On the Detection and Functional Significance of the Protein–Protein Interactions of Mitochondrial Transport Proteins
Abstract
1. Introduction
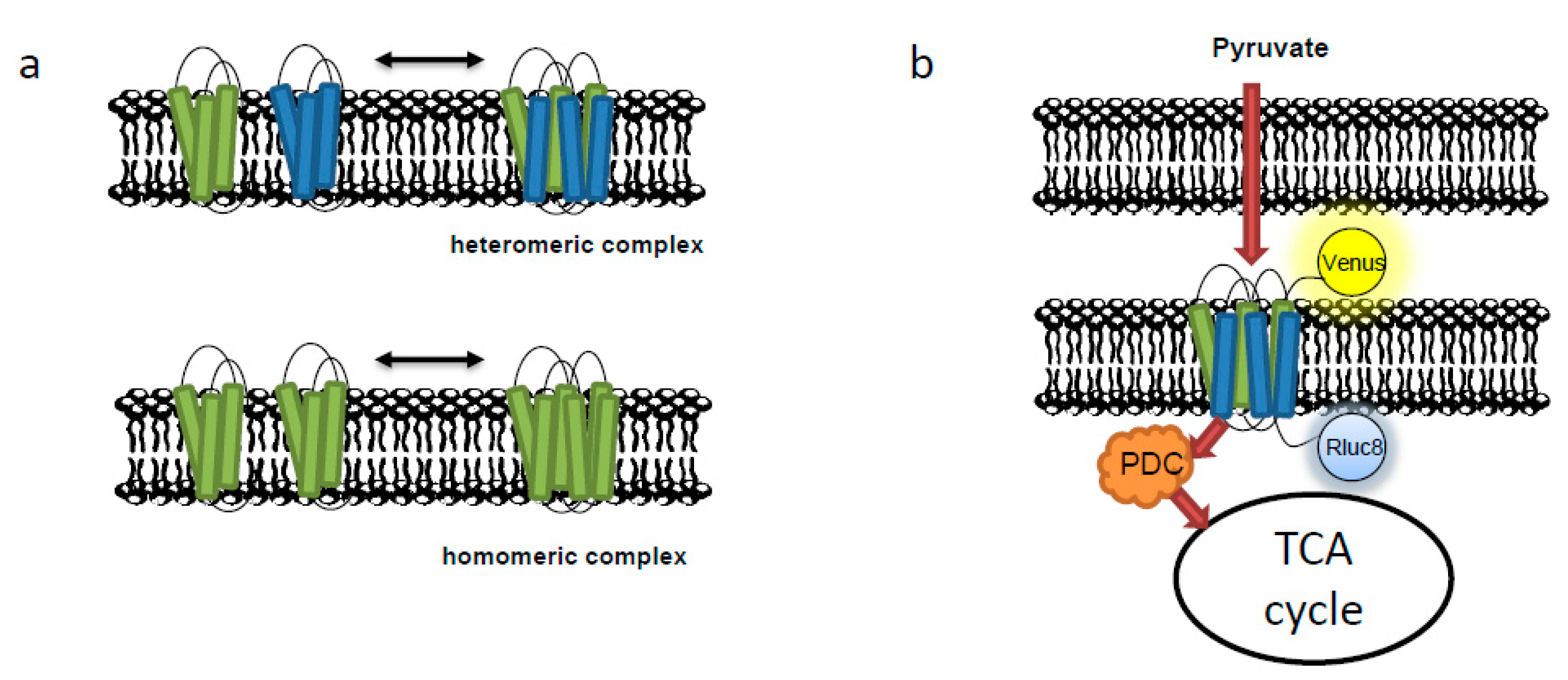
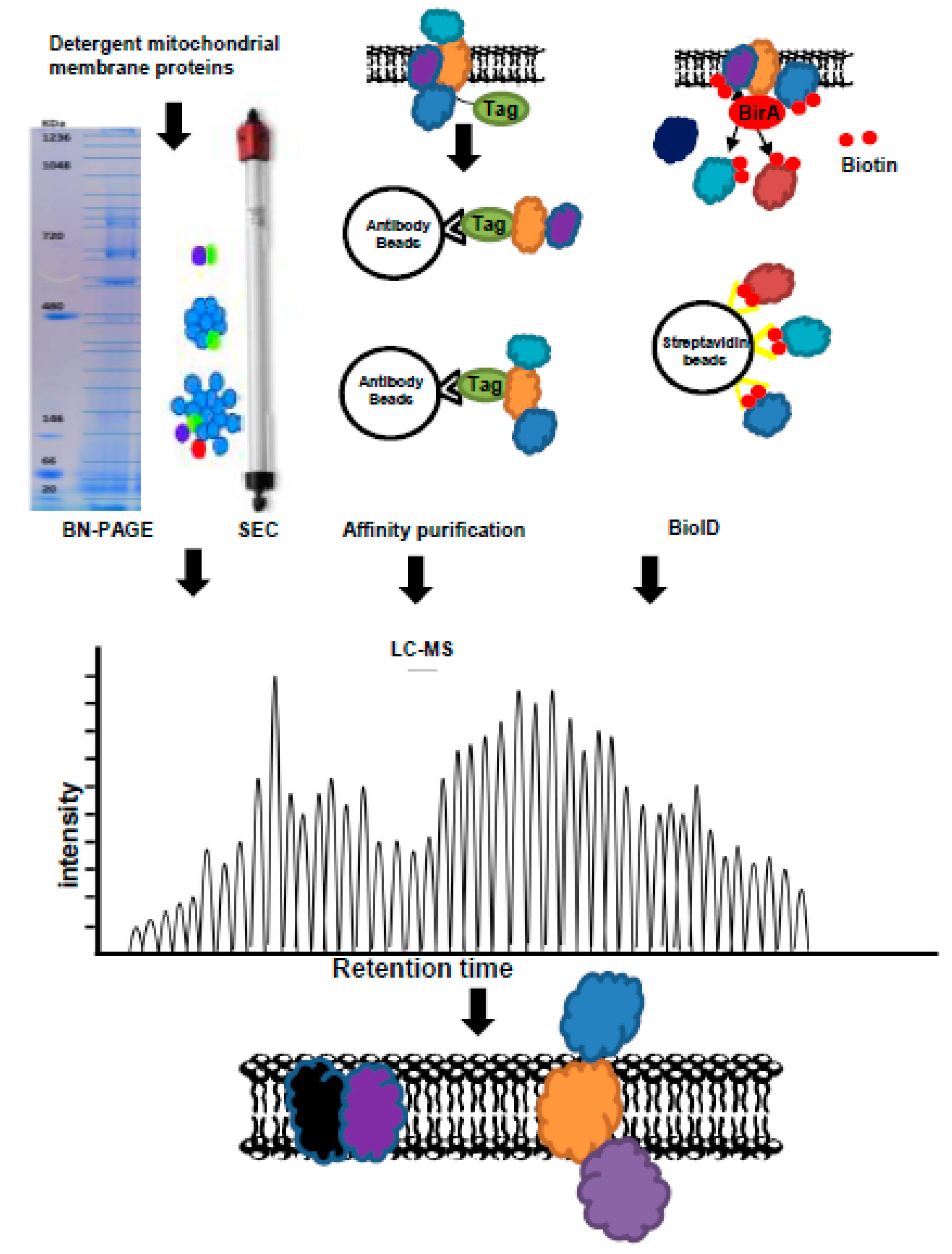
2. Experimental Evaluation of the Protein–Protein Interactions of Mitochondria Carriers
3. Proteins Associated to Mitochondrial Outer Membrane Carrier
4. Proteins Associated to the Inner Membrane of Mitochondria
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Cheng, S.; Zhang, Y.; Sun, Y.; Fernie, A.R.; Kang, K.; Panagiotou, G.; Lo, C.; Lim, B.L. Transcriptomic, proteomic and metabolic changes in arabidopsis thaliana leaves after the onset of illumination. BMC Plant Biol. 2016, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, V.; Macherel, D.; Jaquinod, M.; Douce, R.; Bourguignon, J. Fatty acid and lipoic acid biosynthesis in higher plant mitochondria. J. Biol. Chem. 2000, 275, 5016–5025. [Google Scholar] [CrossRef]
- Fernie, A.R.; Zhang, Y.; Sweetlove, L.J. Passing the baton: Substrate channelling in respiratory metabolism. Research 2018, 2018, 1539325. [Google Scholar] [CrossRef] [PubMed]
- Linka, M.; Weber, A.P. Shuffling ammonia between mitochondria and plastids during photorespiration. Trends Plant Sci. 2005, 10, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.W.; Williams, T.C.; Morgan, M.; Fernie, A.R.; Ratcliffe, R.G.; Sweetlove, L.J. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell 2007, 19, 3723–3738. [Google Scholar] [CrossRef] [PubMed]
- Monné, M.; Miniero, D.V.; Obata, T.; Daddabbo, L.; Palmieri, L.; Vozza, A.; Nicolardi, M.C.; Fernie, A.R.; Palmieri, F. Functional characterization and organ distribution of three mitochondrial atp–mg/pi carriers in arabidopsis thaliana. Biochim. Biophys. Acta BBA Bioenerg. 2015, 1847, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.E.; Franceschi, V.R.; Ku, M.S.B.; Voznesenskaya, E.V.; Pyankov, V.I.; Andreo, C.S. Compartmentation of photosynthesis in cells and tissues of c4 plants. J. Exp. Bot. 2001, 52, 577–590. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Hoek, J.B. Regulation of hexokinase binding to vdac. J. Bioenerg. Biomembr. 2008, 40, 171–182. [Google Scholar] [CrossRef]
- Kanwar, P.; Samtani, H.; Sanyal, S.K.; Srivastava, A.K.; Suprasanna, P.; Pandey, G.K. Vdac and its interacting partners in plant and animal systems: An overview. Crit. Rev. Biotechnol. 2020, 40, 715–732. [Google Scholar] [CrossRef]
- Becker, T.; Vögtle, F.-N.; Stojanovski, D.; Meisinger, C. Sorting and assembly of mitochondrial outer membrane proteins. Biochim. Biophys. Acta BBA Bioenerg. 2008, 1777, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Wagner, R. Mitochondrial outer membrane channels: Emerging diversity in transport processes. BioEssays 2018, 40, 1800013. [Google Scholar] [CrossRef] [PubMed]
- Krüger, V.; Becker, T.; Becker, L.; Montilla-Martinez, M.; Ellenrieder, L.; Vögtle, F.-N.; Meyer, H.E.; Ryan, M.T.; Wiedemann, N.; Warscheid, B. Identification of new channels by systematic analysis of the mitochondrial outer membrane. J. Cell Biol. 2017, 216, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Firment, J.; Vaško, L. Nuclear Encoded Mitochondrial Proteins in Metabolite Transport and Oxidation Pathway Connecting Metabolism of Nutrients. In Mitochondrial Diseases; IntechOpen: London, UK, 2018; pp. 251–289. [Google Scholar]
- Neupert, W.; Brunner, M. The protein import motor of mitochondria. Nat. Rev. Mol. Cell Biol. 2002, 3, 555–565. [Google Scholar] [CrossRef]
- Palmieri, F. Mitochondrial carrier proteins. FEBS Lett. 1994, 346, 48–54. [Google Scholar] [CrossRef]
- Palmieri, F. The mitochondrial transporter family slc25: Identification, properties and physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Toleco, M.R.; Naake, T.; Zhang, Y.J.; Heazlewood, J.L.; Fernie, A.R. Plant mitochondrial carriers: Molecular gatekeepers that help to regulate plant central carbon metabolism. Plants 2020, 9, 117. [Google Scholar] [CrossRef]
