Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L.
Abstract
1. Introduction
2. Material and Methods
2.1. Isolation of Cannabinoids
2.2. High Performance Liquid Chromatography
2.3. Preparation of Standard Solutions
2.4. Solubility Test
2.5. Bacterial Strains
2.6. Antibiotic Susceptibility Test
2.7. Synergy Test
2.8. Optical Density Measurements
2.9. In Vitro Hemolysis Essay on Human Red Blood Cells
2.10. Cell Proliferation Assay—MTS
2.11. Time Kill Essay
2.12. Statistical Analysis
3. Results
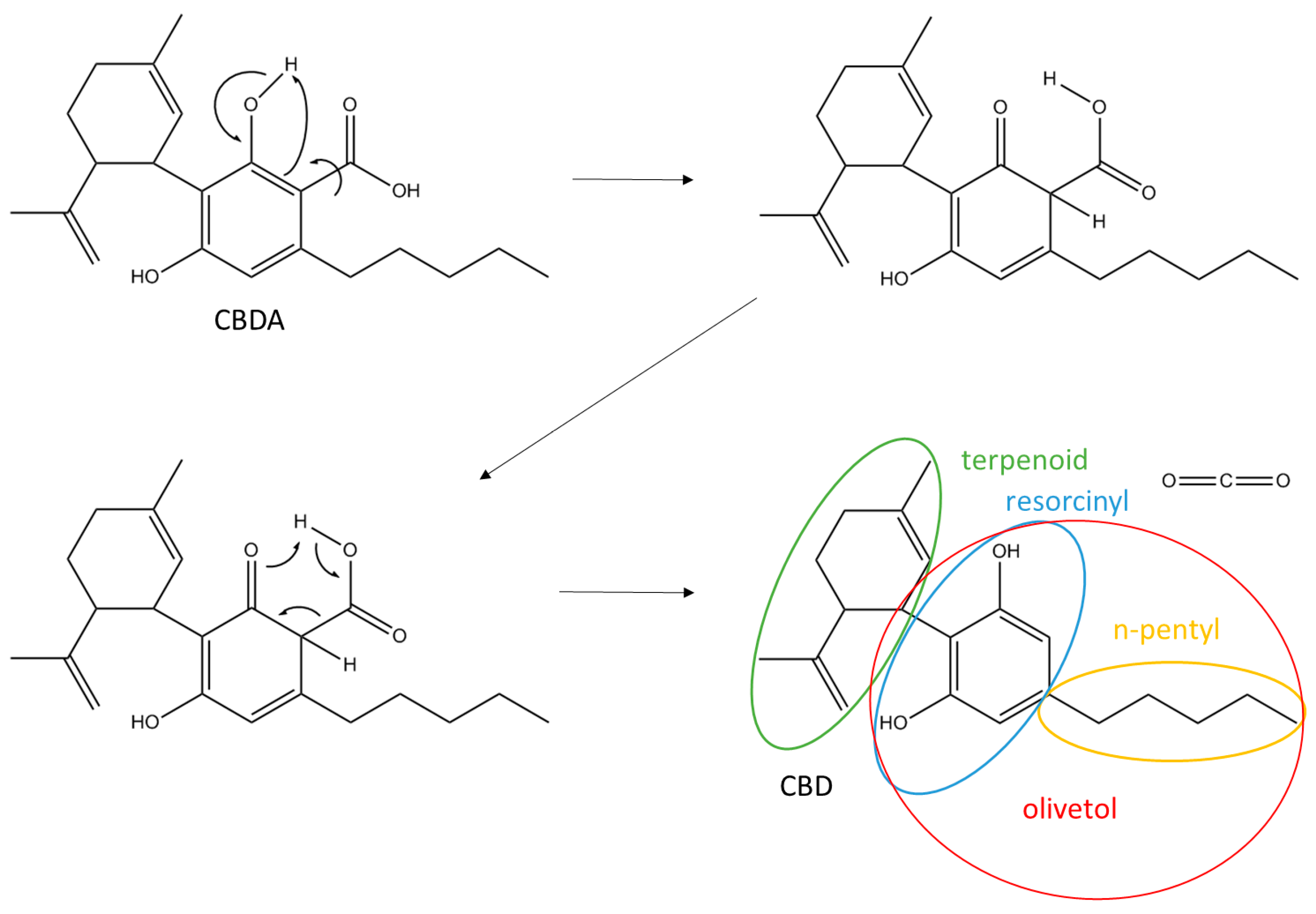
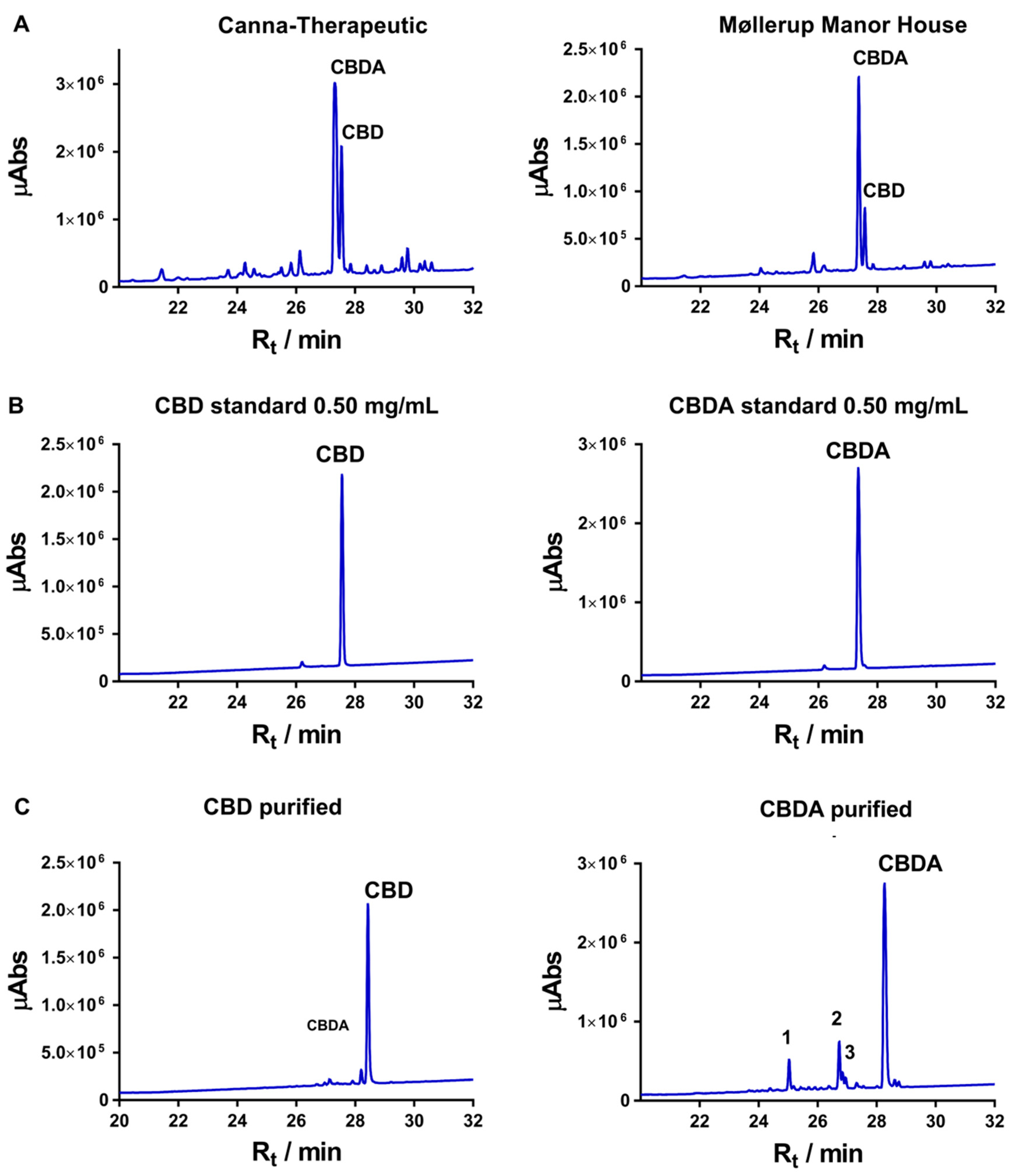
3.1. Isolation and Purification of Cannabidiolic Acid and Cannabidiol
3.2. Minimum Inhibitory Concentration
3.3. Synergy Test
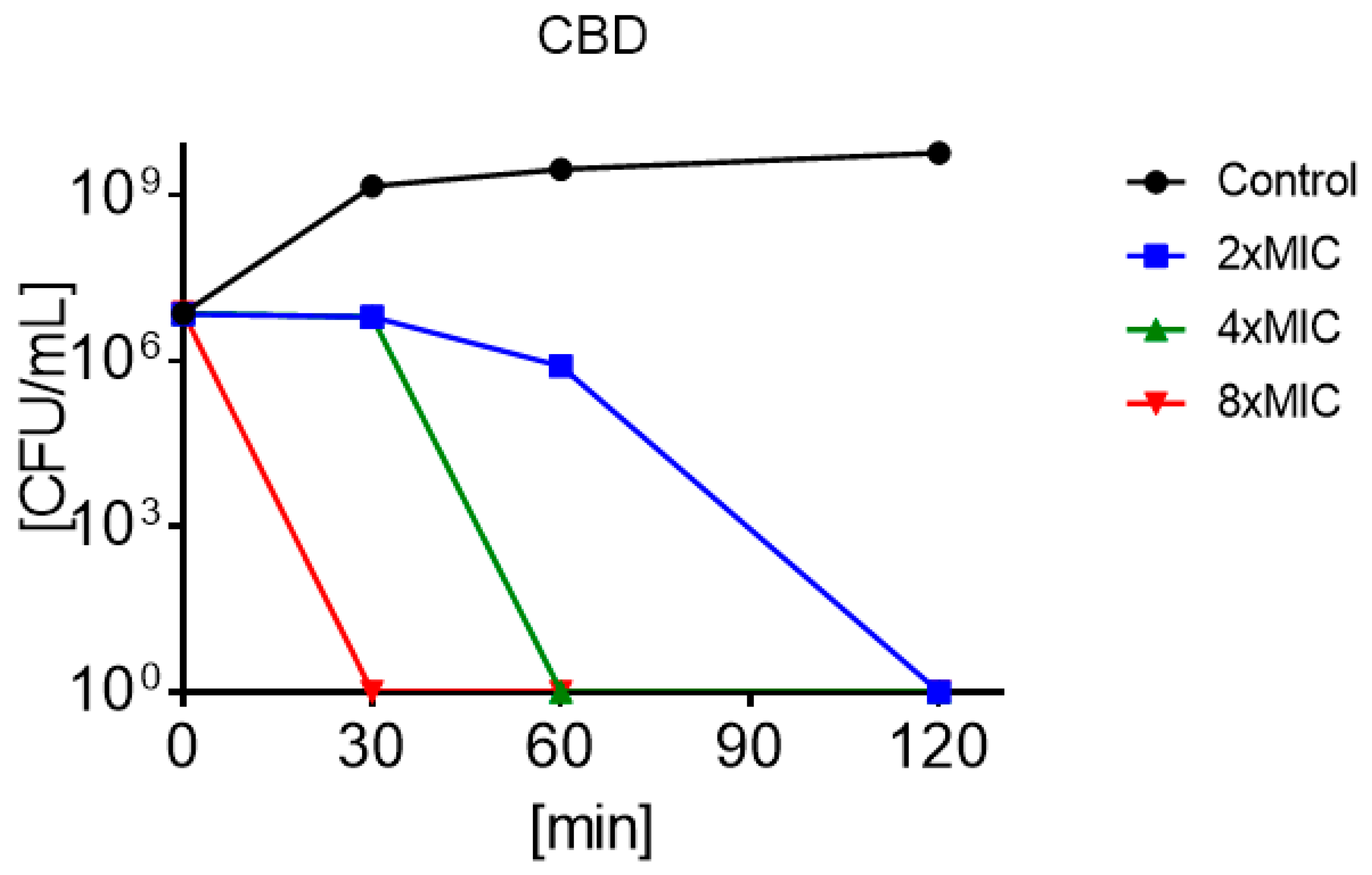
3.4. Minimum Bactericidal Concentration
3.5. Optical Density Measurements
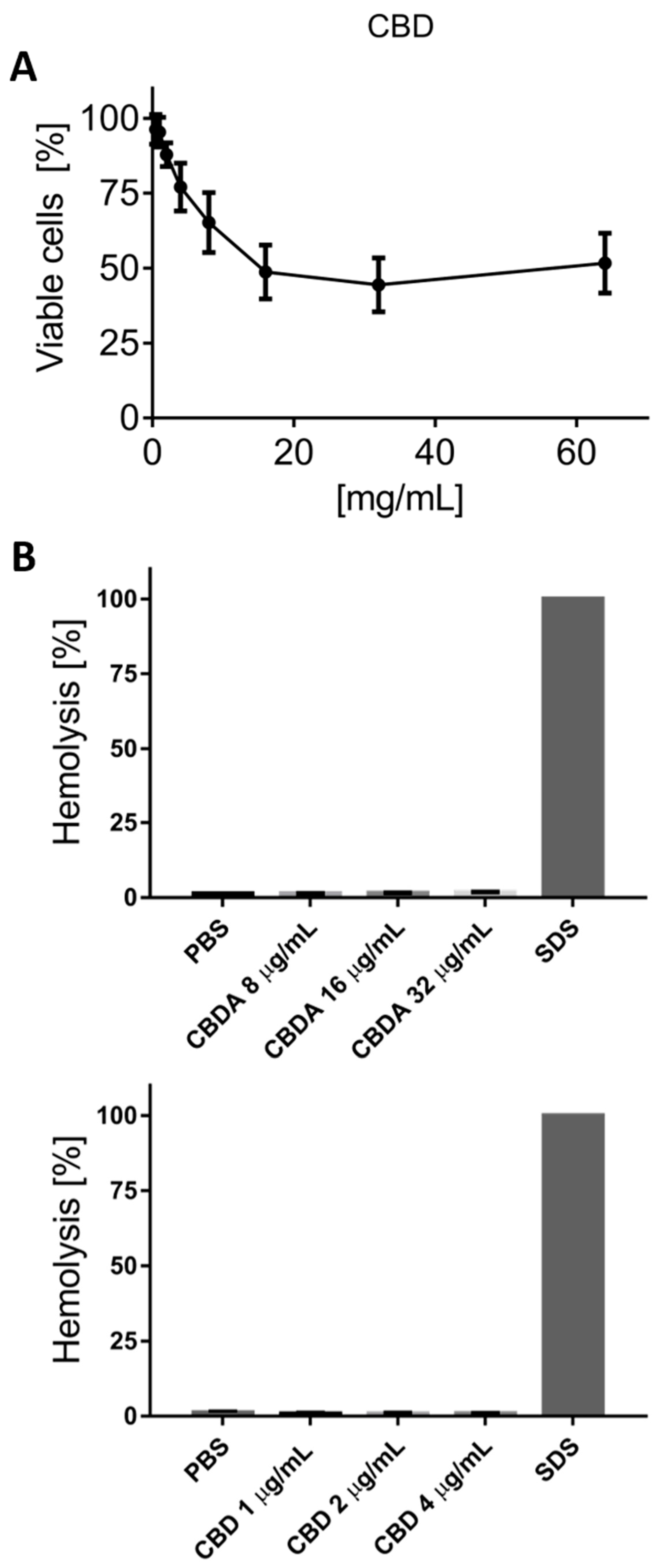
3.6. Cytotoxicity Test on HaCaT Cells
3.7. Hemolysis Test on Red Blood Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baron, E.P.; Lucas, P.; Eades, J.; Hogue, O. Patterns of Medicinal Cannabis Use, Strain Analysis, and Substitution Effect among Patients with Migraine, Headache, Arthritis, and Chronic Pain in a Medicinal Cannabis Cohort. J. Headache Pain 2018, 19, 37. [Google Scholar] [CrossRef]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The Pharmacologic and Clinical Effects of Medical Cannabis. Pharmacotherapy 2013, 33, 195–209. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.; McGlade, E.; Yurgelun-Todd, D. Safety and Toxicology of Cannabinoids. Neurotherapeutics 2015, 12, 735–746. [Google Scholar] [CrossRef]
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Battisti, U.M.; Braghiroli, D.; Ciccarella, G.; Schmid, M.; Vandelli, M.A.; Cannazza, G. A Metabolomic Approach Applied to a Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry Method (HPLC-ESI-HRMS/MS): Towards the Comprehensive Evaluation of the Chemical Composition of Cannabis Medicinal Extracts. Phytochem. Anal. 2018, 29, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene Synthases from Cannabis Sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef]
- Atakan, Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus Aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Bonomo, R.A.; Fowler, V.G. New Molecular Diagnostic Approaches to Bacterial Infections and Antibacterial Resistance. Annu. Rev. Med. 2018, 69, 379–394. [Google Scholar] [CrossRef]
- Barrett, C.T.; Barrett, J.F. Antibacterials: Are the New Entries Enough to Deal with the Emerging Resistance Problems? Curr. Opin. Biotechnol. 2003, 14, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Xu, E.; Mok, I.Y.S.; Song, A.; Karageorgopoulos, D.E.; Armaganidis, A.; Lipman, J.; Tsiodras, S. Novel Antibiotics for Multidrug-Resistant Gram-Positive Microorganisms. Microorganisms 2019, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Grare, M.; Demoré, B. Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules 2019, 24, 3152. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, N.; Autore, G.; Capasso, F.; Menghini, A.; Fasulo, M.P. Biological Screening of Italian Medicinal Plants for Anti-inflammatory Activity. Phyther. Res. 1987, 1, 28–31. [Google Scholar] [CrossRef]
- Wagner, H. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a Medicinal Plant: Antimicrobial Action of Berberine Potentiated by 5′-Methoxyhydnocarpin, a Multidrug Pump Inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef]
- Casciaro, B.; Calcaterra, A.; Cappiello, F.; Mori, M.; Loredo, M.R.; Ghirga, F.; Mangoni, M.L.; Botta, B.; Quaglio, D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins 2019, 11, 511. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, H.T.; Wu, Z.J.; Jhan, Y.L.; Shyu, C.L.; Chou, C.H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef]
- Siddique, H.; Pendry, B.; Rahman, M.M. Terpenes from Zingiber montanum and Their Screening against Multi-Drug Resistant and Methicillin Resistant Staphylococcus aureus. Molecules 2019, 24, 385. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis Sativa: A Structure-Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Almagboul, A. Antimicrobial Activity of Cannabis Sativa L. J. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef]
- Chakraborty, S.; Afaq, N.; Singh, N.; Majumdar, S. Antimicrobial Activity of Cannabis Sativa, Thuja Orientalis and Psidium Guajava Leaf Extracts against Methicillin-Resistant Staphylococcus Aureus. J. Integr. Med. 2018, 16, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Azam, S.; Rehman, P.; Khadim, J. Evaluation of Antimicrobial Activity and Ethnobotanical Study of Cannabis Sativa. Pure Appl. Biol. 2018, 7, 706–713. [Google Scholar] [CrossRef]
- Ardon, C.B.; Prens, E.P.; Fuursted, K.; Ejaz, R.N.; Shailes, J.; Jenssen, H.; Jemec, G.B.E. Biofilm Production and Antibiotic Susceptibility of Staphylococcus Epidermidis Strains from Hidradenitis Suppurativa Lesions. J. Eur. Acad. Dermatol. Venereol. 2018, 33, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mojsoska, B.; Carretero, G.; Larsen, S.; Mateiu, R.V.; Jenssen, H. Peptoids Successfully Inhibit the Growth of Gram Negative E. coli Causing Substantial Membrane Damage. Sci. Rep. 2017, 7, 42332. [Google Scholar] [CrossRef]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy Tests by E Test and Checkerboard Methods of Antimicrobial Combinations against Brucella Melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef]
- Fehri, L.F.; Wróblewski, H.; Blanchard, A. Activities of Antimicrobial Peptides and Synergy with Enrofloxacin against Mycoplasma Pulmonis. Antimicrob. Agents Chemother. 2007, 51, 468–474. [Google Scholar] [CrossRef]
- Mojsoska, B.; Zuckermann, R.N.; Jenssen, H. Structure-Activity Relationship Study of Novel Peptoids That Mimic the Structure of Antimicrobial Peptides. Antimicrob. Agents Chemother. 2015, 59, 4112–4120. [Google Scholar] [CrossRef]
- Godballe, T.; Mojsoska, B.; Nielsen, H.M.; Jenssen, H. Antimicrobial Activity of GN Peptides and Their Mode of Action. Biopolymers 2016, 106, 172–183. [Google Scholar] [CrossRef]
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative Development and Validation of an HPLC/DAD Method for the Qualitative and Quantitative Determination of Major Cannabinoids in Cannabis Plant Material. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Buchanan, B.; Zuccolo, J.; Poulin, M.M.; Gabriele, J.; Baranowski, D.C. A Reliable and Validated LC-MS/MS Method for the Simultaneous Quantification of 4 Cannabinoids in 40 Consumer Products. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Santamaría, A.; Patarroyo, M.E.; Curtidor, H. Designing and Optimizing New Antimicrobial Peptides: All Targets Are Not the Same. Crit. Rev. Clin. Lab Sci. 2019, 56, 351–373. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a New Extraction Technique and HPLC Method for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis Sativa L. (Hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Tipparat, P.; Natakankitkul, S.; Chamnivikaipong, P.; Chutiwat, S. Characteristics of Cannabinoids Composition of Cannabis Plants Grown in Northern Thailand and Its Forensic Application. Forensic Sci. Int. 2012, 215, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Sotto, A.D.; Giacomo, S.D.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.U.; Cianfaglione, K.; Maggi, F.; Sut, S.; Dall’Acqua, S. Chemical Characterization of Leaves, Male and Female Flowers from Spontaneous Cannabis (Cannabis sativa L.) Growing in Hungary. Chem. Biodiversity 2019, 16, e1800562. [Google Scholar] [CrossRef]
- Delgado-Povedanoa, M.M.; Callado, C.S.-M.; Priego-Capote, F.; Ferreiro-Vera, C. Untargeted characterization of extracts from Cannabis sativa L. cultivars by gas and liquid chromatography coupled to mass spectrometry in high resolution mode. Talanta 2020, 208, 120384. [Google Scholar] [CrossRef]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1394. [Google Scholar] [CrossRef]
- Arabski, M.; Wȩgierek-Ciuk, A.; Czerwonka, G.; Lankoff, A.; Kaca, W. Effects of Saponins against Clinical E. Coli Strains and Eukaryotic Cell Line. J. Biomed. Biotechnol. 2012, 2012, 286216. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Nonejuie, P.; Munguia, J.; Hollands, A.; Olson, J.; Dam, Q.; Kumaraswamy, M.; Rivera, H.; Corriden, R.; Rohde, M.; et al. Azithromycin Synergizes with Cationic Antimicrobial Peptides to Exert Bactericidal and Therapeutic Activity Against Highly Multidrug-Resistant Gram-Negative Bacterial Pathogens. EBioMedicine 2015, 2, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Nuding, S.; Frasch, T.; Schaller, M.; Stange, E.F.; Zabel, L.T. Synergistic Effects of Antimicrobial Peptides and Antibiotics against Clostridium Difficile. Antimicrob. Agents Chemother. 2014, 58, 5719–5725. [Google Scholar] [CrossRef] [PubMed]
- Guclu-Ustundag, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Morais, S.L.; Guimaraes, F.S.; Mechoulam, R. Antipsychotic Effect of Cannabidiol. J. Clin. Psychiatry 1995, 56, 485–486. [Google Scholar] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Zuardi, A.W.; Crippa, J.A.S. Safety and Side Effects of Cannabidiol, a Cannabis Sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, H.J.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug Resistant Seizure in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K. Randomized, Dose-Ranging Safety Trial of Cannabidiol in Dravet Syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G.A. Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef]
| Minimal Inhibitory Concentration [MIC µg/mL] | ||||||
|---|---|---|---|---|---|---|
| Staphylococcus Aureus | Staphylococcus Epidermidis | Escherichia Coli | Pseudomonas Aeruginosa | |||
| (ATCC 25923) | MRSA (USA300) | (CA#71) | (ATCC 51625) | (ATCC 25922) | (PA01) | |
| CBDA | 2 | 4 | 4 | 4 | >64 | >64 |
| CBD | 1 | 1 | 2 | 2 | >64 | >64 |
| Clindamycin | >128 | 128 | >128 | 0.06 | 8 | >128 |
| Tobramycin | 0.25 | 1 | 0.06 | 1 | 2 | 0.12 |
| Meropenem | 0.06 | 16 | 0.12 | 2 | 0.06 | 0.5 |
| Ofloxacin | 0.5 | 64 | 0.25 | 1 | 0.06 | 1 |
| Drug Combination | FICindex |
|---|---|
| CBD + Clindamycin | 1.03 |
| CBD + Vancomycin | 1.13 |
| CBD + Tobramycin | 1.50 |
| CBD + Teicoplanin | 1.13 |
| CBD + Ofloxacin | 1.50 |
| CBD + Methicillin | 1.25 |
| CBD + Meropenem | 1.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. https://doi.org/10.3390/biom10060900
Martinenghi LD, Jønsson R, Lund T, Jenssen H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules. 2020; 10(6):900. https://doi.org/10.3390/biom10060900
Chicago/Turabian StyleMartinenghi, Laura Daniela, Rie Jønsson, Torben Lund, and Håvard Jenssen. 2020. "Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L." Biomolecules 10, no. 6: 900. https://doi.org/10.3390/biom10060900
APA StyleMartinenghi, L. D., Jønsson, R., Lund, T., & Jenssen, H. (2020). Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules, 10(6), 900. https://doi.org/10.3390/biom10060900