Inflammatory Breast Carcinoma: Elevated microRNA miR-181b-5p and Reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p Expression as Potential Biomarkers with Diagnostic Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Human Breast Tissue Samples
2.2. Total RNA Extraction and Human Breast Cancer miRNA PCR Array
2.3. Quantitative Real-Time PCR
2.4. Cell Culture
2.5. Prediction of miRNA Target Genes and GO Function and KEGG Pathway Analysis
2.6. Integration of the PPI Network and Identification of Significant Candidate Genes (Hub Genes) and Pathways
2.7. Kaplan-Meier Plots and Survival Analysis
2.8. Statistical Analysis
3. Results
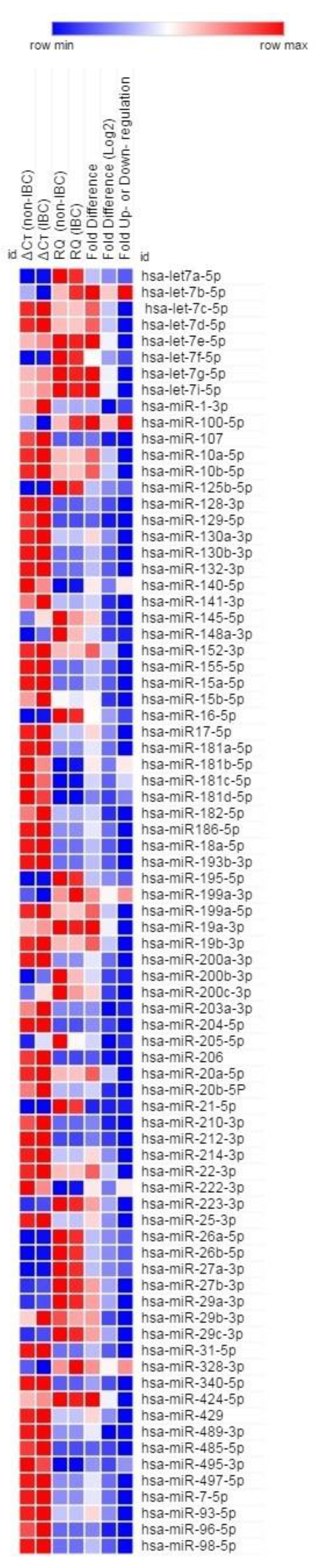
3.1. A Subset of miRNAs Is Differentially Expressed in IBC Tumors
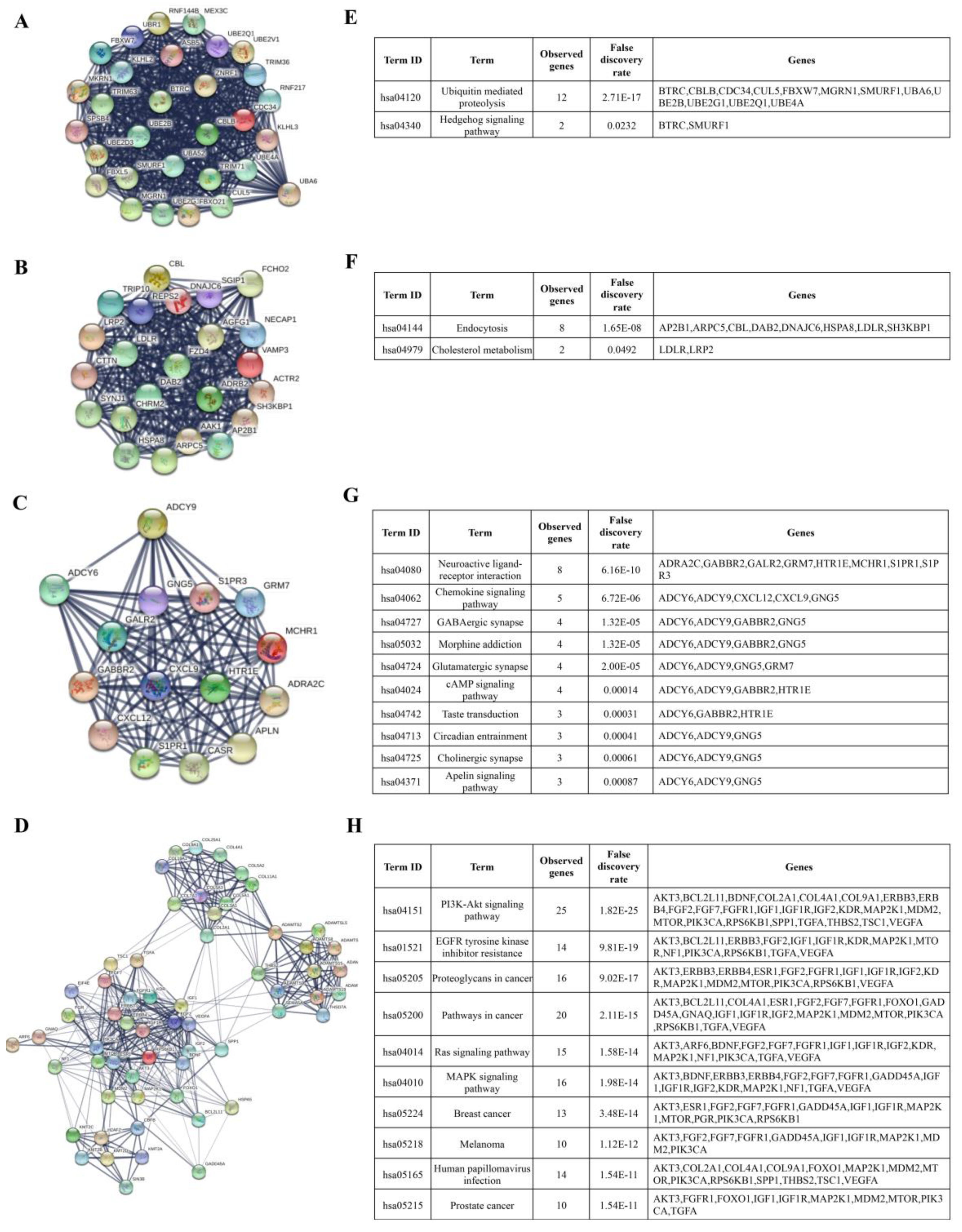
3.2. Prediction of miRNA Target Genes and Enrichment Analyses
3.3. Identification of Hub Genes and Enrichment Pathways from DEG PPI Networks
3.4. Validation of Subsets of Candidate miRNAs in Carcinoma Tissue of IBC vs. Non-IBC
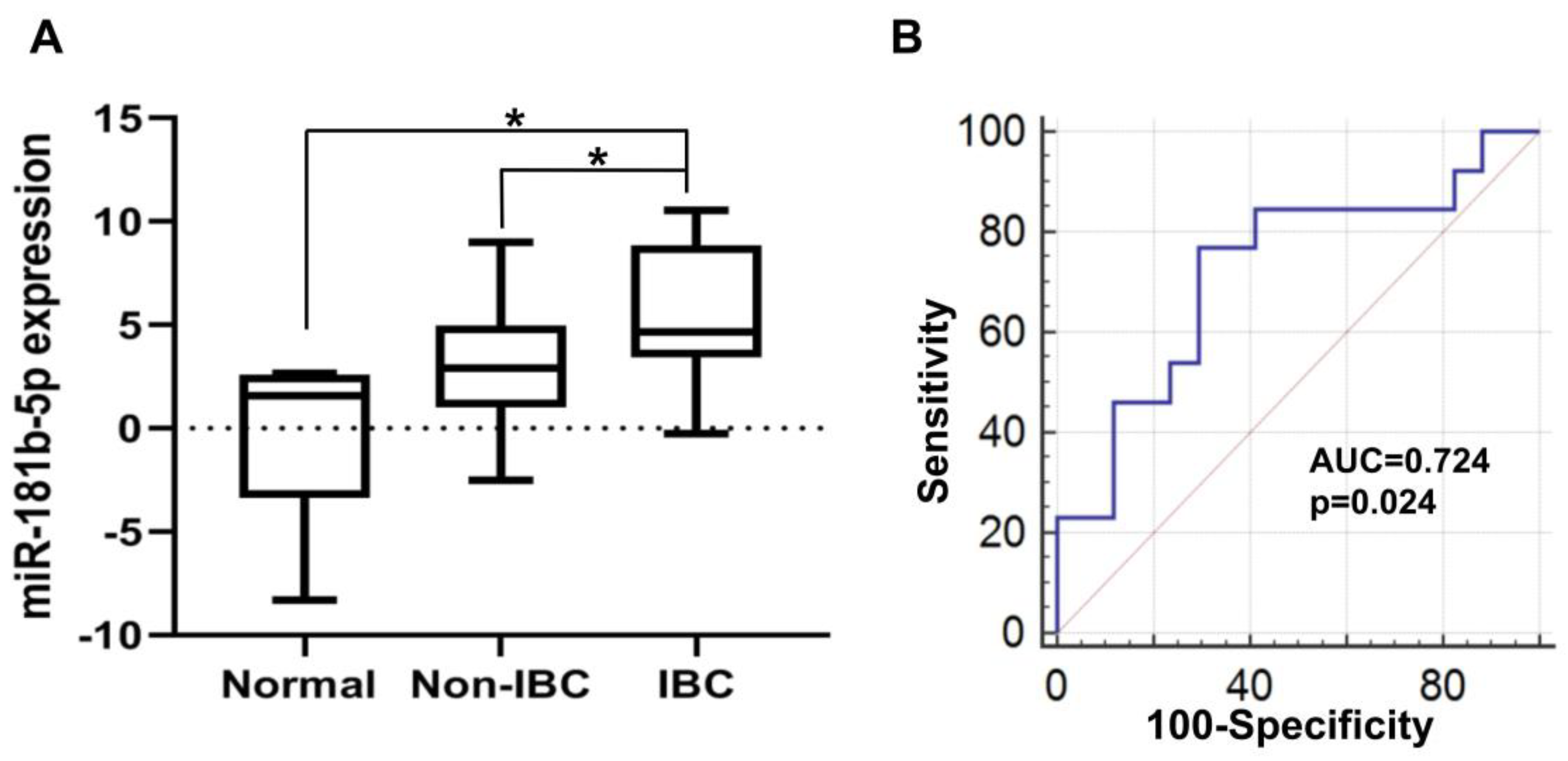
3.4.1. Elevated miR-181b-5p Expression in IBC
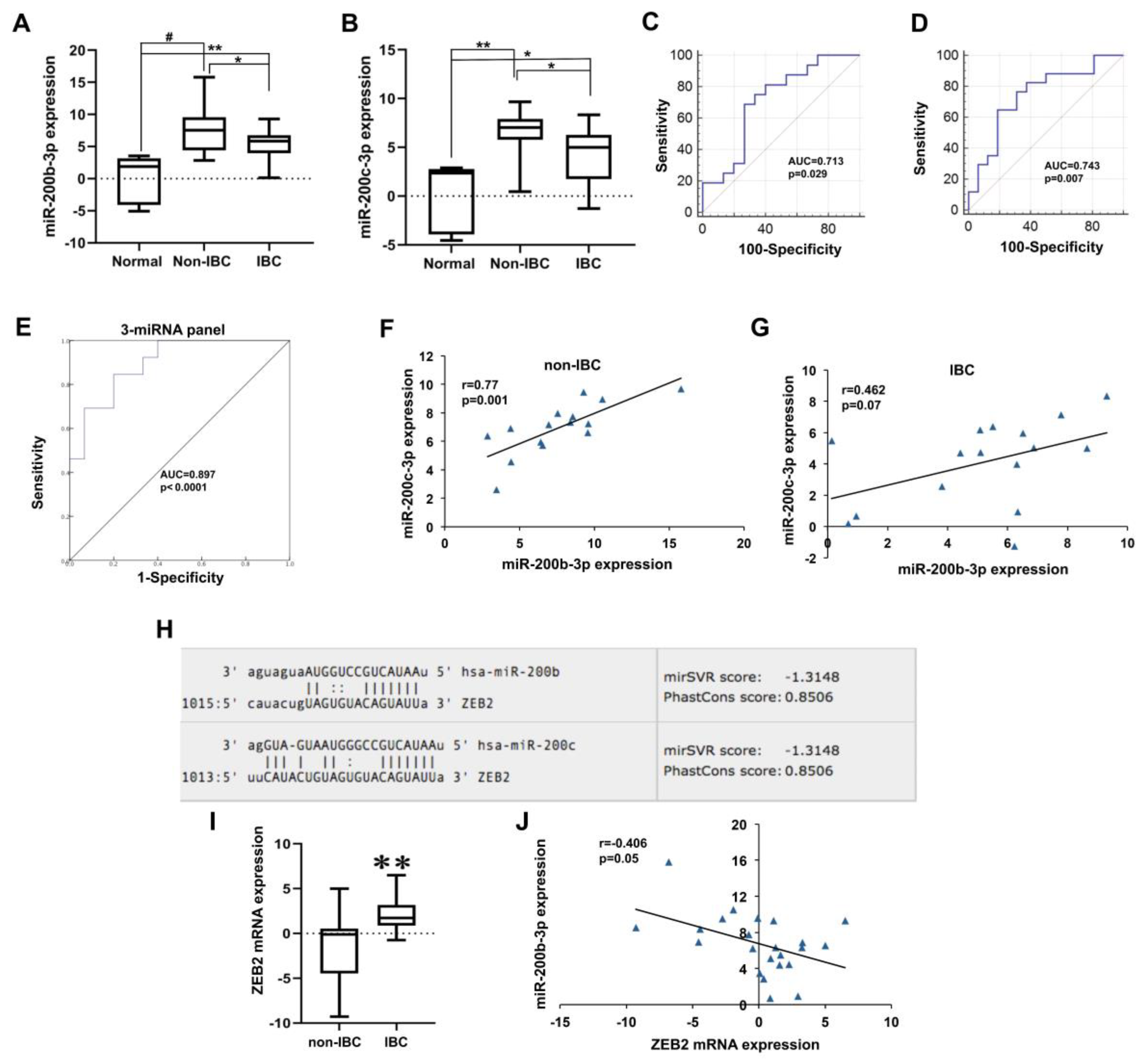
3.4.2. Low miR-200b-3p and miR-200c-3p Expression in IBC
3.4.3. Low miR-203a-3p Expression in IBC
3.4.4. miR-1-3p Expression in IBC
3.5. Expression of miR-1-3p and miR-200b in Non-IBC and IBC Cell Lines
3.6. Overall Survival Status of Breast Cancer Patients with Low and High Levels of the Identified miRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- van Golen, K. Inflammatory Breast Cancer: A Panoramic Overview. J. Rare Dis. Res. Treat. 2018, 3, 37–43. [Google Scholar] [CrossRef]
- van Uden, D.J.P.; van Laarhoven, H.W.M.; Westenberg, A.H.; de Wilt, J.H.W.; Blanken-Peeters, C.F.J.M. Inflammatory breast cancer: An overview. Crit. Rev. Oncol. Hematol. 2015, 93, 116–126. [Google Scholar] [CrossRef]
- Mamouch, F.; Berrada, N.; Aoullay, Z.; El Khanoussi, B.; Errihani, H. Inflammatory Breast Cancer: A Literature Review. World J. Oncol. 2018, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, X.; Kong, X.; Zhai, J.; Fang, Y.; Guan, X.; Wang, J. Expression signatures and roles of microRNAs in inflammatory breast cancer. Cancer Cell Int. 2019, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.; Banerjee, M.; Omar, S.; Khaled, H.; Anwar, N.; Zaghloul, M.S.; Eissa, S.; Kleer, C.G.; Dey, S.; Merajver, S.D.; et al. Survival of inflammatory breast cancer patients compared to non-inflammatory breast cancer patients in egypt. Breast J. 2011, 17, 545–547. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Rougemont, J.; Charafe-Jauffret, E.; Nasser, V.; Loriod, B.; Camerlo, J.; Tagett, R.; Tarpin, C.; Houvenaeghel, G.; et al. Gene expression profiling for molecular characterization of inflammatory breast cancer and prediction of response to chemotherapy. Cancer Res. 2004, 64, 8558–8565. [Google Scholar] [CrossRef]
- Laere, S.J.V.; Ueno, N.T.; Finetti, P.; Vermeulen, P.; Robertson, F.M.; Marsan, M.; Iwamoto, T.; Dam, P.V.; Woodward, W.A.; Viens, P.; et al. Uncovering the Molecular Secrets of Inflammatory Breast Cancer Biology: An Integrated Analysis of Three Distinct Affymetrix Gene Expression Datasets. Clin. Cancer Res. 2018, 19, 4685–4696. [Google Scholar] [CrossRef] [PubMed]
- Van Der Auwera, I.; Limame, R.; Van Dam, P.; Vermeulen, P.B.; Dirix, L.Y.; Van Laere, S.J. Integrated miRNA and mRNA expression profiling of the inflammatory breast cancer subtype. Br. J. Cancer 2010, 103, 532–541. [Google Scholar] [CrossRef]
- Huo, L.; Wang, Y.; Gong, Y.; Krishnamurthy, S.; Wang, J.; Diao, L.; Liu, C.G.; Liu, X.; Lin, F.; Symmans, W.F.; et al. MicroRNA expression profiling identifies decreased expression of miR-205 in inflammatory breast cancer. Mod. Pathol. 2016, 29, 330–346. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Hassan, H.; Götte, M. MicroRNA regulation of proteoglycan function in cancer. FEBS J. 2014, 281, 5009–5022. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Y.P. MicroRNAs in breast cancer: Oncogene and tumor suppressors with clinical potential. J. Zhejiang Univ. Sci. B 2015, 16, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Avery, T.; Cristofanilli, M. Biomarkers and Therapeutic Targets in Inflammatory Breast Cancer (IBC). Curr. Breast Cancer Rep. 2014, 6, 245–250. [Google Scholar] [CrossRef]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef]
- van Schooneveld, E.; Wildiers, H.; Vergote, I.; Vermeulen, P.B.; Dirix, L.Y.; Van Laere, S.J. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015, 17, 21. [Google Scholar] [CrossRef]
- Maltseva, D.V.; Galatenko, V.V.; Samatov, T.R.; Zhikrivetskaya, S.O.; Khaustova, N.A.; Nechaev, I.N.; Shkurnikov, M.U.; Lebedev, A.E.; Mityakina, I.A.; Kaprin, A.D.; et al. MiRNome of inflammatory breast cancer. BMC Res. Notes 2014, 7, 871. [Google Scholar] [CrossRef]
- Lerebours, F.; Cizeron-Clairac, G.; Susini, A.; Vacher, S.; Mouret-Fourme, E.; Belichard, C.; Brain, E.; Alberini, J.L.; Spyratos, F.; Lidereau, R.; et al. MiRNA expression profiling of inflammatory breast cancer identifies a 5-miRNA signature predictive of breast tumor aggressiveness. Int. J. Cancer 2013, 133, 1614–1623. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J. Extracell. Vesicles 2017, 6, 1321455. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Györffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res. Treat. 2010, 123, 725–731. [Google Scholar] [CrossRef]
- Spirin, V.; Mirny, L.A. Protein complexes and functional modules in molecular networks. Proc. Natl. Acad. Sci. USA 2003, 100, 12123–12128. [Google Scholar] [CrossRef]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, 149–153. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef]
- Lánczky, A.; Nagy, Á.; Bottai, G.; Munkácsy, G.; Szabó, A.; Santarpia, L.; Győrffy, B. miRpower: A web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. 2016, 160, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Tuomarila, M.; Luostari, K.; Soini, Y.; Kataja, V.; Kosma, V.M.; Mannermaa, A. Overexpression of microRNA-200c predicts poor outcome in patients with PR-negative breast cancer. PLoS ONE 2014, 9, e109508. [Google Scholar] [CrossRef]
- Papadaki, C.; Stratigos, M.; Markakis, G.; Spiliotaki, M.; Mastrostamatis, G.; Nikolaou, C.; Mavroudis, D.; Agelaki, S. Circulating microRNAs in the early prediction of disease recurrence in primary breast cancer. Breast Cancer Res. 2018, 20, 72. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Hassan, H.; Götte, M. MicroRNA-dependent targeting of the extracellular matrix as a mechanism of regulating cell behavior. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2609–2620. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shaik, S.; Dai, X.; Wu, Q.; Zhou, X.; Wang, Z.; Wei, W. Targeting the Ubiquitin Pathway for Cancer Treatment. Biochim. Biophys. Acta 2015, 1855, 50–60. [Google Scholar] [CrossRef]
- Sari, I.N.; Phi, L.T.H.; Jun, N.; Wijaya, Y.T.; Lee, S.; Kwon, H.Y. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells 2018, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, Y.; Mills, G.B.; Yarden, Y. Derailed endocytosis: An emerging feature of cancer. Nat. Rev. Cancer 2008, 8, 835–850. [Google Scholar] [CrossRef]
- Huang, B.; Song, B.L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef]
- Lim, B.; Woodward, W.A.; Wang, X.; Reuben, J.M.; Ueno, N.T. Inflammatory breast cancer biology: The tumour microenvironment is key. Nat. Rev. Cancer 2018, 18, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galván, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Morrow, R.J.; Etemadi, N.; Yeo, B.; Ernst, M. Challenging a Misnomer? The Role of Inflammatory Pathways in Inflammatory Breast Cancer. Mediat. Inflamm. 2017, 2017, 4754827. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Götte, M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol. Cancer 2017, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- El-Nadi, M.; Hassan, H.; Saleh, M.E.; Nassar, E.; Ismail, Y.M.; Amer, M.; Greve, B.; Götte, M.; El-Shinawi, M.; Ibrahim, S.A. Induction of heparanase via IL-10 correlates with a high infiltration of CD163+ M2-type tumor-associated macrophages in inflammatory breast carcinomas. Matrix Biol. Plus 2020, 6, 100030. [Google Scholar] [CrossRef]
- El-Shinawi, M.; Mohamed, H.T.; Abdel-Fattah, H.H.; Ibrahim, S.A.A.; El-Halawany, M.S.; Nouh, M.A.; Schneider, R.J.; Mohamed, M.M. Inflammatory and Non-inflammatory Breast Cancer: A Potential Role for Detection of Multiple Viral DNAs in Disease Progression. Ann. Surg. Oncol. 2016, 23, 494–502. [Google Scholar] [CrossRef]
- Corbex, M.; Bouzbid, S.; Traverse-Glehen, A.; Aouras, H.; McKay-Chopin, S.; Carreira, C.; Lankar, A.; Tommasino, M.; Gheit, T. Prevalence of papillomaviruses, polyomaviruses, and herpesviruses in triple-negative and inflammatory breast tumors from Algeria compared with other types of breast cancer tumors. PLoS ONE 2014, 9, e114559. [Google Scholar] [CrossRef]
- Liang, X.; Vacher, S.; Boulai, A.; Bernard, V.; Baulande, S.; Bohec, M.; Bièche, I.; Lerebours, F.; Callens, C. Targeted next-generation sequencing identifies clinically relevant somatic mutations in a large cohort of inflammatory breast cancer. Breast Cancer Res. 2018, 20, 1–12. [Google Scholar]
- Jhaveri, K.; Teplinsky, E.; Silvera, D.; Valeta-Magara, A.; Arju, R.; Giashuddin, S.; Sarfraz, Y.; Alexander, M.; Darvishian, F.; Levine, P.H.; et al. Hyperactivated mTOR and JAK2/STAT3 Pathways: Molecular Drivers and Potential Therapeutic Targets of Inflammatory and Invasive Ductal Breast Cancers after Neoadjuvant Chemotherapy. Clin. Breast Cancer 2016, 16, 113–122. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhang, J.H. miR-181c promotes proliferation via suppressing PTEN expression in inflammatory breast cancer. Int. J. Oncol. 2015, 46, 2011–2020. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Tsuyada, A.; Ren, X.; Wu, X.; Stubblefield, K.; Rankin-Gee, E.K.; Wang, S.E. Transforming growth factor-Β regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene 2011, 30, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Neel, J.C.; Lebrun, J.J. Activin and TGFβ regulate expression of the microRNA-181 family to promote cell migration and invasion in breast cancer cells. Cell. Signal. 2013, 25, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, J.X.; He, D.; Zhang, G.J. Bioluminescence Imaging for Monitoring miR-200c Expression in Breast Cancer Cells and its Effects on Epithelial-Mesenchymal Transition Progress in Living Animals. Mol. Imaging Biol. 2018, 20, 761–770. [Google Scholar] [CrossRef]
- Tang, H.; Song, C.; Ye, F.; Gao, G.; Ou, X.; Zhang, L.; Xie, X.; Xie, X. miR-200c suppresses stemness and increases cellular sensitivity to trastuzumab in HER2+ breast cancer. J. Cell. Mol. Med. 2019, 23, 8114–8127. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.J.; Christenson, J.L.; Greene, L.I.; O’Neill, K.I.; Williams, M.M.; Gordon, M.A.; Nemkov, T.; D’Alessandro, A.; Degala, G.D.; Shin, J.; et al. Reversal of Triple-Negative Breast Cancer EMT by miR-200c Decreases Tryptophan Catabolism and a Program of Immunosuppression. Mol. Cancer Res. 2019, 17, 30–41. [Google Scholar] [CrossRef]
- Zheng, Q.; Cui, X.; Zhang, D.; Yang, Y.; Yan, X.; Liu, M.; Niang, B.; Aziz, F.; Liu, S.; Yan, Q.; et al. miR-200b inhibits proliferation and metastasis of breast cancer by targeting fucosyltransferase IV and α1,3-fucosylated glycans. Oncogenesis 2017, 6, e358. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, H.; Song, H.; Xu, H.; Zhao, B.; Wu, C.; Hu, J.; Wu, T.; Xie, D.; Zhao, J.; et al. The microRNAs miR-200b-3p and miR-429-5p target the LIMK1/CFL1 pathway to inhibit growth and motility of breast cancer cells. Oncotarget 2017, 8, 85276–85289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhou, Y.; Yang, H.; Li, L.; Deng, X.; Cheng, C.; Xie, Y.; Luo, X.; Fang, W.; Liu, Z. A directly negative interaction of miR-203 and ZEB2 modulates tumor stemness and chemotherapy resistance in nasopharyngeal carcinoma. Oncotarget 2016, 7, 67288–67301. [Google Scholar] [CrossRef]
- Sánchez-Cid, L.; Pons, M.; Lozano, J.J.; Rubio, N.; Guerra-Rebollo, M.; Soriano, A.; Paris-Coderch, L.; Segura, M.F.; Fueyo, R.; Arguimbau, J.; et al. MicroRNA-200, associated with metastatic breast cancer, promotes traits of mammary luminal progenitor cells. Oncotarget 2017, 8, 83384–83406. [Google Scholar] [CrossRef]
- Korpal, M.; Ell, B.J.; Buffa, F.M.; Ibrahim, T.; Blanco, M.A.; Mercatali, L.; Khan, Z.; Goodarzi, H.; Hua, Y.; Wei, Y.; et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 2012, 17, 1101–1108. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Wu, Y.; Xie, H.; Yu, F.; Lal, A.; Petrocca, F.; Martinvalet, D.; Song, E.; Lim, B.; Lieberman, J. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS ONE 2009, 4, e7181. [Google Scholar] [CrossRef]
- Braicu, C.; Raduly, L.; Morar-Bolba, G.; Cojocneanu, R.; Jurj, A.; Pop, L.A.; Pileczki, V.; Ciocan, C.; Moldovan, A.; Irimie, A.; et al. Aberrant miRNAs expressed in HER-2 negative breast cancers patient. J. Exp. Clin. Cancer Res. 2018, 37, 257. [Google Scholar] [CrossRef]
- Park, S.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Tryndyak, V.P.; Beland, F.A.; Pogribny, I.P. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int. J. Cancer 2010, 126, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.C.; Martino, V.; Reinbold, R.; Schäfer, S.D.; Kiesel, L.; Starzinski-Powitz, A.; Schüring, A.N.; Kemper, B.; Greve, B.; Götte, M. MicroRNA miR-200b affects proliferation, invasiveness and stemness of endometriotic cells by targeting ZEB1, ZEB2 and KLF4. Reprod. Biomed. Online 2016, 32, 434–445. [Google Scholar] [CrossRef]
- Kleer, C.G.; Van Golen, K.L.; Braun, T.; Merajver, S.D. Persistent E-cadherin expression in inflammatory breast cancer. Mod. Pathol. 2001, 14, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tellez, J.D.; Durazo, M.; Belcher, M.; Yearsley, K.; Barsky, S.H. E-cadherin accumulation within the lymphovascular embolus of inflammatory breast cancer is due to altered trafficking. Anticancer Res. 2010, 30, 3903–3910. [Google Scholar]
- Jolly, M.K.; Boareto, M.; Debeb, B.G.; Aceto, N.; Farach-Carson, M.C.; Woodward, W.A.; Levine, H. Inflammatory breast cancer: A model for investigating cluster-based dissemination. NPJ Breast Cancer 2017, 3, 1–7. [Google Scholar]
- Zhang, Z.; Zhang, B.; Li, W.; Fu, L.; Fu, L.; Zhu, Z.; Dong, J.T. Epigenetic Silencing of miR-203 Upregulates SNAI2 and Contributes to the Invasiveness of Malignant Breast Cancer Cells. Genes Cancer 2011, 2, 782–791. [Google Scholar] [CrossRef]
- Duan, X.; Fu, Z.; Gao, L.; Zhou, J.; Deng, X.; Luo, X.; Fang, W.; Luo, R. Direct interaction between miR-203 and ZEB2 suppresses epithelial-mesenchymal transition signaling and reduces lung adenocarcinoma chemoresistance. Acta Biochim. Biophys. Sin. 2016, 48, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Majid, S.; Yamamura, S.; Tabatabai, L.; Suh, S.O.; Shahryari, V.; Chen, Y.; Deng, G.; Tanaka, Y.; Dahiya, R. Regulatory role of mir-203 in prostate cancer progression and metastasis. Clin. Cancer Res. 2011, 17, 5287–5298. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.; Li, L. MiR-124 and miR-203 synergistically inactivate EMT pathway via coregulation of ZEB2 in clear cell renal cell carcinoma (ccRCC). J. Transl. Med. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Gong, P.; Qiao, F.; Wu, H.; Cui, H.; Li, Y.; Zheng, Y.; Zhou, M.; Fan, H. LncRNA UCA1 promotes tumor metastasis by inducing miR-203/ZEB2 axis in gastric cancer. Cell Death Dis. 2018, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Han, J.; Zheng, L.; Yang, Z.; Zhao, L.; Lv, Y. MicroRNA-203 regulates growth and metastasis of breast cancer. Cell. Physiol. Biochem. 2015, 37, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, X.; Shen, C.; Shi, Y. MicroRNA-203 suppresses cell proliferation and migration by targeting BIRC5 and LASP1 in human triple-negative breast cancer cells. J. Exp. Clin. Cancer Res. 2012, 31, 58. [Google Scholar] [CrossRef]
- Zhao, G.; Guo, Y.; Chen, Z.; M, L.; Yue, J. miR-203 Functions as a Tumor Suppressor by Inhibiting Epithelial to Mesenchymal Transition in Ovarian Cancer. J. Cancer Sci. Ther. 2015, 7, 34–43. [Google Scholar] [PubMed]
- Deng, B.; Wang, B.; Fang, J.; Zhu, X.; Cao, Z.; Lin, Q.; Zhou, L.; Sun, X. MiRNA-203 suppresses cell proliferation, migration and invasion in colorectal cancer via targeting of EIF5A2. Sci. Rep. 2016, 6, 28301. [Google Scholar] [CrossRef]
- Jiang, N.; Jiang, X.; Chen, Z.; Song, X.; Wu, L.; Zong, D.; Song, D.; Yin, L.; Wang, D.; Chen, C.; et al. MiR-203a-3p suppresses cell proliferation and metastasis through inhibiting LASP1 in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Boll, K.; Reiche, K.; Kasack, K.; Mörbt, N.; Kretzschmar, A.K.; Tomm, J.M.; Verhaegh, G.; Schalken, J.; Von Bergen, M.; Horn, F.; et al. MiR-130a, miR-203 and miR-205 jointly repress key oncogenic pathways and are downregulated in prostate carcinoma. Oncogene 2013, 32, 277–285. [Google Scholar] [CrossRef]
- Siu, M.K.; Abou-Kheir, W.; Yin, J.J.; Chang, Y.S.; Barrett, B.; Suau, F.; Casey, O.; Chen, W.Y.; Fang, L.; Hynes, P.; et al. Loss of EGFR signaling regulated miR-203 promotes prostate cancer bone metastasis and tyrosine kinase inhibitors resistance. Oncotarget 2014, 5, 3770–3784. [Google Scholar] [CrossRef]
- DeCastro, A.J.; Dunphy, K.A.; Hutchinson, J.; Balboni, A.L.; Cherukuri, P.; Jerry, D.J.; DiRenzo, J. MiR203 mediates subversion of stem cell properties during mammary epithelial differentiation via repression of δnP63α and promotes mesenchymal-to-epithelial transition. Cell Death Dis. 2013, 4, e514. [Google Scholar] [CrossRef]
- He, S.; Zhang, G.; Dong, H.; Ma, M.; Sun, Q. miR-203 facilitates tumor growth and metastasis by targeting fibroblast growth factor 2 in breast cancer. Onco. Targets. Ther. 2016, 9, 6203–6210. [Google Scholar] [CrossRef] [PubMed]
- Panoutsopoulou, K.; Avgeris, M.; Mavridis, K.; Dreyer, T.; Dorn, J.; Reinthaller, A.; Michaelidou, K.; Obermay, E.; Mahner, S. miR-203 is an independent molecular predictor of prognosis and treatment outcome in ovarian cancer: A multi-institutional study Konstantina. Carcinogenesis 2020, 41, 442–451. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Lai, Y.; Liao, Y.; Liu, R.; Qiu, W. Hsa-miR-1 suppresses breast cancer development by down-regulating K-ras and long non-coding RNA MALAT1. Int. J. Biol. Macromol. 2015, 81, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, T.; He, D.; Li, X.; Jiang, Y. miR-1 inhibits the proliferation of breast cancer stem cells by targeting EVI-1. Onco. Targets. Ther. 2018, 11, 8773–8781. [Google Scholar] [CrossRef]
- Chou, J.; Wang, B.; Zheng, T.; Li, X.; Zheng, L.; Hu, J.; Zhang, Y.; Xing, Y.; Xi, T. MALAT1 induced migration and invasion of human breast cancer cells by competitively binding MIR-1 with cdc42. Biochem. Biophys. Res. Commun. 2016, 472, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yan, B.; Lu, Q.; Lin, Y.; Ma, L. Reciprocal regulation of Hsa-miR-1 and long noncoding RNA MALAT1 promotes triple-negative breast cancer development. Tumor Biol. 2016, 37, 7383–7394. [Google Scholar] [CrossRef]
- Minemura, H.; Takagi, K.; Miki, Y.; Shibahara, Y.; Nakagawa, S.; Ebata, A.; Watanabe, M.; Ishida, T.; Sasano, H.; Suzuki, T. Abnormal expression of miR-1 in breast carcinoma as a potent prognostic factor. Cancer Sci. 2015, 106, 1642–1650. [Google Scholar] [CrossRef]
- Ye, F.; Tang, H.; Liu, Q.; Xie, X.; Wu, M.; Liu, X.; Chen, B.; Xie, X. miR-200b as a prognostic factor in breast cancer targets multiple members of RAB family. Transl. Med. 2014, 21, 12–17. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, J.; Shen, Z.; Yao, R.; Liu, S.; Li, Y.; Cong, H.; Wang, X.; Qiu, W.; Yue, L. MiR-200b expression in breast cancer: A prognostic marker and act on cell proliferation and apoptosis by targeting Sp1. J. Cell. Mol. Med. 2015, 19, 760–769. [Google Scholar] [CrossRef]
- Cai, K.T.; Feng, C.X.; Zhao, J.C.; He, R.Q.; Ma, J.; Zhong, J.C. Upregulated miR-203a-3p and its potential molecular mechanism in breast cancer: A study based on bioinformatics analyses and a comprehensive meta-analysis. Mol. Med. Rep. 2018, 18, 4994–5008. [Google Scholar] [CrossRef]
- Song, X.; Zhang, C.; Liu, Z.; Liu, Q.; He, K.; Yu, Z. Characterization of ceRNA network to reveal potential prognostic biomarkers in triple-negative breast cancer. PeerJ 2019, 2019, e7522. [Google Scholar] [CrossRef] [PubMed]
- Cobos Jiménez, V.; Willemsen, A.M.; Bradley, E.J.; Baas, F.; van Kampen, A.H.C.; Kootstra, N.A. Next-generation sequencing of microRNAs in primary human polarized macrophages. Genom. Data 2014, 2, 181–183. [Google Scholar] [CrossRef] [PubMed]

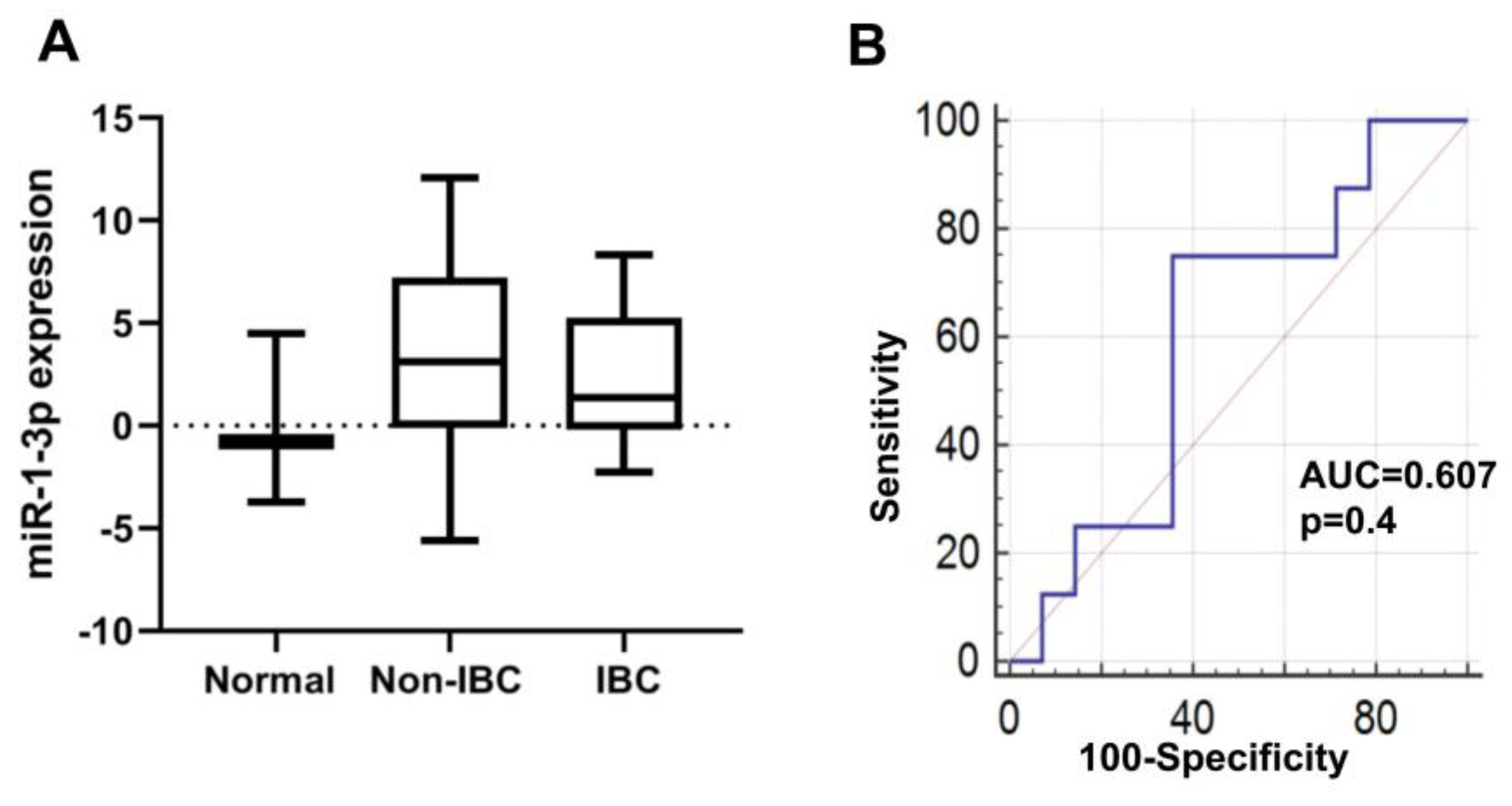
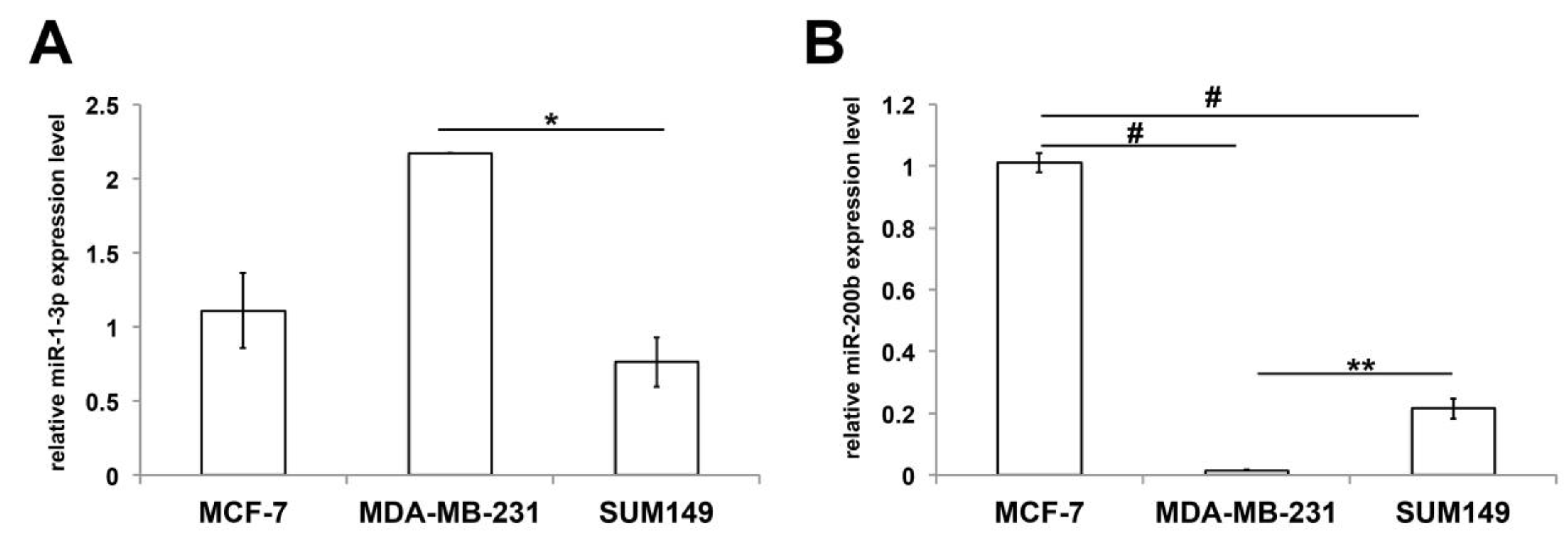

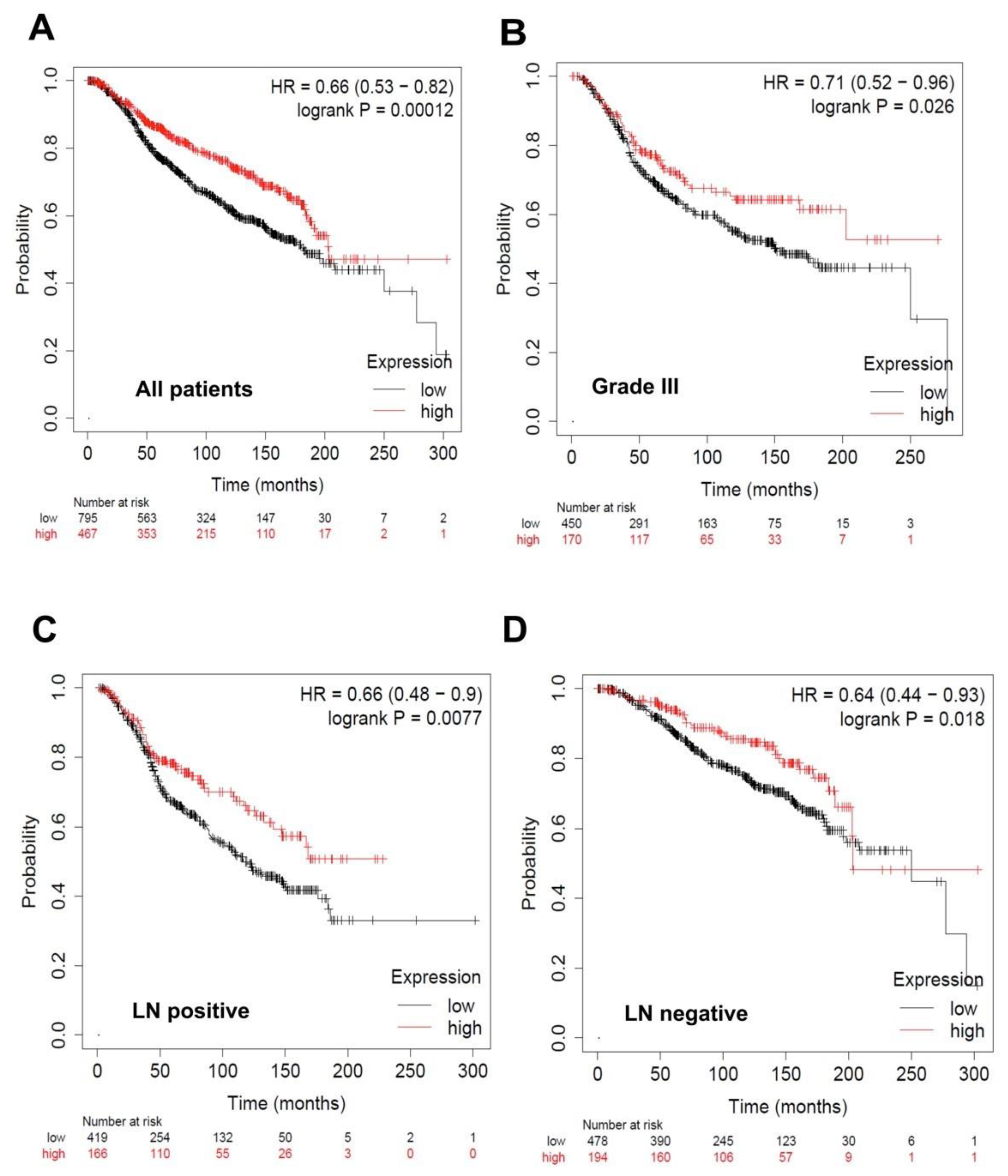
| Characteristic | IBC (n = 17) | Non-IBC (n = 18) | p Value |
|---|---|---|---|
| Age (years) | |||
| Range | 33–82 | 32–73 | 0.806 a |
| Mean ± SEM | 54.4 ± 3.2 | 53.3 ± 2.7 | |
| Tumor size (cm), n (%) | |||
| ≤4 | 6 (35) | 11(61) | 0.11 b |
| >4 | 9 (53) | 5 (28) | |
| NA | 2 (12) | 2 (11) | |
| Lymph node status, n (%) | |||
| <4 | 5 (29) | 12 (67) | 0.02 * b |
| ≥4 | 10 (59) | 4 (22) | |
| NA | 2 (12) | 2 (11) | |
| Tumor grade, n (%) | |||
| Grade I | 0 (0) | 1 (5) | 0.14 b |
| Grade II | 11 (65) | 12 (67) | |
| Grade III | 4 (23) | 2 (11) | |
| NA | 2 (12) | 3 (17) | |
| Lymphovascular invasion, n (%) | |||
| Negative | 4 (23) | 11 (61) | 0.005 * b |
| Positive | 11 (65) | 3 (17) | |
| NA | 2 (12) | 4 (22) | |
| ER, n (%) | |||
| Negative | 8 (47) | 9 (50) | 0.85 b |
| Positive NA | 7 (41) 2 (12) | 9 (50) 0 (0) | |
| PR, n (%) | |||
| Negative | 10 (59) | 10 (56) | 0.83 b |
| Positive NA | 7 (41) 0 (0) | 6 (33) 2 (11) | |
| Her2, n (%) | |||
| Negative | 10 (59) | 12 (66) | 0.28 b |
| Positive NA | 6 (35) 1 (6) | 3 (17) 3 (17) |
| miRNAs | Fold Change (log2) |
|---|---|
| Upregulated in IBC | |
| let-7b-5p | 0.82 |
| miR-100-5p | 0.82 |
| miR-140-5p | 0.82 |
| miR-181b-5p | 0.83 |
| miR-181c-5p | 0.82 |
| miR-181d-5p | 0.83 |
| miR-199a-3p | 0.84 |
| miR-222-3p | 0.83 |
| miR-328-3p | 0.81 |
| miR-495-3p | 0.82 |
| Downregulated in IBC | |
| miR-1-3p | −3.18 |
| miR-107 | −1.18 |
| miR-129-5p | −1.15 |
| miR-141-3p | −1.17 |
| miR-145-5p | −1.16 |
| miR-148a-3p | −1.15 |
| miR-15b-5p | −1.18 |
| miR-182-5p | −1.17 |
| miR-200b-3p | −1.16 |
| miR-200c-3p | −1.17 |
| miR-203a-3p | −2.17 |
| miR-205-5p | −2.18 |
| miR-206-5p | −1.22 |
| miR-20b-5p | −1.17 |
| miR-210-3p | −1.17 |
| miR-29b-3p | −1.16 |
| miR-485-5p | −1.16 |
| miR-96-5p | −1.17 |
| miRNA Regulation | Category | Term | Count | % | p-Value |
|---|---|---|---|---|---|
| Upregulated | GOTERM_ BP_FAT | GO: 0006355 regulation of transcription | 102 | 2.8 | 3.00 × 10−11 |
| GOTERM_ BP_FAT | GO:0006351 transcription | 80 | 2.2 | 5.00 × 10−08 | |
| GOTERM_ BP_FAT | GO: 0051252 regulation of RNA metabolic process | 67 | 1.8 | 2.90 × 10−06 | |
| GOTERM_ BP_FAT | GO: 0006355 regulation of transcription, DNA-dependent | 63 | 1.7 | 2.30 × 10−05 | |
| GOTERM_ BP_FAT | GO: 0050808 synapse organization | 9 | 0.2 | 3.60 × 10−05 | |
| GOTERM_ CC_FAT | GO: 0043228 non-membrane-bounded organelle | 57 | 1.6 | 3.30 × 10−02 | |
| GOTERM_ CC_FAT | GO: 0043232 intracellular non-membrane-bounded organelle | 57 | 1.6 | 3.30 × 10−02 | |
| GOTERM_ CC_FAT | GO: 0045177 apical part of cell | 8 | 0.2 | 3.50 × 10−02 | |
| GOTERM_ CC_FAT | GO: 0031982 membrane-bounded vesicle | 17 | 0.5 | 3.50 × 10−02 | |
| GOTERM_ CC_FAT | GO: 0008023 transcription elongation factor complex | 3 | 0.1 | 3.70 × 10−02 | |
| GOTERM_ MF_FAT | GO: 0046872 metal ion binding | 133 | 3.6 | 1.90 × 10−07 | |
| GOTERM_ MF_FAT | GO: 0043167 ion binding | 135 | 3.7 | 2.50 × 10−07 | |
| GOTERM_ MF_FAT | GO: 0043169 cation binding | 133 | 3.6 | 3.50 × 10−07 | |
| GOTERM_ MF_FAT | GO: 0008270 zinc ion binding | 81 | 2.2 | 1.00 × 10−05 | |
| GOTERM_ MF_FAT | GO: 0046914 transition metal ion binding | 93 | 2.5 | 1.20 × 10−05 | |
| Downregulated | GOTERM_ BP_FAT | GO: 0007507 heart development | 36 | 0.3 | 1.80 × 10−08 |
| GOTERM_ BP_FAT | GO: 0010629 negative regulation of gene expression | 62 | 0.6 | 1.90 × 10−08 | |
| GOTERM_ BP_FAT | GO: 0006468 protein amino acid phosphorylation | 75 | 0.7 | 2.40 × 10−08 | |
| GOTERM_ BP_FAT | GO: 0006351 transcription | 176 | 1.6 | 5.60 × 10−08 | |
| GOTERM_ BP_FAT | GO: 0045892 negative regulation of transcription | 56 | 0.5 | 1.30 × 10−07 | |
| GOTERM_ CC_FAT | GO: 0031981 nuclear lumen | 116 | 1 | 9.80 × 10−07 | |
| GOTERM_ CC_FAT | GO: 0005794 Golgi apparatus | 76 | 0.7 | 6.10 × 10−06 | |
| GOTERM_ CC_FAT | GO: 0005581 collagen | 11 | 0.1 | 6.80 × 10−06 | |
| GOTERM_ CC_FAT | GO: 0005583 fibrillar collagen | 7 | 0.1 | 1.30 × 10−05 | |
| GOTERM_ CC_FAT | GO: 0012505 endomembrane system | 68 | 0.6 | 2.20 × 10−05 | |
| GOTERM_ MF_FAT | GO: 0004672 protein kinase activity | 68 | 0.6 | 2.60 × 10−07 | |
| GOTERM_ MF_FAT | GO: 0030528 transcription regulator activity | 135 | 1.2 | 2.90 × 10−07 | |
| GOTERM_ MF_FAT | GO: 0004674 protein serine/threonine kinase activity | 50 | 0.4 | 4.90 × 10−06 | |
| GOTERM_ MF_FAT | GO: 0003700 transcription factor activity | 90 | 0.8 | 1.20 × 10−05 | |
| GOTERM_ MF_FAT | GO: 0016564 transcription repressor activity | 39 | 0.3 | 1.70 × 10−05 |
| miRNA Regulation | Category | Term | Count | % | p-Value |
|---|---|---|---|---|---|
| Upregulated | KEGG_ PATHWAY | hsa04512: ECM-receptor interaction | 7 | 0.2 | 6.50 × 10−03 |
| KEGG_ PATHWAY | hsa05200: Pathways in cancer | 14 | 0.4 | 1.30 × 10−02 | |
| KEGG_ PATHWAY | hsa05218: Melanoma | 6 | 0.2 | 1.30 × 10−02 | |
| KEGG_ PATHWAY | hsa05214: Glioma | 5 | 0.1 | 3.70 × 10−02 | |
| KEGG_ PATHWAY | hsa04115: p53 signaling pathway | 5 | 0.1 | 4.70 × 10−02 | |
| Downregulated | KEGG_ PATHWAY | hsa04360: Axon guidance | 24 | 0.2 | 9.00 × 10−07 |
| KEGG_ PATHWAY | hsa05200: Pathways in cancer | 40 | 0.4 | 8.70 × 10−06 | |
| KEGG_ PATHWAY | hsa04010: MAPK signaling pathway | 34 | 0.3 | 2.00 × 10−05 | |
| KEGG_ PATHWAY | hsa04510: Focal adhesion | 28 | 0.3 | 2.80 × 10−05 | |
| KEGG_ PATHWAY | hsa05215: Prostate cancer | 17 | 0.2 | 3.90 × 10−05 |
| miR-1-3p Expression | Pearson-Chi Square (p) | ||
|---|---|---|---|
| Negative (N) | Positive (N) | ||
| Non-IBC | 4 | 14 | 0.06 |
| IBC | 9 | 8 | |
| miRNA | ER | Her2 | |||||||
| Positive (n = 979) | Negative (n = 283) | Positive (n = 157) | Negative (n = 1105) | ||||||
| HR 95% CI | p Value | HR 95% CI | p Value | HR 95% CI | p Value | HR 95% CI | p Value | ||
| Upregulated | let-7b-5p | 0.72 (0.56–0.92) | 0.0083 | 1.38 (0.94–2.02) | 0.096 | 0.56 (0.35–0.91) | 0.018 | 0.75 (0.6–0.93) | 0.01 |
| miR-100-5p | 0.65 (0.52–0.8) | 0.00025 | 0.78 (0.52–1.19) | 0.25 | 0.53 (0.32–0.87) | 0.011 | 0.7 (0.57–0.88) | 0.0015 | |
| miR-140-5p | 0.66 (0.53–0.84) | 0.00048 | 0.67 (0.46–0.98) | 0.039 | 0.68 (0.4–1.14) | 0.14 | 0.67 (0.54–0.84) | 0.00043 | |
| miR-181b-5p | 1.56 (1.21–2.01) | 0.00046 | 1.45 (0.97–2.16) | 0.066 | 0.76 (0.45–1.15) | 0.16 | 1.48 (1.17–1.86) | 0.00092 | |
| miR-181c-5p | 0.57 (0.45–0.72) | 2.5 × 10−06 | 1.24 (0.85–1.82) | 0.26 | 0.68 (0.42–1.11) | 0.12 | 0.66 (0.53–0.82) | 0.00014 | |
| miR-181d-5p | 0.7 (0.55–0.88) | 0.0022 | 1.78 (1.09–2.92) | 0.02 | 0.63 (0.46–0.86) | 0.0039 | 0.77 (0.62–0.95) | 0.015 | |
| miR-199a-3p | 0.66 (0.51–0.84) | 0.00067 | 1.29 (0.85–1.94) | 0.23 | 1.3 (0.81–2.08) | 0.28 | 0.66 (0.53–0.82) | 0.00014 | |
| miR-222-3p | 1.44 (1.09–1.91) | 0.011 | 1.35 (0.93–1.96) | 0.11 | 1.55 (0.96–2.51) | 0.073 | 1.38 (1.06–1.79) | 0.016 | |
| miR-328-3p | 0.76 (0.57–1.02) | 0.065 | 0.69 (0.45–1.07) | 0.098 | 0.6 (0.37–0.98) | 0.039 | 0.8 (0.64–1.01) | 0.056 | |
| miR-495-3p | 0.66 (0.52–0.84) | 0.00079 | 0.84 (0.58–1.21) | 0.35 | 0.68 (0.42–1.09) | 0.11 | 0.66 (0.53–0.84) | 0.00043 | |
| Downregulated | miR-1-3p | 0.64 (0.49–0.85) | 0.0015 | 0.73 (0.46–1.16) | 0.18 | 0.62 (0.38–1.02) | 0.059 | 0.64 (0.49–0.83) | 0.00077 |
| miR-107 | 1.24 (0.97–1.58) | 0.091 | 0.79 (0.52–1.19) | 0.25 | 0.75 (0.47–1.22) | 0.25 | 1.22 (0.97–1.54) | 0.092 | |
| miR-129-5p | 1.45 (1.13–1.86) | 0.0037 | 0.83 (0.56–1.25) | 0.37 | 0.68 (0.41–1.14) | 0.14 | 1.36 (1.07–1.71) | 0.011 | |
| miR-141-3p | 1.53 (1.21–1.93) | 0.00029 | 1.47 (1–2.17) | 0.049 | 1.63 (0.98–2.69) | 0.055 | 1.45 (1.17–1.8) | 0.00071 | |
| miR-145-5p | 0.81 (0.63–1.03) | 0.084 | 1.15 (0.79–1.68) | 0.45 | 1.32 (0.78–2.22) | 0.3 | 0.79 (0.63–0.98) | 0.033 | |
| miR-148a-3p | 0.61 (0.48–0.78) | 4.3 × 10−05 | 1.51 (0.98–2.31) | 0.058 | 0.72 (0.43–1.2) | 0.2 | 0.65 (0.52–0.82) | 0.00028 | |
| miR-15b-5p | 1.26 (0.98–1.62) | 0.075 | 0.82 (0.54–1.24) | 0.34 | 0.64 (0.4–1.04) | 0.067 | 1.3 (1.04–1.64) | 0.024 | |
| miR-182-5p | 0.87 (0.67-1.13) | 0.31 | 1.48 (1–2.19) | 0.049 | 0.62 (0.37–0.96) | 0.033 | 0.84 (0.65–1.08) | 0.18 | |
| miR-200b-3p | 0.9 (0.71–1.13) | 0.36 | 1.72 (1.12–2.66) | 0.013 | 1.4 (0.83–2.36) | 0.2 | 1.15 (0.91–1.46) | 0.25 | |
| miR-200c-3p | 1.5 (1.18–1.9) | 0.00083 | 1.49 (1.01–2.2) | 0.045 | 1.48 (0.92–2.4) | 0.1 | 1.53 (1.21–1.92) | 0.00028 | |
| miR-203a-3p | NO DATA FOUND | ||||||||
| miR-205-5p | 0.66 (0.52–0.84) | 0.00055 | 1.4 (0.94–2.07) | 0.094 | 1.47 (0.89–2.44) | 0.13 | 0.72 (0.58–0.9) | 0.0036 | |
| miR-206-5p | 1.31 (1–1.71) | 0.051 | 1.3 (0.84–2) | 0.23 | 1.36 (0.85–2.19) | 0.2 | 1.34 (1.04–1.72) | 0.022 | |
| miR-20b-5p | 0.8 (0.61–1.05) | 0.11 | 1.17 (0.8–1.71) | 0.41 | 1.46 (0.89–2.38) | 0.13 | 1.11 (0.87–1.41) | 0.42 | |
| miR-210-3p | 1.62 (1.26–2.09) | 0.00014 | 1.97 (1.36–2.85) | 0.00025 | 1.39 (0.86–2.26) | 0.18 | 1.6 (1.26–2.02) | 9.3×10−05 | |
| miR-29b-3p | 0.66 (0.52–0.85) | 0.00091 | 0.63 (0.43–0.91) | 0.014 | 1.26 (0.76–2.09) | 0.37 | 0.6 (0.47–0.76) | 2.1 × 10−05 | |
| miR-485-5p | 0.62 (0.48–0.8) | 0.00015 | 1.19 (0.78–1.84) | 0.42 | 0.77 (0.47–1.28) | 0.32 | 0.61 (0.49–0.76) | 1.2 × 10−05 | |
| miR-96-5p | 0.75 (0.59–0.96) | 0.02 | 1.2 (0.79–1.81) | 0.39 | 0.6 (0.37–0.98) | 0.038 | 0.76 (0.61–0.95) | 0.018 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahim, S.A.; Abdullah, M.S.; Espinoza-Sánchez, N.A.; Hassan, H.; Ibrahim, A.M.; Ahmed, S.H.; Shakir, G.; Badawy, M.A.; Zakhary, N.I.; Greve, B.; et al. Inflammatory Breast Carcinoma: Elevated microRNA miR-181b-5p and Reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p Expression as Potential Biomarkers with Diagnostic Value. Biomolecules 2020, 10, 1059. https://doi.org/10.3390/biom10071059
Fahim SA, Abdullah MS, Espinoza-Sánchez NA, Hassan H, Ibrahim AM, Ahmed SH, Shakir G, Badawy MA, Zakhary NI, Greve B, et al. Inflammatory Breast Carcinoma: Elevated microRNA miR-181b-5p and Reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p Expression as Potential Biomarkers with Diagnostic Value. Biomolecules. 2020; 10(7):1059. https://doi.org/10.3390/biom10071059
Chicago/Turabian StyleFahim, Sarah Atef, Mahmoud Salah Abdullah, Nancy A. Espinoza-Sánchez, Hebatallah Hassan, Ayman M. Ibrahim, Sarah Hamdy Ahmed, George Shakir, Mohamed A. Badawy, Nadia I. Zakhary, Burkhard Greve, and et al. 2020. "Inflammatory Breast Carcinoma: Elevated microRNA miR-181b-5p and Reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p Expression as Potential Biomarkers with Diagnostic Value" Biomolecules 10, no. 7: 1059. https://doi.org/10.3390/biom10071059
APA StyleFahim, S. A., Abdullah, M. S., Espinoza-Sánchez, N. A., Hassan, H., Ibrahim, A. M., Ahmed, S. H., Shakir, G., Badawy, M. A., Zakhary, N. I., Greve, B., El-Shinawi, M., Götte, M., & Ibrahim, S. A. (2020). Inflammatory Breast Carcinoma: Elevated microRNA miR-181b-5p and Reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p Expression as Potential Biomarkers with Diagnostic Value. Biomolecules, 10(7), 1059. https://doi.org/10.3390/biom10071059







