The Effect of Escitalopram on Central Serotonergic and Dopaminergic Systems in Patients with Cervical Dystonia, and Its Relationship with Clinical Treatment Effects: A Double-Blind Placebo-Controlled Trial
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Ethical Compliance
2.3. Experimental Design and Treatment
2.4. Scoring Neurological and Psychiatric Symptoms
2.5. SPECT Imaging
2.6. Statistical Analysis
3. Results
3.1. Difference between Baseline Scans and Scans after Treatment
3.2. Differences between SSRI and Placebo
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarsy, D.; Simon, D.K. Dystonia. N. Engl. J. Med. 2006, 355, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Agusti, I.; Parees, I.; Kojovic, M.; Stamelou, M.; Saifee, T.A.; Charlesworth, G.; Sheerin, U.M.; Edwards, M.J.; Bhatia, K.P. Tremulous cervical dystonia is likely to be familial: Clinical characteristics of a large cohort. Parkinsonism Relat. Disord. 2013, 19, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Gundel, H.; Wolf, A.; Xidara, V.; Busch, R.; Ladwig, K.H.; Jacobi, F.; von, R.M.; Ceballos-Baumann, A.O. High psychiatric comorbidity in spasmodic torticollis: A controlled study. J. Nerv. Ment. Dis. 2003, 191, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Kuiper, A.; Han, V.; Jiawan, V.C.; Douma, G.; van Harten, B.; Oen, J.M.; Pouwels, M.E.; Dieks, H.J.; Bartels, A.L.; et al. Psychiatric co-morbidity is highly prevalent in idiopathic cervical dystonia and significantly influences health-related quality of life: Results of a controlled study. Parkinsonism Relat. Disord. 2016, 30, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, J.S.; Mink, J.W. Dysfunction of dopaminergic pathways in dystonia. Adv. Neurol. 2004, 94, 163–170. [Google Scholar] [PubMed]
- Smit, M.; Bartels, A.L.; van Faassen, M.; Kuiper, A.; Niezen-Koning, K.E.; Kema, I.P.; Dierckx, R.A.; de Koning, T.J.; Tijssen, M.A. Serotonergic perturbations in dystonia disorders-a systematic review. Neurosci. