The English (H6R) Mutation of the Alzheimer’s Disease Amyloid-β Peptide Modulates Its Zinc-Induced Aggregation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Peptide Solutions and Preparation of Zinc-Induced Aβ42 Aggregates
2.3. Circular Dichroism Spectroscopy
2.4. Turbidimetry and Dynamic Light Scattering
2.5. Sedimentation Assay
2.6. Fluorimetry
3. Results
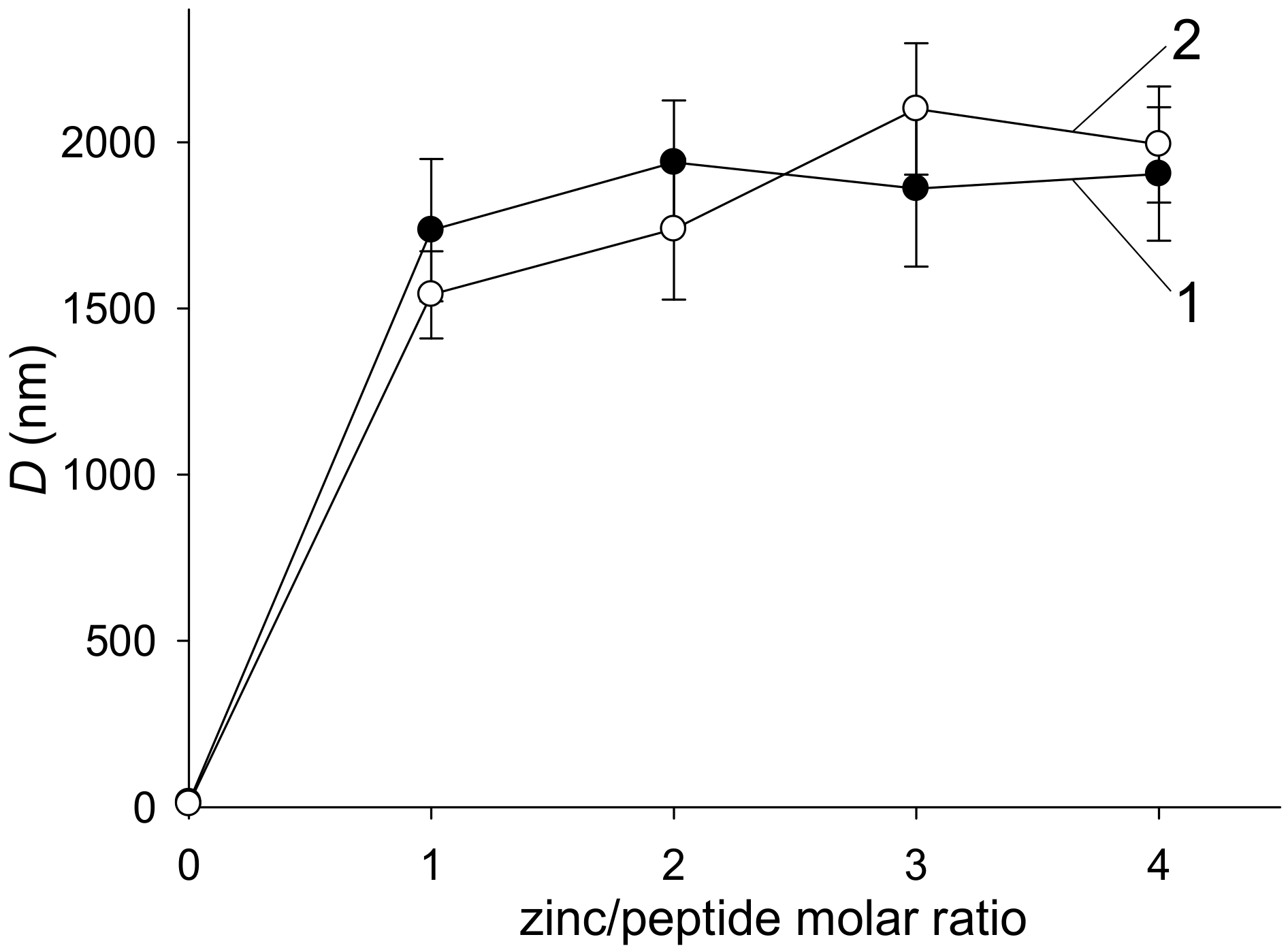
3.1. The Zn2+-Induced Aggregation of Aβ42 and H6R-Aβ42 Peptides Measured with Turbidity, DLS, and Sedimentation Methods
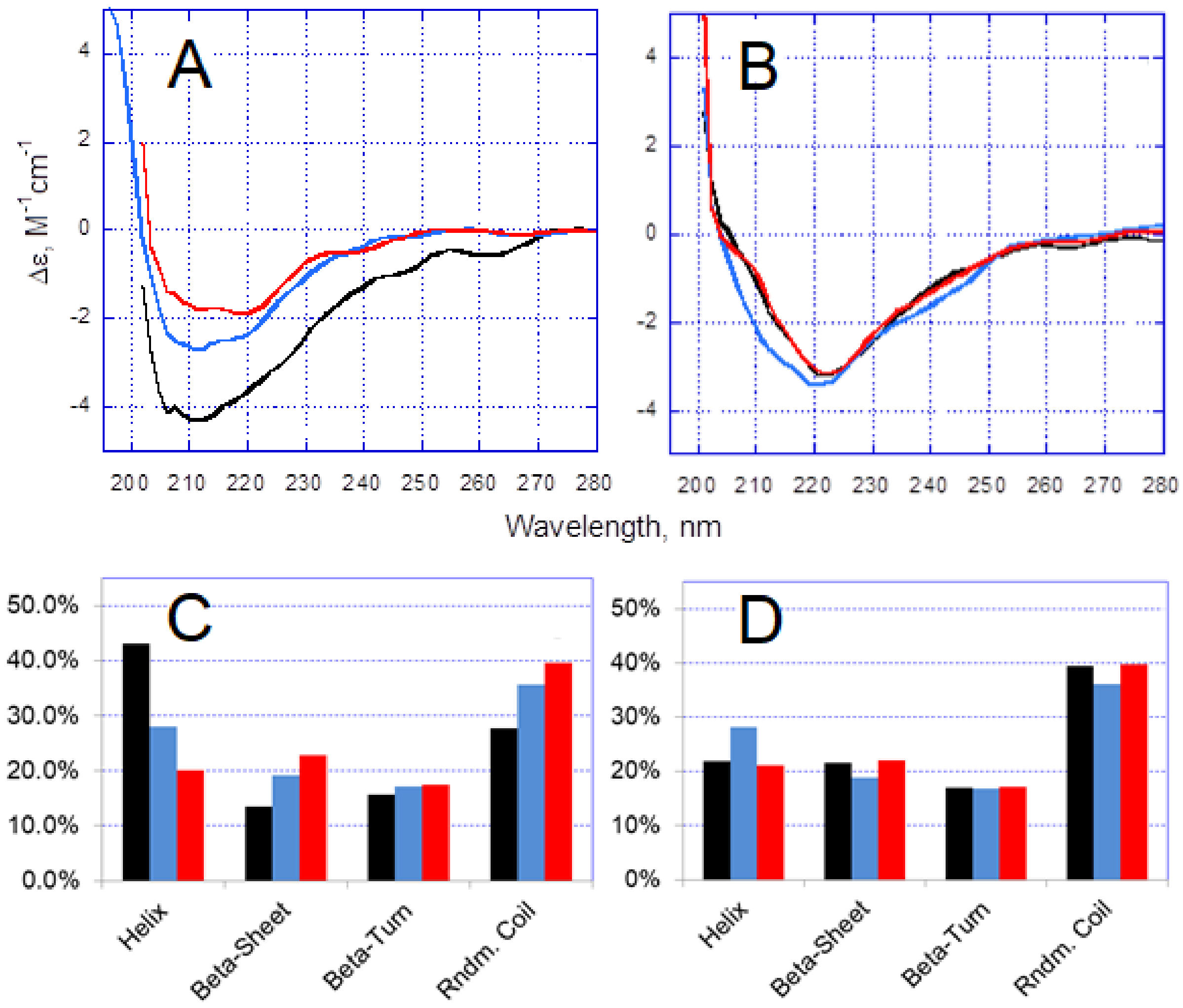
3.2. Effect of the H6R Mutation on Zn2+-Induced Conformational Changes in Aβ Peptides
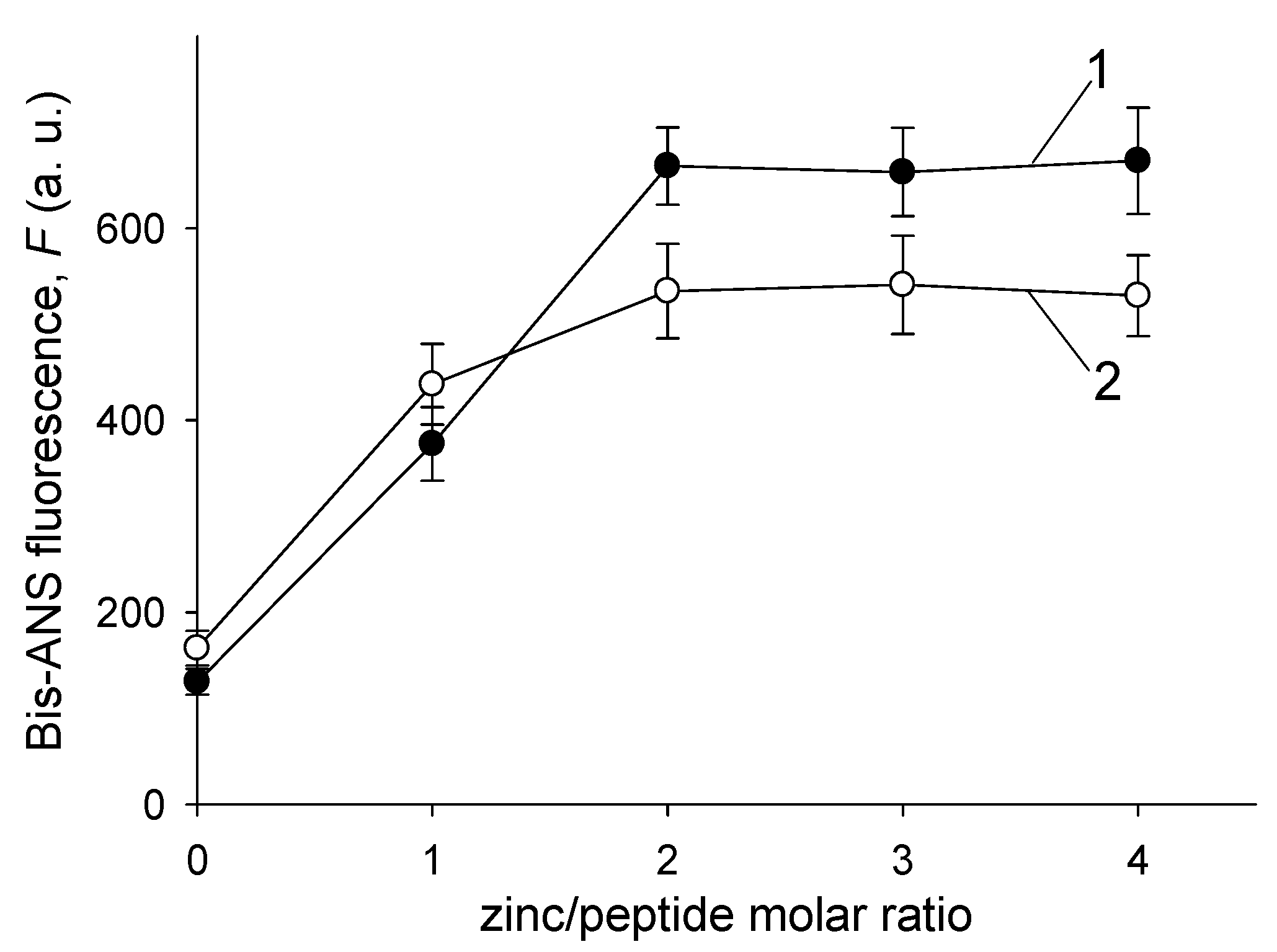
3.3. Zn2+-Induced Aggregation of Aβ Isoforms Tested with ThT and Bis-ANS Fluorescence Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penke, B.; Bogár, F.; Fulop, L. β-Amyloid and the Pathomechanisms of Alzheimer’s Disease: A Comprehensive View. Molecules 2017, 22, 1692. [Google Scholar] [CrossRef]
- Eisele, Y.S.; Duyckaerts, C. Propagation of Aß pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2015, 131, 5–25. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Monteleone, D.; Manassero, G.; Vasciaveo, V.; Tabaton, M. The Unexpected Role of Aβ1-42 Monomers in the Pathogenesis of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1241–1245. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance. Co-ord. Chem. Rev. 2018, 371, 38–55. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.-Y. Metal ions influx is a double edged sword for the pathogenesis of Alzheimer’s disease. Ageing Res. Rev. 2017, 35, 265–290. [Google Scholar] [CrossRef]
- Bush, A.I.; Pettingell, W.; Multhaup, G.; Paradis, M.D.; Vonsattel, J.; Gusella, J.; Beyreuther, K.; Masters, C.; Tanzi, R. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Miller, L.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, T.; Miklossy, J.; Miklossy, J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with β-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 2006, 155, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of Metal Ions in the Self-assembly of the Alzheimer’s Amyloid-β Peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Cole, T.B.; Palmiter, R.D.; Suh, S.W.; Koh, J.