? In Citrin Deficiency—A Unique Disease that Many Doctors Don’t Know; Fueisha: Osaka, Japan, 2017; pp. 53–58. [Google Scholar]
- Sinasac, D.S.; Moriyama, M.; Jalil, M.A.; Begum, L.; Li, M.X.; Iijima, M.; Horiuchi, M.; Robinson, B.H.; Kobayashi, K.; Saheki, T.; et al. Slc25a13-knockout mice harbor metabolic deficits but fail to display hallmarks of adult-onset type II citrullinemia. Mol. Cell. Biol. 2004, 24, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Iijima, M.; Li, M.X.; Kobayashi, K.; Horiuchi, M.; Ushikai, M.; Okumura, F.; Meng, X.J.; Inoue, I.; Tajima, A.; et al. Citrin/mitochondrial glycerol-3-phosphate dehydrogenase double-knockout mice recapitulate features of human citrin deficiency. J. Biol. Chem. 2007, 282, 25041–25052. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Inoue, K.; Ono, H.; Fujimoto, Y.; Furuie, S.; Yamamura, K.; Kuroda, E.; Ushikai, M.; Asakawa, A.; Inui, A.; et al. Oral aversion to dietary sugar, ethanol and glycerol correlates with alterations in specific hepatic metabolites in a mouse model of human citrin deficiency. Mol. Genet. Metab. 2017, 120, 306–316. [Google Scholar] [CrossRef]
- Saheki, T.; Inoue, K.; Ono, H.; Tushima, A.; Katsura, N.; Yokogawa, M.; Yoshidumi, Y.; Kuhara, T.; Ohse, M.; Eto, K.; et al. Metabolomic analysis reveals hepatic metabolite perturbations in citrin/mitochondrial glycerol-3-phosphate dehydrogenase double-knockout mice, a model of human citrin deficiency. Mol. Genet. Metab. 2011, 104, 492–500. [Google Scholar] [CrossRef]
- Yazaki, M.; Takei, Y.; Kobayashi, K.; Saheki, T.; Ikeda, S. Risk of worsened encephalopathy after intravenous glycerol therapy in patients with adult-onset type II citrullinemia (CTLN2). Intern. Med. 2005, 44, 188–195. [Google Scholar] [CrossRef][Green Version]
- Todo, S.; Starzel, T.E.; Tzakis, A.; Benkov, K.J.; Kalousek, F.; Saheki, T.; Tanikawa, K.; Fenton, W.A. Orthotopic liver transplantation for urea cycle enzyme deficiency. Hepatology 1992, 15, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Yazaki, M.; Takei, Y.; Ikegami, T.; Hashikura, Y.; Kawasaki, S.; Iwai, M.; Kobayashi, K.; Saheki, T. Type II (adult onset) citrullinaemia: Clinical pictures and the therapeutic effect of liver transplantation. J. Neurol. Neurosurg. Psychiatry 2001, 71, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Yazaki, M.; Nakamura, M.; Tanaka, N.; Kobayashi, K.; Saheki, T.; Takei, H.; Ikeda, S. Conventional diet therapy for hyperammonemia is risky in the treatment of hepatic encephalopathy associated with citrin deficiency. Intern. Med. 2010, 49, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Kobayashi, K.; Shibatou, T.; Aburada, S.; Tahara, K.; Kubozono, O.; Saheki, T. Effectiveness of carbohydrate-restricted diet and arginine granules therapy for adult-onset type II citrullinemia: A case report of siblings showing homozygous SLC25A13 mutation with and without the disease. Hepatol. Res. 2003, 26, 68–72. [Google Scholar] [CrossRef]
- Tamakawa, S.; Nakamura, H.; Katano, T.; Yoshizawa, M.; Ohtake, K.; Kubota, T. Hyperalimentation therapy produces a comatose state in a patient with citrullinemia. J. Jpn. Soc. Intensive. Care. Med. 1994, 1, 37–41. [Google Scholar] [CrossRef][Green Version]
- Takahashi, H.; Kagawa, T.; Kobayashi, K.; Hirabayashi, H.; Yui, M.; Begum, L.; Mine, T.; Takagi, S.; Saheki, T.; Shinohara, Y. A Case of adult-onset type ii citrullinemia—Deterioration of clinical course after infusion of hyperosmotic and high sugar solutions. Med. Sci. Monit. 2006, 12, CS13–CS15. [Google Scholar] [PubMed]
- Mutoh, K.; Kurokawa, K.; Kobayashi, K.; Saheki, T. Treatment of a citrin-deficient patient at the early stage of adult-onset type II citrullinaemia with arginine and sodium pyruvate. J. Inherit. Metab. Dis. 2008, 31, S343–S347. [Google Scholar] [CrossRef]
- Saheki, T.; Inoue, K.; Tushima, A.; Mutoh, K.; Kobayashi, K. Citrin deficiency and current treatment concepts. Mol. Genet. Metab. 2010, 100, S59–S64. [Google Scholar] [CrossRef]
- Saheki, T.; Inoue, K.; Ono, H.; Katsura, N.; Yokogawa, M.; Yoshidumi, Y.; Furuie, S.; Kuroda, E.; Ushikai, M.; Asakawa, A.; et al. Effects of supplementation on food intake, body weight and hepatic metabolites in the citrin/mitochondrial glycerol-3-phosphate dehydrogenase double-knockout mouse model of human citrin deficiency. Mol. Genet. Metab. 2012, 107, 322–329. [Google Scholar] [CrossRef]
- Saheki, T.; Moriyama, M.; Kuroda, E.; Funahashi, A.; Yasuda, I.; Setogawa, Y.; Gao, Q.; Ushikai, M.; Furuie, S.; Yamamura, K.; et al. Pivotal role of inter-organ aspartate metabolism for treatment of mitochondrial aspartate-glutamate carrier 2 (citrin) deficiency, based on the mouse model. Sci. Rep. 2019, 9, 4179. [Google Scholar] [CrossRef]
- Moriyama, M.; Li, M.X.; Kobayashi, K.; Sinasac, D.S.; Kannan, Y.; Iijima, M.; Horiuchi, M.; Tsui, L.C.; Tanaka, M.; Nakamura, Y.; et al. Pyruvate ameliorates the defect in ureogenesis from ammonia in citrin-deficient mice. J. Hepatol. 2006, 44, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.; McNelly, S.; Buscher, H.P.; Häussinger, D. Functional hepatocyte heterogeneity in glutamate, aspartate and alpha-ketoglutarate uptake: A histoautoradiographical study. Hepatology 1991, 13, 247–253. [Google Scholar] [CrossRef]
- Neame, K.D.; Wiseman, G. The transamination of glutamic and aspartic acids during absorption by the small intestine of the dog in vivo. J. Physiol. 1957, 135, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.S.; Volman-Mitchell, H. The transamination of glutamate and aspartate during absorption in vitro by small intestine of chicken, guinea-pig and rat. J. Physiol. 1974, 239, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Windmueller, H.G.; Spaeth, A.E. Metabolism of absorbed aspartate, asparagine, and arginine by rat small intestine in vivo. Arch. Biochem. Biophys. 1976, 175, 670–676. [Google Scholar] [CrossRef]
- Hoshi, M.; Mukai, S.; Shinzawa, J.; Watanabe, S.; Kasukawa, R.; Orikasa, H.; Kobayashi, K.; Saheki, T. A case of adult-onset type II citrullinemia in which oral administration of L-arginine granules improved the patient’s encephalopathy and the increased level of ammonia. Kanzo 2002, 43, 492–497. [Google Scholar] [CrossRef]
- Yazaki, M.; Kinoshita, M.; Ogawa, S.; Fujimi, S.; Matsushima, A.; Hineno, A.; Tazawa, K.; Fukushima, K.; Kimura, R.; Yanagida, M.; et al. A 73-year-old patient with adult-onset type II citrullinemia successfully treated by sodium pyruvate and arginine. Clin. Neurol. Neurosurg. 2013, 115, 1542–1545. [Google Scholar] [CrossRef]
- Okano, Y.; Ohura, T.; Sakamoto, O.; Inui, A. Current treatment for citrin deficiency during NICCD and adaptation/compensatin stages: Strategy to prevent CTLN2. Mol. Genet. Meatab. 2019, 127, 175–185. [Google Scholar] [CrossRef]
- Hayasaka, K.; Numakura, C.; Toyota, K.; Kakizaki, S.; Watanabe, H.; Haga, H.; Takahashi, H.; Takahashi, Y.; Kaneko, M.; Yamakawa, M.; et al. Medium-chain triglyceride supplementation under a low-carbohydrate formula is a promising therapy for adult-onset type II citrullinemia. Mol. Genet. Metab. Rep. 2014, 1, 42–50. [Google Scholar] [CrossRef]
- Saheki, T.; Funahashi, A.; Kuroda, E.; Yasuda, I.; Gao, Q.; Setogawa, Y.; Ushikai, M.; Horiuchi, M.; Moriyama, M. Effect of MCT on hyperammonemia in citrin deficiency. In Japanese Journal for Inherited Metabolic Diseases Volume 35, Akita, Japan, 24–26 October 2019; LETTERPRESS: Hiroshima, Japan, 2019; p. 159. [Google Scholar]
- Moriyama, M.; Kuroda, E.; Funahashi, A.; Yasuda, I.; Gao, Q.; Setogawa, Y.; Ushikai, M.; Horiuchi, M.; Saheki, T. Effect of MCT on ureagenesis from ammonia in perfused liver of citrin-deficiency model mouse. Japanese Journal for Inherited Metabolic Diseases Volume 35, Akita, Japan, 24–26 October 2019; LETTERPRESS: Hiroshima, Japan, 2019; p. 157. [Google Scholar]




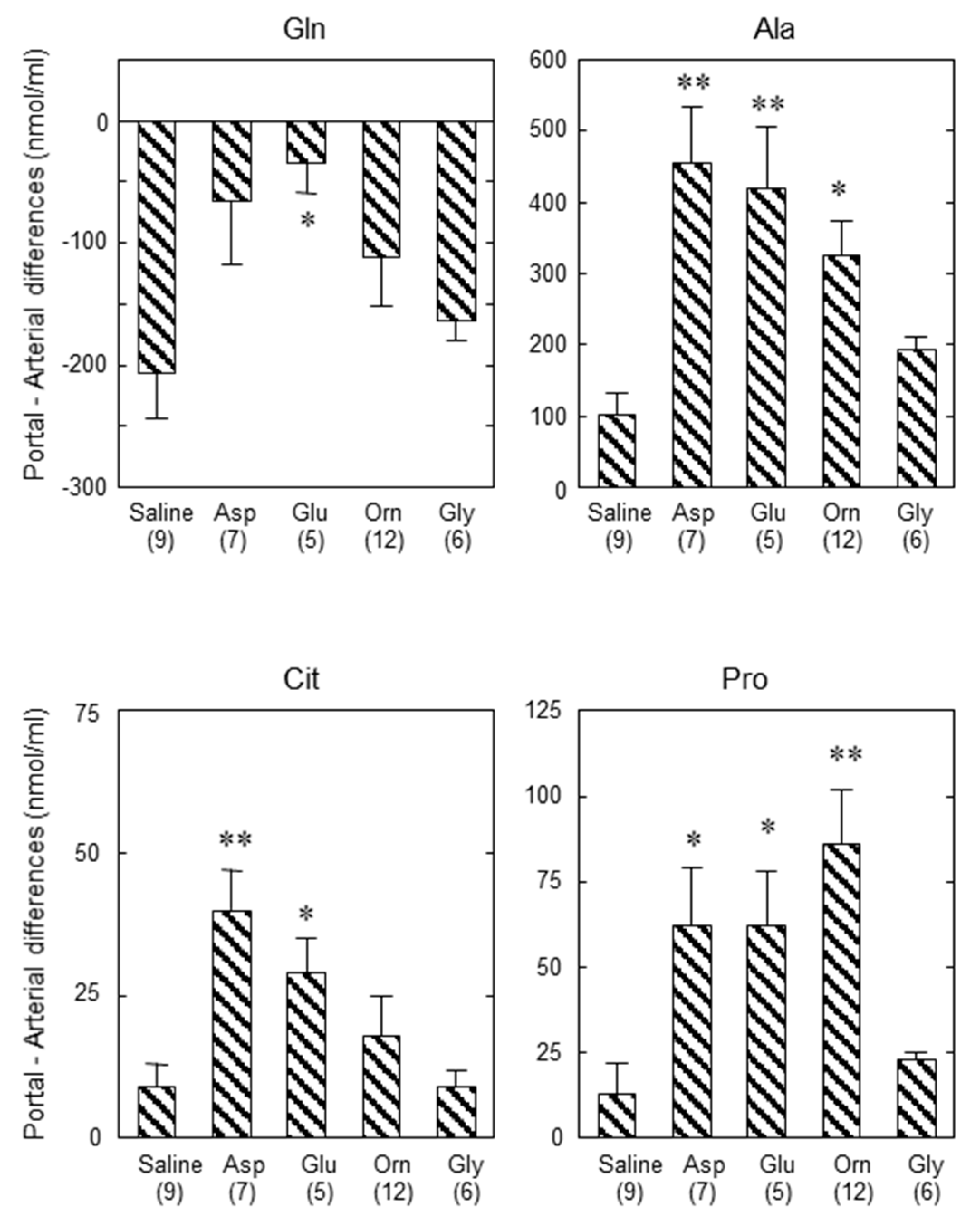
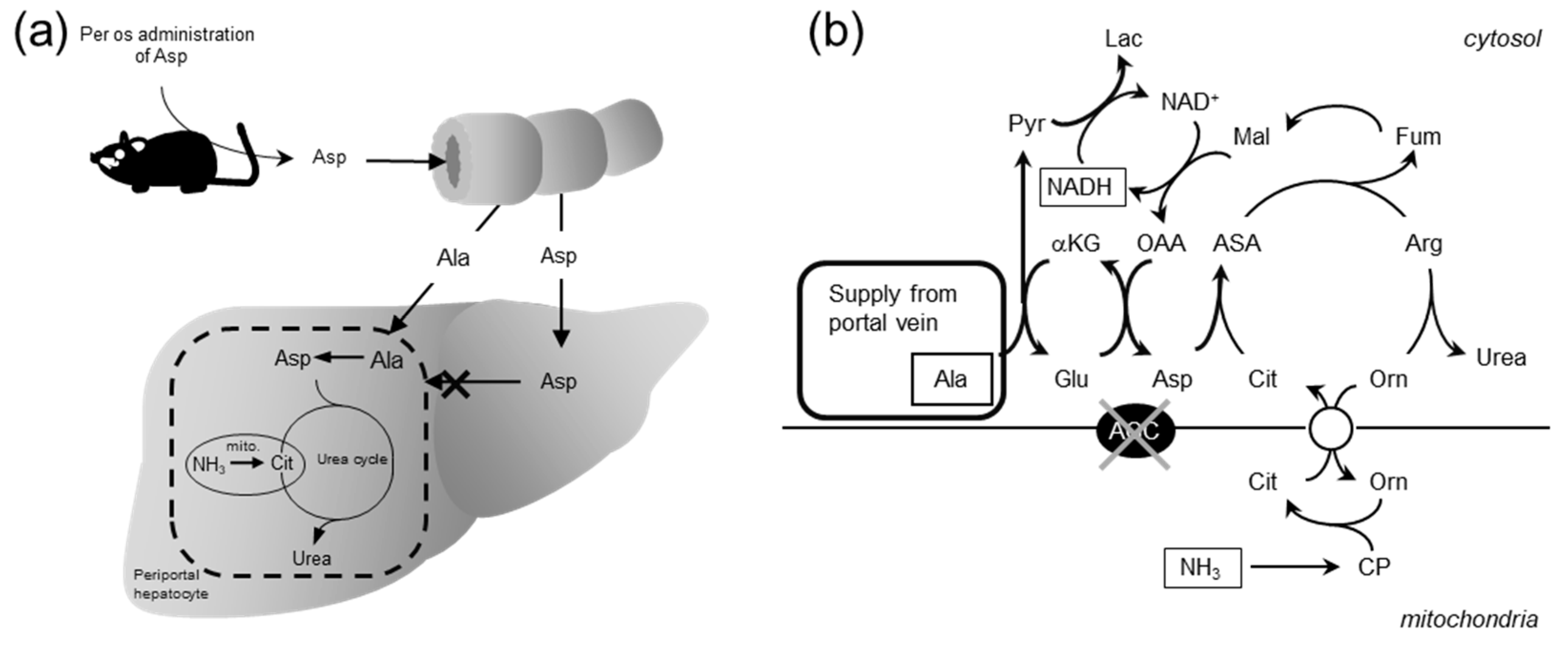
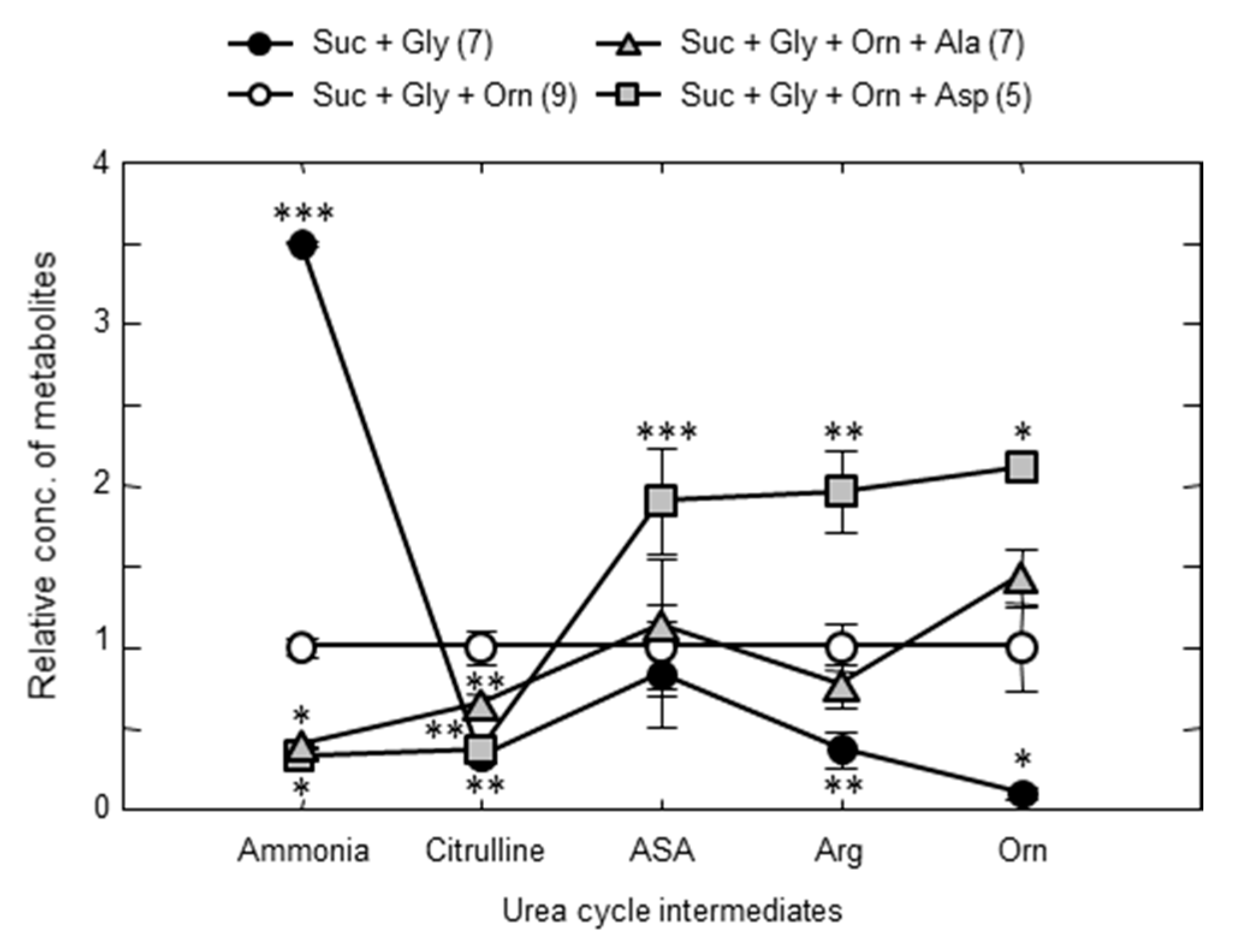
| Type of Enzyme Abnormality We Named: | Type I | Type III | Type II |
|---|---|---|---|
| ASS activity or enzyme amount: | Abnormal kinetics | Almost null | Decrease of normal ASS |
| Organ specificity | All cells | All cells | Liver-specific |
| Type of abnormality | Qualitative | Quantitative | |
| Disease: | CTLN1 (Classical citrullinemia) | CTLN2 (Adult-onset type II citrullinemia) | |
| Gene: | ASS1 | SLC25A13 (Citrin deficiency) | |
| Change from CE-2 to | Body Weight | Food Intake |
|---|---|---|
| AIN93M | Dec | Dec |
| (Effect of supplements) | ||
| Protein (+8%) | Inc | Inc |
| Ala (5%) | Inc | Inc |
| Na-Glu (5%) | Inc | Inc |
| Na-Pyr (10%) | Inc | Inc |
| MCT (6%) | Inc | Inc |
| Soybean oil (6%) | no | no |
| Lard (6%) | no | no |
| Olive oil (6%) | no | no |
| Fish oil (6%) | no | no |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saheki, T.; Moriyama, M.; Funahashi, A.; Kuroda, E. AGC2 (Citrin) Deficiency—From Recognition of the Disease till Construction of Therapeutic Procedures. Biomolecules 2020, 10, 1100. https://doi.org/10.3390/biom10081100
Saheki T, Moriyama M, Funahashi A, Kuroda E. AGC2 (Citrin) Deficiency—From Recognition of the Disease till Construction of Therapeutic Procedures. Biomolecules. 2020; 10(8):1100. https://doi.org/10.3390/biom10081100
Chicago/Turabian StyleSaheki, Takeyori, Mitsuaki Moriyama, Aki Funahashi, and Eishi Kuroda. 2020. "AGC2 (Citrin) Deficiency—From Recognition of the Disease till Construction of Therapeutic Procedures" Biomolecules 10, no. 8: 1100. https://doi.org/10.3390/biom10081100
APA StyleSaheki, T., Moriyama, M., Funahashi, A., & Kuroda, E. (2020). AGC2 (Citrin) Deficiency—From Recognition of the Disease till Construction of Therapeutic Procedures. Biomolecules, 10(8), 1100. https://doi.org/10.3390/biom10081100





