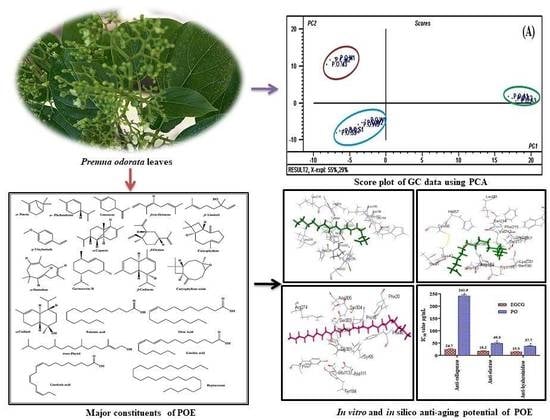
Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Essential Oil Samples
2.3. GC/FID and GC/MS Analyses
2.4. Chemometric Analysis
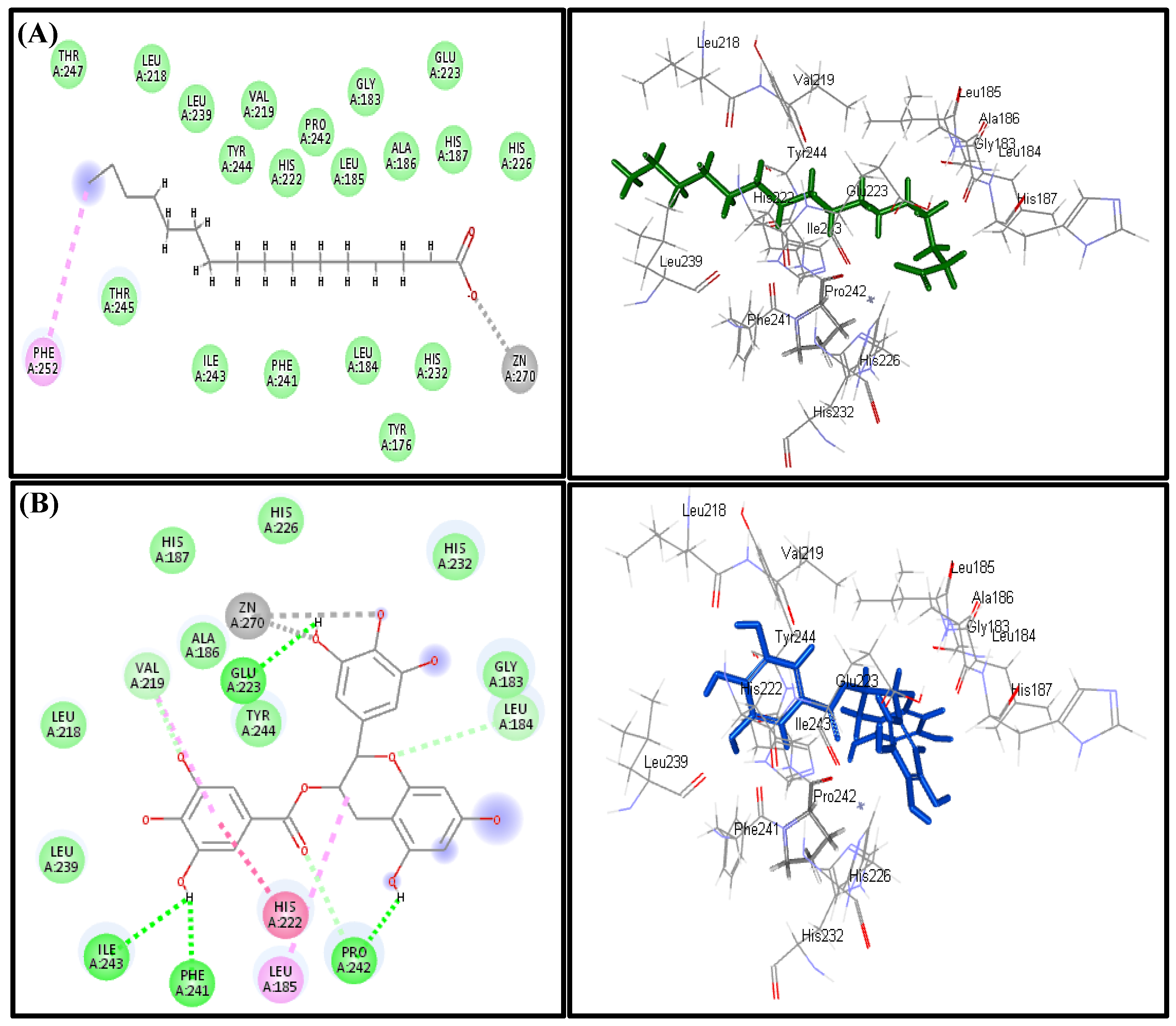
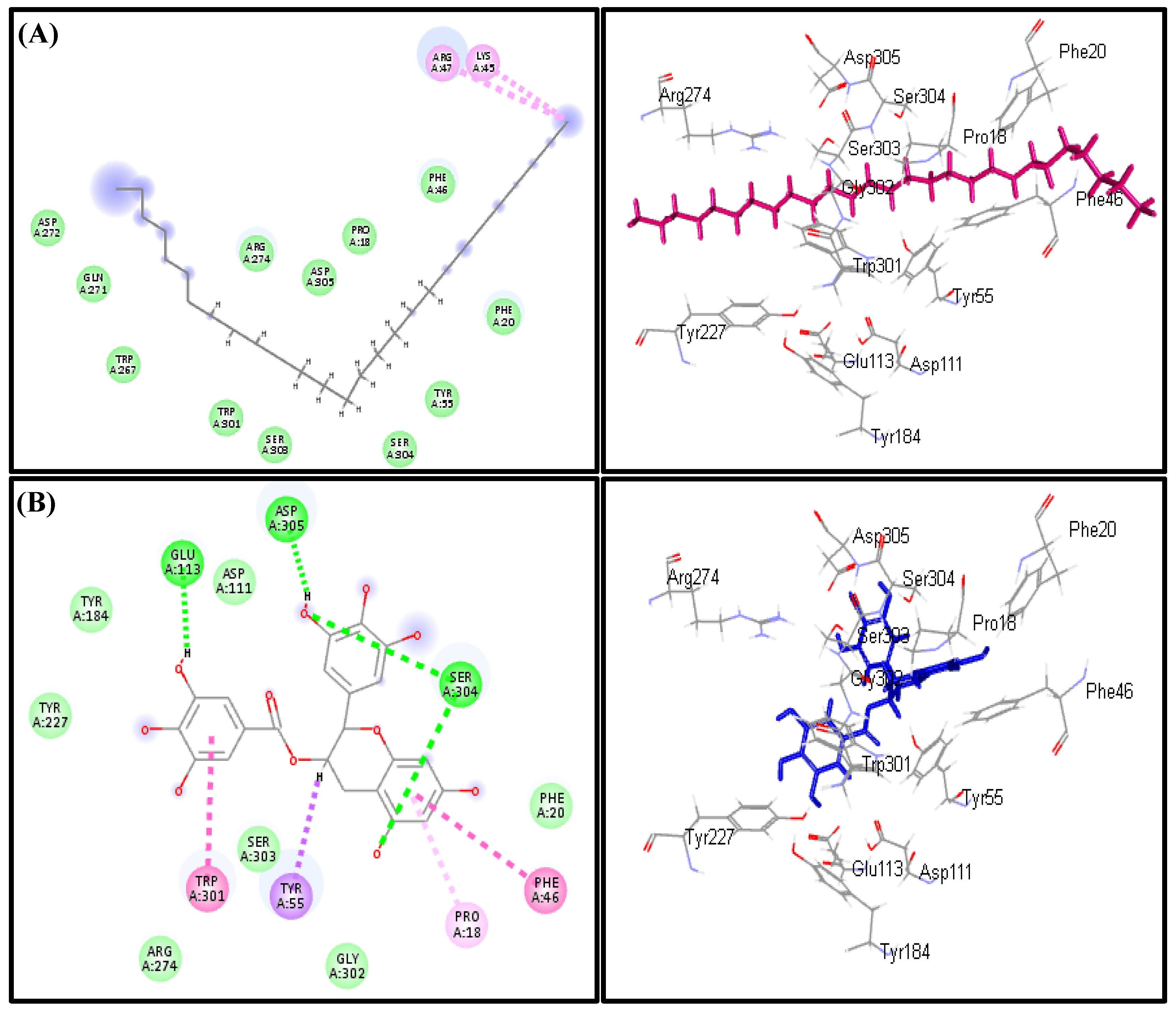
2.5. In Silico Virtual Studies
2.6. Assessment of the Anti-Ageing Potential of Premna Odorata Leaves Essential Oil
2.6.1. Anti-Collagenase Evaluation
2.6.2. Anti-Elastase Evaluation
2.6.3. Anti-Hyaluronidase Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. GC/FID and GC/MS Analyses
3.2. Chemometric Analysis
3.3. In Silico Virtual Studies
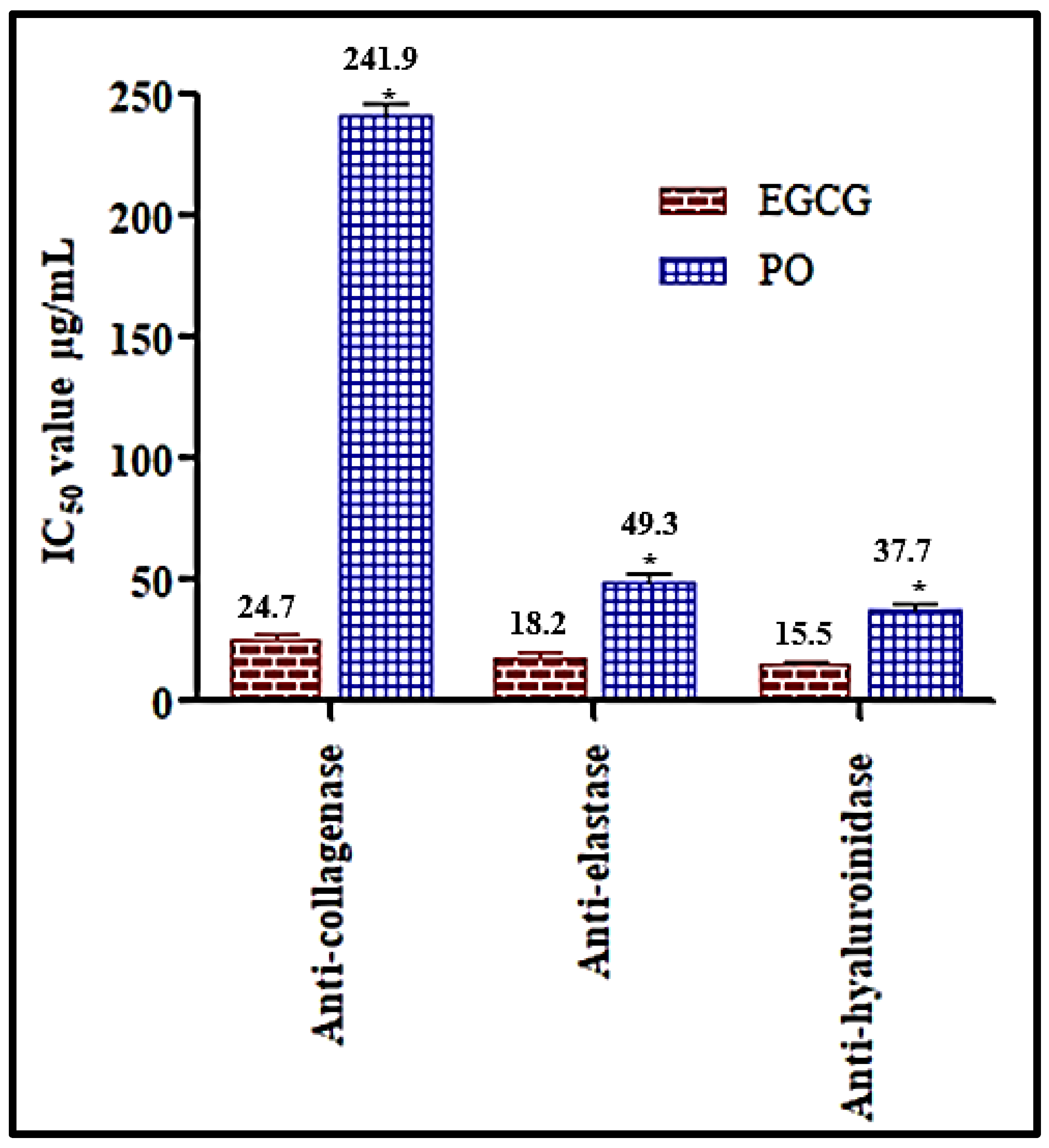
3.4. In Vitro Assessment of the Anti-Ageing Potential of Premna odorata Leaves Essential Oil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.; Richa, P.; Babu, S.; Rao, M. A phytochemistry of the genus Premna: A review. Int. J. Pharm. Chem. Sci. 2015, 4, 317–325. [Google Scholar]
- Pinzon, L.C.; Uy, M.M.; Sze, K.H.; Wang, M.; Chu, I.K. Isolation and characterization of antimicrobial, anti-inflammatory and chemopreventive flavones from Premna odorata Blanco. J. Med. Plant Res. 2011, 5, 2729–2735. [Google Scholar]
- Youssef, F.S.; Hamoud, R.; Ashour, M.L.; Singab, A.N.; Wink, M. Volatile oils from the aerial parts of Eremophila maculata and their antimicrobial activity. Chem. Biodivers. 2014, 11, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, M.I.; Youssef, F.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Comparative analysis of volatile constituents of Pachira aquatica Aubl. and Pachira glabra Pasq., their anti-Mycobacterial and anti-Helicobacter pylori activities and their metabolic discrimination using chemometrics. J. Essent. Oil Bear. Plants 2018, 21, 1550–1567. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Youssef, F.S.; El-Shazly, M.; Ashour, M.L.; Singab, A.N.B.; Wink, M. Volatile constituents of Dietes bicolor (Iridaceae) and their antimicrobial activity. Z. Nat. C 2015, 70, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Sarker, S.D.; Nahar, L.; Al-Azizi, M.M.; Ashour, M.L. Authentication and discrimination of green tea samples using UV-vis, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2019, 164, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Thabet, A.A.; Youssef, F.S.; El-Shazly, M.; Singab, A.N.B. GC-MS and GC-FID analyses of the volatile constituents of Brachychiton rupestris and Brachychiton discolor, their biological activities and their differentiation using multivariate data analysis. Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; El-Readi, M.Z.; Bouzabata, A.; Abuzeid, R.M.; Sobeh, M.; Wink, M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018, 70, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Janibekov, A.A.; Youssef, F.S.; Ashour, M.L.; Mamadalieva, N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crops Prod. 2018, 118, 142–148. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)−catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, W.D.; Abeysekera, W.P.K.M.; Ratnasooriya, C.T.D. In vitro anti-hyaluronidase activity of Sri Lankan low grown orthodox orange pekoe grade black tea (Camellia sinensis L.). Asian Pac. J. Trop. Biomed. 2014, 4, 959–963. [Google Scholar] [CrossRef]
- Shoko, T.; Maharaj, V.J.; Naidoo, D.; Tselanyane, M.; Nthambeleni, R.; Khorombi, E.; Apostolides, Z. Anti-ageing potential of extracts from Sclerocarya birrea (A. Rich.) Hochst and its chemical profiling by UPLC-Q-TOF-MS. BMC Complement. Altern. Med. 2018, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-ageing potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Putri, T.; Raya, I.; Natsir, H.; Mayasari, E. Chlorella sp: Extraction of fatty acid by using avocado oil as solvent and its application as an anti-ageing cream. J. Phys. 2018, 979, 012009. [Google Scholar] [CrossRef]
- Pant, A.; Pandey, R. Ocimum Species: A Longevity Elixir. In The Ocimum Genome; Shasany, A.K., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 9–24. [Google Scholar]
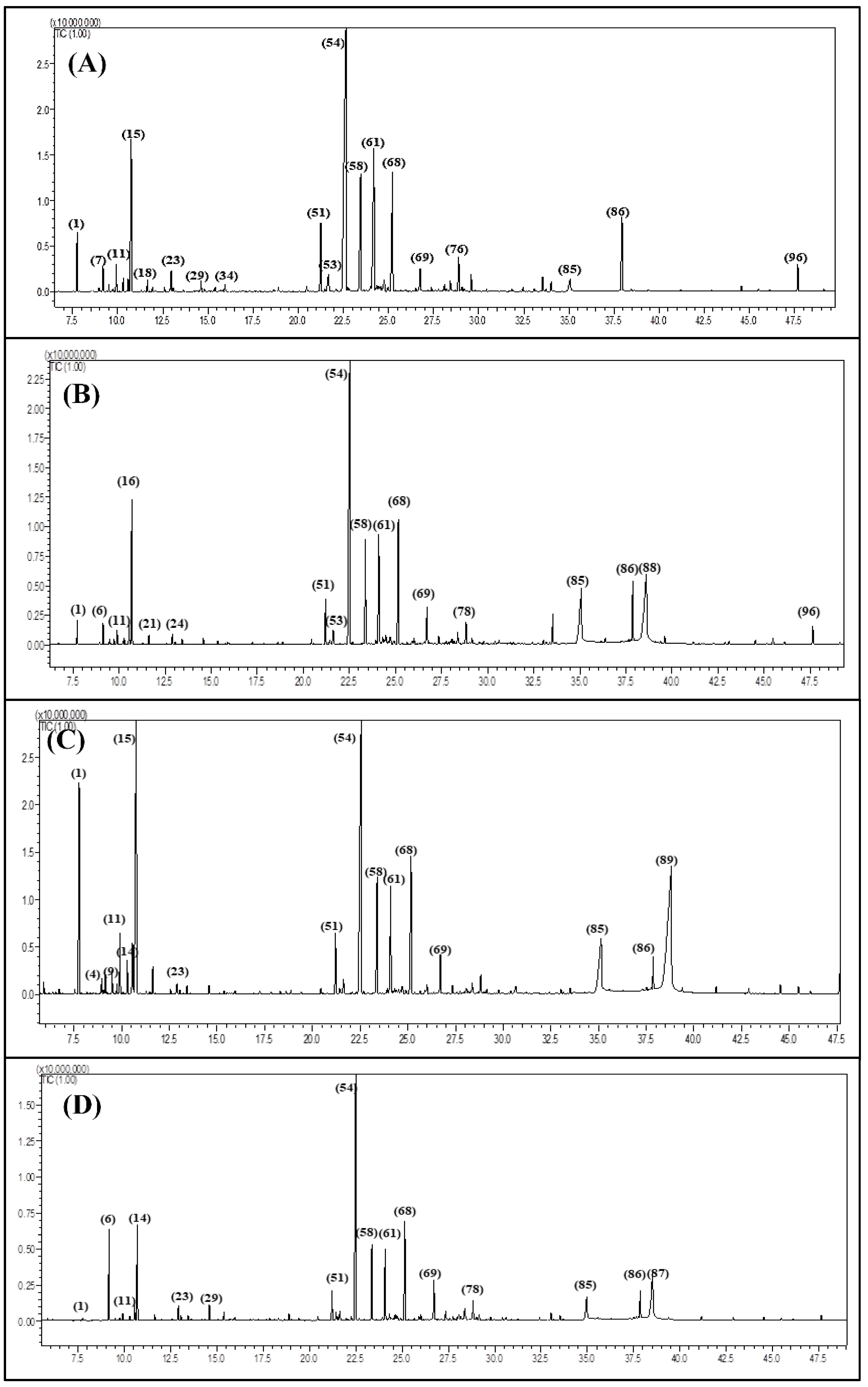
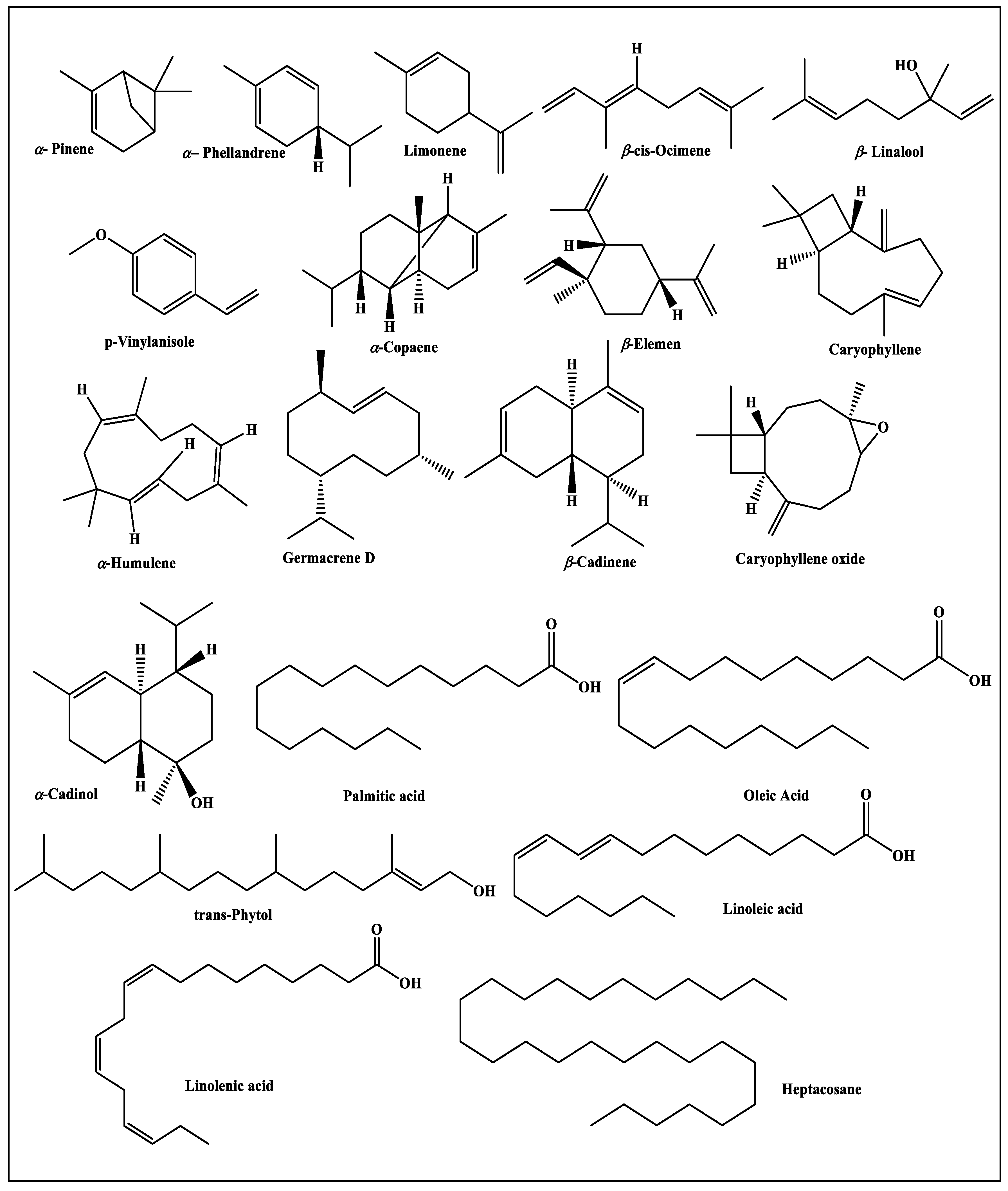

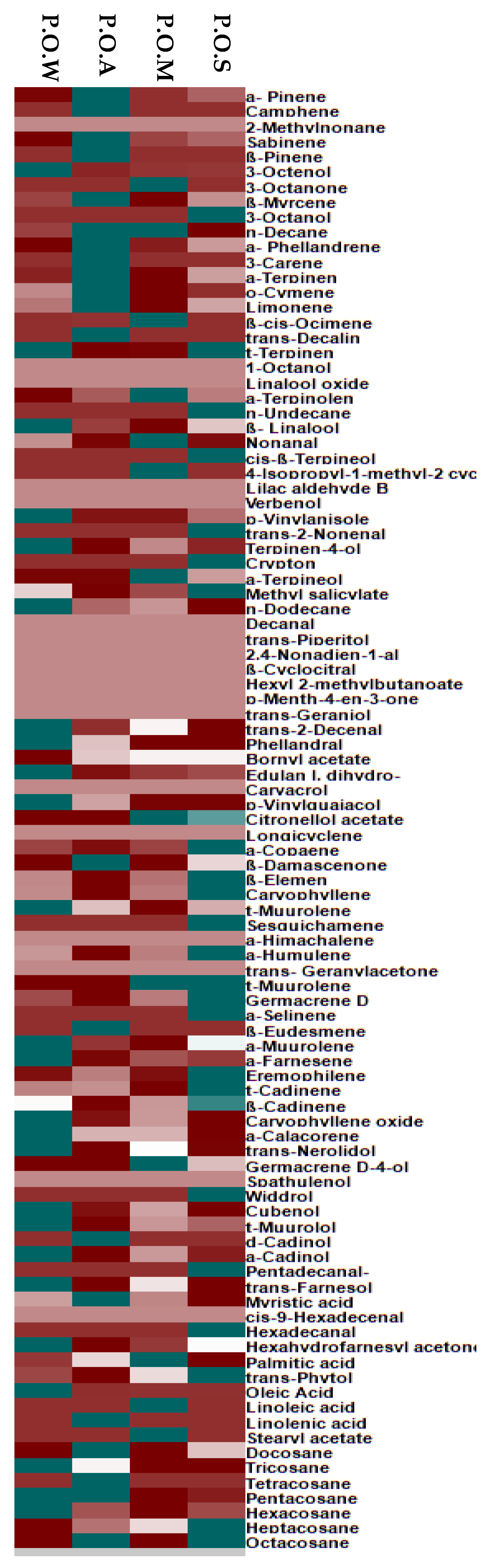

| Compound | RI | Content [%] | Identification Method | |||||
|---|---|---|---|---|---|---|---|---|
| Cal. | Rep. | POS | POM | POA | POW | |||
| 1. | α-Pinene | 927 | 927 | 1.95 | 0.89 | 6.85 | 0.15 | MS, RI, AU |
| 2. | Camphene | 944 | 946 | - | - | 0.04 | - | MS, RI, AU |
| 3. | 2-Methylnonane | 960 | 962 | - | - | tr | - | MS, RI |
| 4. | Sabinene | 971 | 971 | 0.13 | 0.07 | 0.36 | - | MS, RI |
| 5. | β-Pinene | 975 | 975 | tr | - | 0.08 | - | MS, RI, AU |
| 6. | 3-Octenol | 979 | 979 | 0.87 | 0.81 | 0.58 | 6.52 | MS, RI |
| 7. | 3-Octanone | 987 | 987 | tr | 0.2 | - | - | MS, RI |
| 8. | β-Myrcene | 992 | 992 | 0.24 | - | 0.55 | 0.14 | MS, RI, AU |
| 9. | 3-Octanol | 997 | 997 | 0.05 | - | - | tr | MS, RI |
| 10. | n-Decane | 1000 | 1000 | 0.08 | 0.23 | 0.23 | 0.14 | MS, RI |
| 11. | α-Phellandrene | 1005 | 1005 | 0.95 | 0.61 | 1.57 | 0.49 | MS, RI, AU |
| 12. | 3-Carene | 1011 | 1011 | tr | - | 0.05 | - | MS, RI |
| 13. | α-Terpinene | 1018 | 1018 | 0.47 | 0.15 | 0.86 | 0.25 | MS, RI, AU |
| 14. | o-Cymene | 1027 | 1028 | 0.57 | 0.26 | 2.16 | 0.99 | MS, RI |
| 15. | Limonene | 1032 | 1032 | 8.34 | 0.82 | 14 | 6.95 | MS, RI, AU |
| 16. | β-cis-Ocimene | 1050 | 1051 | tr | 7.4 | 0.07 | tr | MS, RI |
| 17. | trans-Decaline | 1056 | 1056 | tr | - | 0.06 | tr | MS, RI |
| 18. | τ-Terpinene | 1061 | 1061 | 0.39 | 0.05 | - | 0.38 | MS, RI |
| 19. | 1-Octanol | 1074 | 1074 | tr | - | - | tr | MS, RI |
| 20. | Linalool oxide | 1075 | 1075 | tr | - | - | tr | MS, RI |
| 21. | α-Terpinolene | 1091 | 1088 | 0.16 | 0.4 | 0.13 | - | MS, RI |
| 22. | n-Undecane | 1101 | 1100 | 0.09 | - | - | - | MS, RI |
| 23. | β-Linalool | 1103 | 1103 | 0.79 | 0.08 | 0.42 | 1.21 | MS, RI, AU |
| 24. | Nonanal | 1107 | 1107 | 0.12 | 0.61 | 0.11 | 0.29 | MS, RI |
| 25. | cis-β-Terpineol | 1125 | 1125 | 0.06 | - | - | - | MS, RI |
| 26. | 4-Isopropyl-1-methyl-2 cyclohexen-1-ol | 1143 | 1142 | tr | 0.11 | tr | tr | MS, RI |
| 27. | Lilac aldehyde B | 1146 | 1148 | tr | - | - | - | MS, RI |
| 28. | Verbenol | 1149 | 1148 | tr | - | - | - | MS, RI |
| 29. | p-Vinylanisole | 1156 | 1159 | 0.43 | 0.24 | 0.24 | 1.04 | MS, RI |
| 30. | trans-2-Nonenal | 1162 | 1162 | 0.08 | - | - | - | MS, RI |
| 31. | Terpinen-4-ol | 1182 | 1182 | 0.16 | 0.28 | 0.1 | 0.58 | MS, RI |
| 32. | Cryptone | 1191 | 1188 | 0.04 | - | - | tr | MS, RI |
| 33. | α-Terpineol | 1195 | 1195 | 0.06 | 0.15 | tr | tr | MS, RI |
| 34. | Methyl salicylate | 1199 | 1198 | 0.28 | 0.1 | tr | 0.19 | MS, RI |
| 35. | n-Dodecane | 1200 | 1200 | - | 0.09 | 0.07 | 0.18 | MS, RI |
| 36. | Decanal | 1207 | 1207 | - | - | tr | - | MS, RI |
| 37. | trans-Piperitol | 1212 | 1211 | tr | - | - | tr | MS, RI |
| 38. | 2,4-Nonadien-1-al | 1218 | 1218 | tr | - | - | - | MS, RI |
| 39. | β-Cyclocitral | 1227 | 1223 | tr | - | tr | - | MS, RI |
| 40. | Hexyl 2-methylbutanoate | 1240 | 1243 | tr | - | - | - | MS, RI |
| 41. | p-Menth-4-en-3-one | 1255 | 1251 | tr | - | - | - | MS, RI |
| 42. | trans-Geraniol | 1258 | 1258 | - | - | tr | tr | MS, RI |
| 43. | trans-2-Decenal | 1266 | 1266 | tr | 0.11 | 0.04 | 0.14 | MS, RI |
| 44. | Phellandral | 1282 | 1276 | - | - | 0.08 | 0.15 | MS, RI |
| 45. | Bornyl acetate | 1293 | 1293 | 0.09 | 0.09 | 0.08 | tr | MS, RI |
| 46. | Edulan I, dihydro- | 1302 | 1292 | 0.16 | 0.14 | 0.1 | 0.49 | MS, RI |
| 47. | Carvacrol | 1307 | 1307 | - | tr | tr | tr | MS, RI |
| 48. | p-Vinylguaiacol | 1320 | 1320 | - | - | 0.08 | 0.19 | MS, RI |
| 49. | Citronellol acetate | 1357 | 1357 | 0.22 | 0.26 | - | - | MS, RI |
| 50. | Longicyclene | 1361 | 1371 | tr | - | - | - | MS, RI |
| 51. | α-Copaene | 1384 | 1383 | 3.04 | 2.21 | 2.08 | 2.21 | MS, RI |
| 52. | β-Damascenone | 1391 | 1391 | 0.14 | - | 0.23 | - | MS, RI |
| 53. | β-Elemene | 1399 | 1398 | 1.26 | 0.91 | 0.66 | 0.94 | MS, RI |
| 54. | Caryophyllene | 1415 | 1415 | 35.37 | 26.39 | 18.84 | 26.97 | MS, RI, AU |
| 55. | τ-Muurolene | 1433 | 1433 | 0.16 | - | 0.17 | 0.23 | MS, RI |
| 56. | Sesquichamene | 1441 | 1443 | 0.09 | - | - | - | MS, RI |
| 57. | α-Himachalene | 1444 | 1438 | tr | - | - | - | MS, RI |
| 58. | α-Humulene | 1453 | 1451 | 7.29 | 5.57 | 4.01 | 5.75 | MS, RI |
| 59. | trans-Geranylacetone | 1458 | 1458 | - | tr | - | - | MS, RI |
| 60. | τ-Muurolene | 1468 | 1468 | 0.24 | 0.24 | - | - | MS, RI |
| 61. | Germacrene D | 1490 | 1491 | 10.21 | 6.35 | 4.02 | 5.65 | MS, RI |
| 62. | α-Selinene | 1496 | 1496 | 0.14 | - | - | - | MS, RI |
| 63. | β-Eudesmene | 1498 | 1495 | - | - | 0.09 | - | MS, RI |
| 64. | α-Muurolene | 1501 | 1500 | 0.34 | - | 0.13 | 0.4 | MS, RI |
| 65. | α-Farnesene | 1512 | 1508 | 0.18 | 0.2 | 0.14 | 0.45 | MS, RI |
| 66. | Eremophilene | 1515 | 1515 | 0.82 | - | 0.24 | - | MS, RI |
| 67. | τ-Cadinene | 1520 | 1530 | 0.33 | - | 0.17 | 0.16 | MS, RI |
| 68. | β-Cadinene | 1534 | 1529 | 7.82 | 7.12 | 5.5 | 7.71 | MS, RI |
| 69. | Caryophyllene oxide | 1537 | 1537 | 1.32 | 2.31 | 1.46 | 3.75 | MS, RI, AU |
| 70. | α-Calacorene | 1554 | 1553 | - | 0.09 | 0.09 | 0.13 | MS, RI |
| 71. | trans-Nerolidol | 1566 | 1566 | 0.04 | 0.31 | - | 0.39 | MS, RI |
| 72. | Germacrene D-4-ol | 1570 | 1570 | 0.14 | 0.27 | - | - | MS, RI |
| 73. | Spathulenol | 1589 | 1589 | - | tr | - | - | MS, RI |
| 74. | Widdrol | 1601 | 1606 | 0.06 | - | - | - | MS, RI |
| 75. | Cubenol | 1642 | 1642 | 0.08 | 0.15 | 0.09 | 0.24 | MS, RI |
| 76. | τ-Muurolol | 1656 | 1655 | 0.37 | 0.41 | 0.24 | 0.58 | MS, RI |
| 77. | δ-Cadinol | 1659 | 1655 | - | - | 0.08 | - | MS, RI |
| 78. | α-Cadinol | 1670 | 1676 | 0.5 | 0.72 | 0.42 | 1.13 | MS, RI |
| 79. | Pentadecanal | 1720 | 1721 | 0.62 | - | - | - | MS, RI |
| 80. | trans-Farnesol | 1729 | 1722 | - | 0.14 | - | 0.21 | MS, RI |
| 81. | Myristic acid | 1765 | 1767 | - | 0.18 | 0.38 | 0.2 | MS, RI |
| 82. | cis-9-Hexadecenal | 1798 | 1800 | tr | - | - | - | MS, RI |
| 83. | Hexadecanal | 1820 | 1819 | 0.08 | - | - | - | MS, RI |
| 84. | Hexahydrofarnesyl acetone | 1849 | 1848 | 0.15 | 0.09 | 0.05 | 0.17 | MS, RI |
| 85. | Palmitic acid | 1973 | 1973 | 1.03 | 7.99 | 5.75 | 3.06 | MS, RI, AU |
| 86. | trans-Phytol | 2127 | 2122 | 3.91 | 3.05 | 0.95 | 2 | MS, RI |
| 87. | Oleic Acid | 2156 | 2152 | 0.07 | 0.08 | - | 8.9 | MS, RI, AU |
| 88. | Linoleic acid | 2161 | 2157 | - | 11.92 | - | - | MS, RI, AU |
| 89. | Linolenic acid | 2170 | 2178 | - | - | 15.76 | - | MS, RI, AU |
| 90. | Stearyl acetate | 2219 | 2122 | - | 0.34 | - | - | MS, RI |
| 91. | Docosane | 2208 | 2200 | 0.07 | - | 0.13 | - | MS, RI |
| 92. | Tricosane | 2305 | 2300 | Tr. | - | 0.2 | 0.27 | MS, RI |
| 93. | Tetracosane | 2398 | 2400 | - | - | 0.14 | - | MS, RI |
| 94. | Pentacosane | 2505 | 2500 | 0.22 | 0.21 | 0.25 | 0.25 | MS, RI |
| 95. | Hexacosane | 2606 | 2600 | 0.08 | - | 0.09 | 0.41 | MS, RI |
| 96. | Heptacosane | 2706 | 2700 | 1.15 | 0.87 | 0.59 | - | MS, RI |
| 97. | Octacosane | 2799 | 2800 | 0.09 | - | 0.09 | - | MS, RI |
| Monoterpene hydrocarbons | 13.26 | 10.65 | 26.72 | 9.35 | ||||
| Oxygenated monoterpene | 1.91 | 1.35 | 1.1 | 3.66 | ||||
| Sesquiterpene hydrocarbons | 67.29 | 49.08 | 36.14 | 50.6 | ||||
| Oxygenated sesquiterpene | 3.28 | 4.4 | 2.34 | 6.47 | ||||
| Others | 8.45 | 26.79 | 25.65 | 22.55 | ||||
| Total identified components | 94.19 | 92.27 | 91.95 | 92.63 | ||||
| Compound | Collagenase | Elastase | Hyaluronidase | |||
|---|---|---|---|---|---|---|
| pH-Based | Rule-Based | pH-Based | Rule-Based | pH-Based | Rule-Based | |
| α-Pinene | 4.99 | 4.99 | 9.63 | 9.63 | 13.44 | 12.22 |
| α-Phellandrene | 6.85 | 6.85 | 6.46 | 6.46 | 16.45 | 16.45 |
| Limonene | 14.69 | 13.92 | 14.65 | 16.74 | 24.90 | 20.40 |
| β-cis-Ocimene | 11.85 | 11.85 | 17.84 | 17.84 | 22.06 | 22.06 |
| β-Linalool | −3.84 | 0.10 | 9.34 | 6.43 | 12.07 | 12.40 |
| p-Vinylanisole | −20.06 | −20.06 | −15.56 | −15.56 | −11.44 | −11.44 |
| β-Elemen | 21.19 | 21.19 | 26.71 | 26.71 | 33.61 | 33.61 |
| Caryophyllene | 11.74 | 11.74 | 11.48 | 11.48 | 28.20 | 28.20 |
| α-Humulene | 46.58 | 46.58 | 49.96 | 50.07 | 56.05 | 56.05 |
| Germacrene D | 1.15 | 1.15 | 6.24 | 6.24 | 11.47 | 11.47 |
| β-Cadinene | 27.90 | 27.90 | 27.03 | 27.03 | 36.47 | 36.47 |
| Caryophyllene oxide | −7.19 | −5.20 | −1.27 | 0.92 | 4.57 | 6.13 |
| α-Cadinol | 7.29 | 7.29 | 15.96 | 15.96 | 22.23 | 22.23 |
| Palmitic acid | −78.27 | −78.27 | −45.77 | −45.77 | −40.85 | −39.19 |
| Oleic Acid | −57.81 | −57.81 | −31.50 | −31.50 | −31.59 | −31.59 |
| trans-Phytol | −28.44 | −29.65 | −16.62 | −15.12 | −4.10 | −4.70 |
| Linoleic acid | −59.31 | −59.31 | −31.51 | −31.51 | −30.48 | −30.48 |
| Linolenic acid | −29.85 | −29.85 | −1.43 | −1.43 | 4.12 | 4.12 |
| Heptacosane | −63.64 | −63.64 | −40.48 | −40.48 | −43.78 | −43.78 |
| Epigallocatechin gallate | −59.27 | −59.27 | −49.35 | −49.35 | −43.70 | −43.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling. Biomolecules 2020, 10, 879. https://doi.org/10.3390/biom10060879
Altyar AE, Ashour ML, Youssef FS. Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling. Biomolecules. 2020; 10(6):879. https://doi.org/10.3390/biom10060879
Chicago/Turabian StyleAltyar, Ahmed E., Mohamed L. Ashour, and Fadia S. Youssef. 2020. "Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling" Biomolecules 10, no. 6: 879. https://doi.org/10.3390/biom10060879
APA StyleAltyar, A. E., Ashour, M. L., & Youssef, F. S. (2020). Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling. Biomolecules, 10(6), 879. https://doi.org/10.3390/biom10060879