-Y. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7705–7710. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Bush, I.A. Metals and Alzheimer’s Disease: How Far Have We Come in the Clinic? J. Alzheimer’s Dis. 2018, 62, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, A.A.; Makarov, A.A.; Kozin, S.A. Roles of zinc ions and structural polymorphism of β-amyloid in the development of Alzheimer’s disease. Mol. Biol. 2015, 49, 217–230. [Google Scholar] [CrossRef]
- Liu, S.-T.; Howlett, G.; Barrow, C.J. Histidine-13 Is a Crucial Residue in the Zinc Ion-Induced Aggregation of the Aβ Peptide of Alzheimer’s Disease. Biochemistry 1999, 38, 9373–9378. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; McLaurin, J.; Qin, K.; Westaway, D.; Fraser, P.E. Examining the zinc binding site of the amyloid-β peptide. J. Biol. Inorg. Chem. 2000, 267, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Tõugu, V.; Karafin, A.; Zovo, K.; Chung, R.; Howells, C.; West, A.K.; Palumaa, P. Zn(II)- and Cu(II)-induced non-fibrillar aggregates of amyloid-β (1-42) peptide are transformed to amyloid fibrils, both spontaneously and under the influence of metal chelators. J. Neurochem. 2009, 110, 1784–1795. [Google Scholar] [CrossRef]
- Janssen, J.; Beck, J.; Campbell, T.; Dickinson, A.; Fox, N.; Harvey, R.; Houlden, H.; Rossor, M.; Collinge, J. Early onset familial Alzheimer’s disease: Mutation frequency in 31 families. Neurology 2003, 60, 235–239. [Google Scholar] [CrossRef]
- Khmeleva, S.; Radko, S.; Kozin, S.A.; Kiseleva, Y.Y.; Mezentsev, Y.V.; Mitkevich, V.A.; Kurbatov, L.K.; Ivanov, A.; Makarov, A.A. Zinc-Mediated Binding of Nucleic Acids to Amyloid-β Aggregates: Role of Histidine Residues. J. Alzheimer’s Dis. 2016, 54, 809–819. [Google Scholar] [CrossRef]
- Radko, S.; Khmeleva, S.A.; Kiseleva, Y.Y.; Kozin, S.A.; Mitkevich, V.A.; Makarov, A.A. Effects of the H6R and D7H Mutations on the Heparin-Dependent Modulation of Zinc-Induced Aggregation of Amyloid β. Mol. Biol. 2019, 53, 922–928. [Google Scholar] [CrossRef]
- Jan, A.; Hartley, D.M.; A Lashuel, H. Preparation and characterization of toxic Aβ aggregates for structural and functional studies in Alzheimer’s disease research. Nat. Protoc. 2010, 5, 1186–1209. [Google Scholar] [CrossRef]
- Böhm, G.; Muhr, R.; Jaenicke, R. Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng. Des. Sel. 1992, 5, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Suprun, E.V.; Khmeleva, S.; Kiseleva, Y.Y.; Radko, S.P.; Archakov, A.I.; Shumyantseva, V. Quantitative Aspects of Electrochemical Detection of Amyloid-β Aggregation. Electroanalysis 2016, 28, 1977–1983. [Google Scholar] [CrossRef]
- Bitan, G.; Kirkitadze, M.D.; Lomakin, A.; Vollers, S.S.; Benedek, G.B.; Teplow, D.B. Amyloid β-protein (Aβ) assembly: Aβ 40 and Aβ 42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA 2002, 100, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, M.; Bertucci, C.; Cavrini, V.; Andrisano, V. β-Amyloid aggregation induced by human acetylcholinesterase: Inhibition studies. Biochem. Pharmacol. 2003, 65, 407–416. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight Into the Kinetic of Amyloid β (1–42) Peptide Self-Aggregation: Elucidation of Inhibitors’ Mechanism of Action. ChemBioChem 2007, 8, 2152–2161. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Wang, X. Dual effects of familial Alzheimer’s disease mutations (D7H, D7N, and H6R) on amyloid β peptide: Correlation dynamics and zinc binding. Proteins: Struct. Funct. Bioinform. 2014, 82, 3286–3297. [Google Scholar] [CrossRef]
- Yang, M.; Teplow, D.B. Amyloid β-Protein Monomer Folding: Free-Energy Surfaces Reveal Alloform-Specific Differences. J. Mol. Biol. 2008, 384, 450–464. [Google Scholar] [CrossRef]
- Coskuner-Weber, O.; Uversky, V.N. Insights into the Molecular Mechanisms of Alzheimer’s and Parkinson’s Diseases with Molecular Simulations: Understanding the Roles of Artificial and Pathological Missense Mutations in Intrinsically Disordered Proteins Related to Pathology. Int. J. Mol. Sci. 2018, 19, 336. [Google Scholar] [CrossRef]
- Hori, Y.; Hashimoto, T.; Wakutani, Y.; Urakami, K.; Nakashima, K.; Condron, M.M.; Tsubuki, S.; Saido, T.C.; Teplow, D.B.; Iwatsubo, T. The Tottori (D7N) and English (H6R) Familial Alzheimer Disease Mutations Accelerate Aβ Fibril Formation without Increasing Protofibril Formation. J. Biol. Chem. 2006, 282, 4916–4923. [Google Scholar] [CrossRef]
- Ono, K.; Condron, M.M.; Teplow, D.B. Effects of the English (H6R) and Tottori (D7N) Familial Alzheimer Disease Mutations on Amyloid β-Protein Assembly and Toxicity. J. Biol. Chem. 2010, 285, 23186–23197. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. Effect of Curcumin on the metal ion induced fibrillization of Amyloid-β peptide. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014, 117, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pauly, T.; Nagel-Steger, L. Stoichiometric Zn2+ interferes with the self-association of Aβ42: Insights from size distribution analysis. Int. J. Biol. Macromol. 2018, 113, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Klajnert-Maculewicz, B.; Marcinkowska, M.; Duchnowicz, P.; Appelhans, D.; Grasso, G.; Deriu, M.; Danani, A.; Cangiotti, M.; Ottaviani, M.F. Multivalent interacting glycodendrimer to prevent amyloid-peptide fibril formation induced by Cu(II): A multidisciplinary approach. Nano Res. 2018, 11, 1204–1226. [Google Scholar] [CrossRef]
- Radko, S.P.; Khmeleva, S.; Suprun, E.V.; Kozin, S.A.; Bodoev, N.V.; Makarov, A.A.; Archakov, A.I.; Shumyantseva, V.V. Physico-chemical methods for studying amyloid-β aggregation. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2015, 9, 258–274. [Google Scholar] [CrossRef]
- Hawe, A.; Sutter, M.; Jiskoot, W. Extrinsic Fluorescent Dyes as Tools for Protein Characterization. Pharm. Res. 2008, 25, 1487–1499. [Google Scholar] [CrossRef]
- Chen, W.-T.; Liao, Y.-H.; Yu, H.-M.; Cheng, I.H.; Chen, Y.-R. Distinct Effects of Zn2+, Cu2+, Fe3+, and Al3+ on Amyloid-β Stability, Oligomerization, and Aggregation. J. Biol. Chem. 2011, 286, 9646–9656. [Google Scholar] [CrossRef]
- Chen, W.-T.; Hong, C.-J.; Lin, Y.-T.; Chang, W.-H.; Huang, H.-T.; Liao, J.-Y.; Chang, Y.-J.; Hsieh, Y.-F.; Cheng, C.-Y.; Liu, H.-C.; et al. Amyloid-Beta (Aβ) D7H Mutation Increases Oligomeric Aβ42 and Alters Properties of Aβ-Zinc/Copper Assemblies. PLOS ONE 2012, 7, e35807. [Google Scholar] [CrossRef]
- Radko, S.P.; Khmeleva, S.; Mantsyzov, A.B.; Kiseleva, Y.Y.; Mitkevich, V.A.; Kozin, S.A.; Makarov, A.A. Heparin Modulates the Kinetics of Zinc-Induced Aggregation of Amyloid-β Peptides. J. Alzheimer’s Dis. 2018, 63, 539–550. [Google Scholar] [CrossRef]
- Hatami, A.; Monjazeb, S.; Milton, S.; Glabe, C.G. Familial Alzheimer’s Disease Mutations within the Amyloid Precursor Protein Alter the Aggregation and Conformation of the Amyloid-β Peptide. J. Biol. Chem. 2017, 292, 3172–3185. [Google Scholar] [CrossRef]
- Grasso, G.; Leanza, L.; Morbiducci, U.; Danani, A.; Deriu, M.A. Aminoacid substitutions in the glycine zipper affect the conformational stability of amyloid beta fibrils. J. Biomol. Struct. Dyn. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Stefani, M.; Taddei, N.; Ramponi, G.; Dobson, C.M. Rationalization of the effects of mutations on peptide andprotein aggregation rates. Nature 2003, 424, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Kulikova, A.A.; Golovin, A.V.; Tkachev, Y.V.; Archakov, A.I.; Kozin, S.A.; Makarov, A.A. Minimal Zn2+ Binding Site of Amyloid-β. Biophys. J. 2010, 99, L84–L86. [Google Scholar] [CrossRef]
- Kozin, S.A.; Kulikova, A.A.; Istrate, A.; Tsvetkov, P.O.; Zhokhov, S.; Mezentsev, Y.V.; Kechko, O.I.; Ivanov, A.; Polshakov, V.; Makarov, A.A. The English (H6R) familial Alzheimer’s disease mutation facilitates zinc-induced dimerization of the amyloid-β metal-binding domain. Metallomics 2015, 7, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Istrate, A.; Kozin, S.A.; Zhokhov, S.S.; Mantsyzov, A.B.; Kechko, O.I.; Pastore, A.; Makarov, A.A.; Polshakov, V. Interplay of histidine residues of the Alzheimer’s disease Aβ peptide governs its Zn-induced oligomerization. Sci. Rep. 2016, 6, 21734. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.; Ma, B.; Nussinov, R. Metal binding sites in amyloid oligomers: Complexes and mechanisms. Co-ord. Chem. Rev. 2012, 256, 2245–2252. [Google Scholar] [CrossRef]
- Lim, K.H.; Kim, Y.K.; Chang, Y.-T. Investigations of the Molecular Mechanism of Metal-Induced Aβ (1−40) Amyloidogenesis†. Biochemistry 2007, 46, 13523–13532. [Google Scholar] [CrossRef]
- Olofsson, A.; Lindhagen-Persson, M.; Vestling, M.; Sauer-Eriksson, A.E.; Öhman, A. Quenched hydrogen/deuterium exchange NMR characterization of amyloid-β peptide aggregates formed in the presence of Cu2+ or Zn2+. FEBS J. 2009, 276, 4051–4060. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radko, S.P.; Khmeleva, S.A.; Kaluzhny, D.N.; Kechko, O.I.; Kiseleva, Y.Y.; Kozin, S.A.; Mitkevich, V.A.; Makarov, A.A. The English (H6R) Mutation of the Alzheimer’s Disease Amyloid-β Peptide Modulates Its Zinc-Induced Aggregation. Biomolecules 2020, 10, 961. https://doi.org/10.3390/biom10060961
Radko SP, Khmeleva SA, Kaluzhny DN, Kechko OI, Kiseleva YY, Kozin SA, Mitkevich VA, Makarov AA. The English (H6R) Mutation of the Alzheimer’s Disease Amyloid-β Peptide Modulates Its Zinc-Induced Aggregation. Biomolecules. 2020; 10(6):961. https://doi.org/10.3390/biom10060961
Chicago/Turabian StyleRadko, Sergey P., Svetlana A. Khmeleva, Dmitry N. Kaluzhny, Olga I. Kechko, Yana Y. Kiseleva, Sergey A. Kozin, Vladimir A. Mitkevich, and Alexander A. Makarov. 2020. "The English (H6R) Mutation of the Alzheimer’s Disease Amyloid-β Peptide Modulates Its Zinc-Induced Aggregation" Biomolecules 10, no. 6: 961. https://doi.org/10.3390/biom10060961
APA StyleRadko, S. P., Khmeleva, S. A., Kaluzhny, D. N., Kechko, O. I., Kiseleva, Y. Y., Kozin, S. A., Mitkevich, V. A., & Makarov, A. A. (2020). The English (H6R) Mutation of the Alzheimer’s Disease Amyloid-β Peptide Modulates Its Zinc-Induced Aggregation. Biomolecules, 10(6), 961. https://doi.org/10.3390/biom10060961