- Saraste, M.; Walker, J.E. Internal sequence repeats and the path of polypeptide in mitochondrial adp/atp translocase. FEBS Lett. 1982, 144, 250–254. [Google Scholar] [CrossRef]
- Palmieri, F.; Bisaccia, F.; Capobianco, L.; Dolce, V.; Fiermonte, G.; Iacobazzi, V.; Zara, V. Transmembrane topology, genes, and biogenesis of the mitochondrial phosphate and oxoglutarate carriers. J. Bioenerg. Biomembr. 1993, 25, 493–501. [Google Scholar] [CrossRef]
- Bisaccia, F.; Capobianco, L.; Brandolin, G.; Palmieri, F. Transmembrane topography of the mitochondrial oxoglutarate carrier assessed by peptide-specific antibodies and enzymic cleavage. Biochemistry 1994, 33, 3705–3713. [Google Scholar] [CrossRef]
- Pebay-Peyroula, E.; Dahout-Gonzalez, C.; Kahn, R.; Trézéguet, V.; Lauquin, G.J.-M.; Brandolin, G. Structure of mitochondrial adp/atp carrier in complex with carboxyatractyloside. Nature 2003, 426, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Monné, M. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 2362–2378. [Google Scholar] [CrossRef] [PubMed]
- Monné, M.; Vozza, A.; Lasorsa, F.M.; Porcelli, V.; Palmieri, F. Mitochondrial carriers for aspartate, glutamate and other amino acids: A review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef]
- Kunji, E.R.; Aleksandrova, A.; King, M.S.; Majd, H.; Ashton, V.L.; Cerson, E.; Springett, R.; Kibalchenko, M.; Tavoulari, S.; Crichton, P.G. The transport mechanism of the mitochondrial adp/atp carrier. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 2379–2393. [Google Scholar] [CrossRef]
- Palmieri, F.; Pierri, C.L.; De Grassi, A.; Nunes-Nesi, A.; Fernie, A.R. Evolution, structure and function of mitochondrial carriers: A review with new insights. Plant J. 2011, 66, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.J.; King, M.S.; Zögg, T.; Aleksandrova, A.A.; Pardon, E.; Crichton, P.G.; Steyaert, J.; Kunji, E.R.S. The molecular mechanism of transport by the mitochondrial adp/atp carrier. Cell 2019, 176, 435–447. [Google Scholar] [CrossRef]
- Springett, R.; King, M.S.; Crichton, P.G.; Kunji, E.R.S. Modelling the free energy profile of the mitochondrial adp/atp carrier. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 906–914. [Google Scholar] [CrossRef]
- Klingenberg, M. The adp, atp shuttle of the mitochondrion. Trends Biochem. Sci. 1979, 4, 249–252. [Google Scholar] [CrossRef]
- Klingenberg, M. The adp and atp transport in mitochondria and its carrier. Biochim. Biophys. Acta BBA Biomembr. 2008, 1778, 1978–2021. [Google Scholar] [CrossRef]
- Indiveri, C.; Tonazzi, A.; Palmieri, F. The reconstituted carnitine carrier from rat liver mitochondria: Evidence for a transport mechanism different from that of the other mitochondrial translocators. Biochim. Biophys. Acta BBA Biomembr. 1994, 1189, 65–73. [Google Scholar] [CrossRef]
- Palmieri, F.; Pierri, C.L. Mitochondrial metabolite transport. Essays Biochem. 2010, 47, 37–52. [Google Scholar] [PubMed]
- Pietropaolo, A.; Pierri, C.L.; Palmieri, F.; Klingenberg, M. The switching mechanism of the mitochondrial adp/atp carrier explored by free-energy landscapes. Biochim. Biophys. Acta BBA Bioenerg. 2016, 1857, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.J.; Kunji, E.R. The slc25 mitochondrial carrier family: Structure and mechanism. Trends Biochem. Sci. 2019, 45, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F. Mitochondrial transporters of the slc25 family and associated diseases: A review. J. Inherit. Metab. Dis. 2014, 37, 565–575. [Google Scholar] [CrossRef]
- Monne, M.; Palmieri, F. Antiporters of the mitochondrial carrier family. In Current Topics in Membranes; Bevensee, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 73, pp. 289–320. [Google Scholar]
- Kunji, E.R.; Crichton, P.G. Mitochondrial carriers function as monomers. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 817–831. [Google Scholar] [CrossRef]
- Bamber, L.; Harding, M.; Monné, M.; Slotboom, D.-J.; Kunji, E.R. The yeast mitochondrial adp/atp carrier functions as a monomer in mitochondrial membranes. Proc. Natl. Acad. Sci. USA 2007, 104, 10830–10834. [Google Scholar] [CrossRef]
- Palmieri, L.; Pardo, B.; Lasorsa, F.M.; del Arco, A.; Kobayashi, K.; Iijima, M.; Runswick, M.J.; Walker, J.E.; Saheki, T.; Satrustegui, J.; et al. Citrin and aralar1 are ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001, 20, 5060–5069. [Google Scholar] [CrossRef]
- Lasorsa, F.M.; Pinton, P.; Palmieri, L.; Fiermonte, G.; Rizzuto, R.; Palmieri, F. Recombinant expression of the ca2+-sensitive aspartate/glutamate carrier increases mitochondrial atp production in agonist-stimulated chinese hamster ovary cells. J. Biol. Chem. 2003, 278, 38686–38692. [Google Scholar] [CrossRef]
- Thangaratnarajah, C.; Ruprecht, J.J.; Kunji, E.R. Calcium-induced conformational changes of the regulatory domain of human mitochondrial aspartate/glutamate carriers. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Compan, V.; Pierredon, S.; Vanderperre, B.; Krznar, P.; Marchiq, I.; Zamboni, N.; Pouyssegur, J.; Martinou, J.-C. Monitoring mitochondrial pyruvate carrier activity in real time using a bret-based biosensor: Investigation of the warburg effect. Mol. Cell 2015, 59, 491–501. [Google Scholar] [CrossRef]
- van der Giezen, M.; Slotboom, D.J.; Horner, D.S.; Dyal, P.L.; Harding, M.; Xue, G.P.; Embley, T.M.; Kunji, E.R.S. Conserved properties of hydrogenosomal and mitochondrial adp/atp carriers: A common origin for both organelles. EMBO J. 2002, 21, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Leroch, M.; Neuhaus, H.E.; Kirchberger, S.; Zimmermann, S.; Melzer, M.; Gerhold, J.; Tjaden, J. Identification of a novel adenine nucleotide transporter in the endoplasmic reticulum of arabidopsis. Plant Cell 2008, 20, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Santoro, A.; Carrari, F.; Blanco, E.; Nunes-Nesi, A.; Arrigoni, R.; Genchi, F.; Fernie, A.R.; Palmieri, F. Identification and characterization of adnt1, a novel mitochondrial adenine nucleotide transporter from arabidopsis. Plant Physiol. 2008, 148, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Belzacq, A.-S.; Vieira, H.L.; Kroemer, G.; Brenner, C. The adenine nucleotide translocator in apoptosis. Biochimie 2002, 84, 167–176. [Google Scholar] [CrossRef]
- Lançar-Benba, J.; Foucher, B.; Saint-Macary, M. Purification of the rat-liver mitochondrial dicarboxylate carrier by affinity chromatography on immobilized malate dehydrogenase. Biochim. Biophys. Acta BBA Biomembr. 1994, 1190, 213–216. [Google Scholar] [CrossRef]
- Krause, F. Detection and analysis of protein–protein interactions in organellar and prokaryotic proteomes by native gel electrophoresis:(membrane) protein complexes and supercomplexes. Electrophoresis 2006, 27, 2759–2781. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. Stable and temporary enzyme complexes and metabolons involved in energy and redox metabolism. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, S.; Kim, D.-K.; Jeong, M.G.; Noh, J.; Kwon, Y.; Zhou, K.; Lee, N.K.; Ryu, S.H. Direct visualization of single-molecule membrane protein interactions in living cells. PLoS Biol. 2018, 16, e2006660. [Google Scholar] [CrossRef]
- Moore, D.T.; Berger, B.W.; DeGrado, W.F. Protein-protein interactions in the membrane: Sequence, structural, and biological motifs. Structure 2008, 16, 991–1001. [Google Scholar] [CrossRef]
- Stansfeld, P.J. Computational studies of membrane proteins: From sequence to structure to simulation. Curr. Opin. Struct. Biol. 2017, 45, 133–141. [Google Scholar] [CrossRef]
- Shimizu, K.; Cao, W.; Saad, G.; Shoji, M.; Terada, T. Comparative analysis of membrane protein structure databases. Biochim. Biophys. Acta BBA Biomembr. 2018, 1860, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, T. Protein-protein interactions of mitochondrial-associated protein via bioluminescence resonance energy transfer. Biophys. Phys. 2015, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, O.; Yoshizumi, T.; Kuboyama, M.; Ishihara, T.; Suzuki, E.; Kawabata, S.-i.; Koshiba, T. A structural perspective of the mavs-regulatory mechanism on the mitochondrial outer membrane using bioluminescence resonance energy transfer. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Baril, M.; Racine, M.-E.; Penin, F.; Lamarre, D. Mavs dimer is a crucial signaling component of innate immunity and the target of hepatitis c virus ns3/4a protease. J. Virol. 2009, 83, 1299–1311. [Google Scholar] [CrossRef]
- Bender, T.; Pena, G.; Martinou, J.C. Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes. EMBO J. 2015, 34, 911–924. [Google Scholar] [CrossRef]
- Fernie, A.R.; Zhang, Y.; Sampathkumar, A. Cytoskeleton architecture regulates glycolysis coupling cellular metabolism to mechanical cues. Trends Biochem. Sci. 2020. [Google Scholar] [CrossRef]
- Park, J.S.; Burckhardt, C.J.; Lazcano, R.; Solis, L.M.; Isogai, T.; Li, L.; Chen, C.S.; Gao, B.; Minna, J.D.; Bachoo, R. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 2020, 578, 621–626. [Google Scholar] [CrossRef]
- Carriba, P.; Navarro, G.; Ciruela, F.; Ferré, S.; Casadó, V.; Agnati, L.; Cortés, A.; Mallol, J.; Fuxe, K.; Canela, E. Detection of heteromerization of more than two proteins by sequential bret-fret. Nat. Methods 2008, 5, 727. [Google Scholar] [CrossRef]
- Crichton, P.G.; Harding, M.; Ruprecht, J.J.; Lee, Y.; Kunji, E.R. Lipid, detergent, and coomassie blue g-250 affect the migration of small membrane proteins in blue native gels mitochondrial carriers migrate as monomers not dimers. J. Biol. Chem. 2013, 288, 22163–22173. [Google Scholar] [CrossRef]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.-C.; Cox, J.E.; Cardon, C.M.; Van Vranken, J.G.; Dephoure, N. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, drosophila, and humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef]
- Eubel, H.; Braun, H.-P.; Millar, A. Blue-native page in plants: A tool in analysis of protein-protein interactions. Plant Methods 2005, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Rampelt, H.; Sucec, I.; Bersch, B.; Horten, P.; Perschil, I.; Martinou, J.-C.; van der Laan, M.; Wiedemann, N.; Schanda, P.; Pfanner, N. The mitochondrial carrier pathway transports non-canonical substrates with an odd number of transmembrane segments. BMC Biol. 2020, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.; Harding, M.; Butler, P.J.G.; Akamine, P. Determination of the molecular mass and dimensions of membrane proteins by size exclusion chromatography. Methods 2008, 46, 62–72. [Google Scholar] [CrossRef]
- Jha, P.; Wang, X.; Auwerx, J. Analysis of mitochondrial respiratory chain supercomplexes using blue native polyacrylamide gel electrophoresis (bn-page). Curr. Protoc. Mouse Biol. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Palmisano, A.; Zara, V.; Honlinger, A.; Vozza, A.; Dekker, P.J.; Pfanner, N.; Palmieri, F. Targeting and assembly of the oxoglutarate carrier: General principles for biogenesis of carrier proteins of the mitochondrial inner membrane. Biochem. J. 1998, 333, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Vozza, A.; Hönlinger, A.; Dietmeier, K.; Palmisano, A.; Zara, V.; Palmieri, F. The mitochondrial dicarboxylate carrier is essential for the growth of saccharomyces cerevisiae on ethanol or acetate as the sole carbon source. Mol. Microbiol. 1999, 31, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, L.; Ferramosca, A.; Zara, V. The mitochondrial tricarboxylate carrier of silver eel: Dimeric structure and cytosolic exposure of both n-and c-termini. J. Protein Chem. 2002, 21, 515–521. [Google Scholar] [CrossRef]
- Tavoulari, S.; Thangaratnarajah, C.; Mavridou, V.; Harbour, M.E.; Martinou, J.C.; Kunji, E.R. The yeast mitochondrial pyruvate carrier is a hetero-dimer in its functional state. EMBO J. 2019, 38, e100785. [Google Scholar] [CrossRef]
- Zhang, Y.; Skirycz, A.; Fernie, A.R. An abundance and interaction encyclopedia of plant protein function. Trends Plant Sci. 2020, 25, 627–630. [Google Scholar] [CrossRef]
- McWhite, C.D.; Papoulas, O.; Drew, K.; Cox, R.M.; June, V.; Dong, O.X.; Kwon, T.; Wan, C.; Salmi, M.L.; Roux, S.J. A pan-plant protein complex map reveals deep conservation and novel assemblies. Cell 2020, 181, 460–474. [Google Scholar] [CrossRef]
- Villa, A.; García-Simón, M.I.; Blanco, P.; Sesé, B.; Bogónez, E.; Satrustegui, J. Affinity chromatography purification of mitochondrial inner membrane proteins with calcium transport activity. Biochim. Biophys. Acta BBA Biomembr. 1998, 1373, 347–359. [Google Scholar] [CrossRef]
- Schroers, A.; Burkovski, A.; Wohlrab, H.; Krämer, R. The phosphate carrier from yeast mitochondria dimerization is a prerequisite for function. J. Biol. Chem. 1998, 273, 14269–14276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Swart, C.; Alseekh, S.; Scossa, F.; Jiang, L.; Obata, T.; Graf, A.; Fernie, A.R. The extra-pathway interactome of the tca cycle: Expected and unexpected metabolic interactions. Plant Physiol. 2018, 177, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Beard, K.F.; Swart, C.; Bergmann, S.; Krahnert, I.; Nikoloski, Z.; Graf, A.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Protein-protein interactions and metabolite channelling in the plant tricarboxylic acid cycle. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Natale, R.; Domingues, A.P.; Toleco, M.R.; Siemiatkowska, B.; Fàbregas, N.; Fernie, A.R. Rapid identification of protein-protein interactions in plants. Curr. Protoc. Plant Biol. 2019, 4, e20099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Siemiatkowska, B.; Toleco, M.R.; Jing, Y.; Strotmann, V.; Zhang, J.; Stahl, Y.; Fernie, A.R. A highly efficient agrobacterium-mediated method for transient gene expression and functional studies in multipe plant species. Plant Commun. 2020, 100028. [Google Scholar] [CrossRef]
- Makowski, M.M.; Willems, E.; Jansen, P.W.; Vermeulen, M. Cross-linking immunoprecipitation-ms (xip-ms): Topological analysis of chromatin-associated protein complexes using single affinity purification. Mol. Cell. Proteom. 2016, 15, 854–865. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. Evidence for metabolons—Enzyme-enzyme assemblies that mediate substrate channeling—And their roles in plants. Plant Commun. 2020, 1, 100081. [Google Scholar] [CrossRef]
- Liu, F.; Rijkers, D.T.; Post, H.; Heck, A.J. Proteome-wide profiling of protein assemblies by cross-linking mass spectrometry. Nat. Methods 2015, 12, 1179–1184. [Google Scholar] [CrossRef]
- Liu, F.; Lössl, P.; Rabbitts, B.M.; Balaban, R.S.; Heck, A.J. The interactome of intact mitochondria by cross-linking mass spectrometry provides evidence for coexisting respiratory supercomplexes. Mol. Cell. Proteom. 2018, 17, 216–232. [Google Scholar] [CrossRef]
- Schweppe, D.K.; Chavez, J.D.; Lee, C.F.; Caudal, A.; Kruse, S.E.; Stuppard, R.; Marcinek, D.J.; Shadel, G.S.; Tian, R.; Bruce, J.E. Mitochondrial protein interactome elucidated by chemical cross-linking mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Back, J.W.; Sanz, M.A.; de Jong, L.; de Koning, L.J.; Nijtmans, L.G.; de Koster, C.G.; Grivell, L.A.; van der Spek, H.; Muijsers, A.O. A structure for the yeast prohibitin complex: Structure prediction and evidence from chemical crosslinking and mass spectrometry. Protein Sci. 2002, 11, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Majima, E.; Takeda, M.; Miki, S.; Shinohara, Y.; Terada, H. Close location of the first loop to the third loop of the mitochondrial adp/atp carrier deduced from cross-linking catalyzed by copper-o-phenanthroline of the solubilized carrier with triton x-100. J. Biochem. 2002, 131, 461–468. [Google Scholar] [CrossRef]
- Klngenberg, M.; Appel, M. The uncoupling protein dimer can form a disulfide cross-link between the mobile c-terminal sh groups. Eur. J. Biochem. 1989, 180, 123–131. [Google Scholar] [CrossRef]
- Bisaccia, F.; Zara, V.; Capobianco, L.; Iacobazzi, V.; Mazzeo, M.; Palmieri, F. The formation of a disulfide cross-link between the two subunits demonstrates the dimeric structure of the mitochondrial oxoglutarate carrier. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1996, 1292, 281–288. [Google Scholar] [CrossRef]
- Varnaitė, R.; MacNeill, S.A. Meet the neighbors: Mapping local protein interactomes by proximity-dependent labeling with bioid. Proteomics 2016, 16, 2503–2518. [Google Scholar] [CrossRef]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef]
- Koshiba, T.; Kosako, H. Mass spectrometry-based methods for analysing the mitochondrial interactome in mammalian cells. J. Biochem. 2020, 167, 225–231. [Google Scholar] [CrossRef]
- Yoshinaka, T.; Kosako, H.; Yoshizumi, T.; Furukawa, R.; Hirano, Y.; Kuge, O.; Tamada, T.; Koshiba, T. Structural basis of mitochondrial scaffolds by prohibitin complexes: Insight into a role of the coiled-coil region. iScience 2019, 19, 1065–1078. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Holmuhamedov, E. Voltage-dependent anion channel (vdac) as mitochondrial governator—Thinking outside the box. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2006, 1762, 181–190. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Lemasters, J.J. Warburg revisited: Regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. J. Pharmacol. Exp. Ther. 2012, 342, 637–641. [Google Scholar] [CrossRef]
- Krasnov, G.S.; Dmitriev, A.A.; Lakunina, V.A.; Kirpiy, A.A.; Kudryavtseva, A.V. Targeting vdac-bound hexokinase ii: A promising approach for concomitant anti-cancer therapy. Expert Opin. Ther. Targets 2013, 17, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Dolder, M.; Wendt, S.; Wallimann, T. Mitochondrial creatine kinase in contact sites: Interaction with porin and adenine nucleotide translocase, role in permeability transition and sensitivity to oxidative damage. Neurosignals 2001, 10, 93–111. [Google Scholar] [CrossRef]
- Lee, C.P.; Millar, A.H. The plant mitochondrial transportome: Balancing metabolic demands with energetic constraints. Trends Plant Sci. 2016, 21, 662–676. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; Hellawell, A.M.; Harding, M.; Crichton, P.G.; McCoy, A.J.; Kunji, E.R. Structures of yeast mitochondrial adp/atp carriers support a domain-based alternating-access transport mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, E426–E434. [Google Scholar] [CrossRef]
- Falconi, M.; Chillemi, G.; Di Marino, D.; D’Annessa, I.; Morozzo della Rocca, B.; Palmieri, L.; Desideri, A. Structural dynamics of the mitochondrial adp/atp carrier revealed by molecular dynamics simulation studies. Proteins Struct. Funct. Bioinform. 2006, 65, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Claypool, S.M.; Oktay, Y.; Boontheung, P.; Loo, J.A.; Koehler, C.M. Cardiolipin defines the interactome of the major adp/atp carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008, 182, 937–950. [Google Scholar] [CrossRef]
- Halestrap, A.P.; McStay, G.P.; Clarke, S.J. The permeability transition pore complex: Another view. Biochimie 2002, 84, 153–166. [Google Scholar] [CrossRef]
- Kokoszka, J.E.; Waymire, K.G.; Levy, S.E.; Sligh, J.E.; Cai, J.; Jones, D.P.; MacGregor, G.R.; Wallace, D.C. The adp/atp translocator is not essential for the mitochondrial permeability transition pore. Nature 2004, 427, 461–465. [Google Scholar] [CrossRef]
- Ko, Y.H.; Delannoy, M.; Hullihen, J.; Chiu, W.; Pedersen, P.L. Mitochondrial atp synthasome cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the atp synthase and carriers for pi and adp/atp. J. Biol. Chem. 2003, 278, 12305–12309. [Google Scholar] [CrossRef]
- Davies, K.M.; Anselmi, C.; Wittig, I.; Faraldo-Gómez, J.D.; Kühlbrandt, W. Structure of the yeast f1fo-atp synthase dimer and its role in shaping the mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2012, 109, 13602–13607. [Google Scholar] [CrossRef] [PubMed]
- Galber, C.; Valente, G.; von Stockum, S.; Giorgio, V. Purification of Functional f-atp Synthase from Blue Native Page. In Calcium Signalling; Springer: Berlin/Heidelberg, Germany, 2019; pp. 233–243. [Google Scholar]
- Dienhart, M.K.; Stuart, R.A. The yeast aac2 protein exists in physical association with the cytochrome bc 1-cox supercomplex and the tim23 machinery. Mol. Biol. Cell 2008, 19, 3934–3943. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, C.S.; Rampelt, H.; Gebert, M.; Oeljeklaus, S.; Schrempp, S.G.; Kochbeck, L.; Guiard, B.; Warscheid, B.; van der Laan, M. The mitochondrial adp/atp carrier associates with the inner membrane presequence translocase in a stoichiometric manner. J. Biol. Chem. 2014, 289, 27352–27362. [Google Scholar] [CrossRef] [PubMed]
- Miniero, D.V.; Cappello, A.R.; Curcio, R.; Ludovico, A.; Daddabbo, L.; Stipani, I.; Robinson, A.J.; Kunji, E.R.; Palmieri, F. Functional and structural role of amino acid residues in the matrix α-helices, termini and cytosolic loops of the bovine mitochondrial oxoglutarate carrier. Biochim. Biophys. Acta BBA Bioenerg. 2011, 1807, 302–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sweetlove, L.J.; Lytovchenko, A.; Morgan, M.; Nunes-Nesi, A.; Taylor, N.L.; Baxter, C.J.; Eickmeier, I.; Fernie, A.R. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19587–19592. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Ratcliffe, R.G.; Sweetlove, L.J. Activation and function of mitochondrial uncoupling protein in plants. J. Biol. Chem. 2004, 279, 51944–51952. [Google Scholar] [CrossRef]
- Monné, M.; Daddabbo, L.; Gagneul, D.; Obata, T.; Hielscher, B.; Palmieri, L.; Miniero, D.V.; Fernie, A.R.; Weber, A.P.M.; Palmieri, F. Uncoupling proteins 1 and 2 (ucp1 and ucp2) fromarabidopsis thaliana are mitochondrial transporters of aspartate, glutamate, and dicarboxylates. J. Biol. Chem. 2018, 293, 4213–4227. [Google Scholar] [CrossRef]
- Palmieri, F.; Quagliariello, E.; Klingenberg, M. Quantitative correlation between the distribution of anions and the ph difference across the mitochondrial membrane. Eur. J. Biochem. 1970, 17, 230–238. [Google Scholar] [CrossRef]
- Fiermonte, G.; Dolce, V.; Palmieri, F. Expression in escherichia coli, functional characterization, and tissue distribution of isoforms a and b of the phosphate carrier from bovine mitochondria. J. Biol. Chem. 1998, 273, 22782–22787. [Google Scholar] [CrossRef]
- Leung, A.W.; Varanyuwatana, P.; Halestrap, A.P. The mitochondrial phosphate carrier interacts with cyclophilin d and may play a key role in the permeability transition. J. Biol. Chem. 2008, 283, 26312–26323. [Google Scholar] [CrossRef]
- García-Valencia, L.E.; Bravo-Alberto, C.E.; Wu, H.-M.; Rodríguez-Sotres, R.; Cheung, A.Y.; Cruz-García, F. Sipp, a novel mitochondrial phosphate carrier, mediates in self-incompatibility. Plant Physiol. 2017, 175, 1105–1120. [Google Scholar] [CrossRef]
- Srere, P.A. The metabolon. Trends Biochem. Sci. 1985, 10, 109–110. [Google Scholar] [CrossRef]
- Vélot, C.; Mixon, M.B.; Teige, M.; Srere, P.A. Model of a quinary structure between krebs tca cycle enzymes: A model for the metabolon. Biochemistry 1997, 36, 14271–14276. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Stipani, I.; Quagliariello, E.; Klingenberg, M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur. J. Biochem. 1972, 26, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bisaccia, F.; De Palma, A.; Palmieri, F. Identification and purification of the tricarboxylate carrier from rat liver mitochondria. Biochim. Biophys. Acta BBA Bioenerg. 1989, 977, 171–176. [Google Scholar] [CrossRef]
- Bisaccia, F.; De Palma, A.; Dierks, T.; Krämer, R.; Palmieri, F. Reaction mechanism of the reconstituted tricarboxylate carrier from rat liver mitochondria. Biochim. Biophys. Acta BBA Bioenerg. 1993, 1142, 139–145. [Google Scholar] [CrossRef]
- Lin, C.; Hackenberg, H.; Klingenberg, E. The uncoupling protein from brown adipose tissue mitochondria is a dimer. A hydrodynamic study. FEBS Lett. 1980, 113, 304–306. [Google Scholar] [CrossRef]
- Jakobs, P.; Braun, A.; Jezek, P.; Trommer, W. Binding of atp to uncoupling protein of brown fat mitochondria as studied by means of spin-labeled atp derivatives. FEBS Lett. 1991, 284, 195–198. [Google Scholar] [CrossRef]
- Winkler, E.; Klingenberg, M. Photoaffinity labeling of the nucleotide-binding site of the uncoupling protein from hamster brown adipose tissue. Eur. J. Biochem. 1992, 203, 295–304. [Google Scholar] [CrossRef]
- Lee, Y.; Willers, C.; Kunji, E.R.; Crichton, P.G. Uncoupling protein 1 binds one nucleotide per monomer and is stabilized by tightly bound cardiolipin. Proc. Natl. Acad. Sci. USA 2015, 112, 6973–6978. [Google Scholar] [CrossRef]
- Crichton, P.G.; Lee, Y.; Kunji, E.R. The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism. Biochimie 2017, 134, 35–50. [Google Scholar] [CrossRef]
- Arriza, J.L.; Eliasof, S.; Kavanaugh, M.P.; Amara, S.G. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. USA 1997, 94, 4155–4160. [Google Scholar] [CrossRef] [PubMed]
- Menga, A.; Iacobazzi, V.; Infantino, V.; Avantaggiati, M.L.; Palmieri, F. The mitochondrial aspartate/glutamate carrier isoform 1 gene expression is regulated by creb in neuronal cells. Int. J. Biochem. Cell Biol. 2015, 60, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Marquardt, K.; Lash, L.H.; Linseman, D.A. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic. Biol. Med. 2012, 52, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Brandina, I.; Graham, J.; Lemaitre-Guillier, C.; Entelis, N.; Krasheninnikov, I.; Sweetlove, L.; Tarassov, I.; Martin, R.P. Enolase takes part in a macromolecular complex associated to mitochondria in yeast. Biochim. Biophys. Acta BBA Bioenerg. 2006, 1757, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Nelson, J.C.; Bend, E.G.; Rodríguez-Laureano, L.; Tueros, F.G.; Cartagenova, L.; Underwood, K.; Jorgensen, E.M.; Colón-Ramos, D.A. Glycolytic enzymes localize to synapses under energy stress to support synaptic function. Neuron 2016, 90, 278–291. [Google Scholar] [CrossRef] [PubMed]

| Mitochondria Carrier | Interactor | Reference |
|---|---|---|
| VDAC | Tubulin, Desmin, Vimentin, plectin, hexokinase and creatine kinases, MtCK, ANT, cardiolipin | [10,42,43] |
| Acyl-dihydroxyacetone phosphate reductase | Unclear | [13] |
| ADP/ATP carrier | Previous suggested as dimer, and now convincedly proved as monomers | [44] |
| Phosphate carrier | ATP synthase, mitochondrial peptidyl-prolyl cis-trans isomerase, NaStEP, Citrate synthase, isocitrate dehydrogenase and oxoglutarate dehydrogenase | [25,45,46,47] |
| Pyruvate carrier | Heterodimer | [48,49] |
| Tricarboxylate carrier | Homodimer | [50] |
| Dicarboxylate carrier | Homodimer | [51] |
| Oxoglutarate carrier | Homodimer, BCL2 | [52,53] |
| Glutamate transporter | Synaptic protein | [54] |
| Aspartate/glutamate carrier | Homodimer | [36,39,55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fernie, A.R. On the Detection and Functional Significance of the Protein–Protein Interactions of Mitochondrial Transport Proteins. Biomolecules 2020, 10, 1107. https://doi.org/10.3390/biom10081107
Zhang Y, Fernie AR. On the Detection and Functional Significance of the Protein–Protein Interactions of Mitochondrial Transport Proteins. Biomolecules. 2020; 10(8):1107. https://doi.org/10.3390/biom10081107
Chicago/Turabian StyleZhang, Youjun, and Alisdair R. Fernie. 2020. "On the Detection and Functional Significance of the Protein–Protein Interactions of Mitochondrial Transport Proteins" Biomolecules 10, no. 8: 1107. https://doi.org/10.3390/biom10081107
APA StyleZhang, Y., & Fernie, A. R. (2020). On the Detection and Functional Significance of the Protein–Protein Interactions of Mitochondrial Transport Proteins. Biomolecules, 10(8), 1107. https://doi.org/10.3390/biom10081107






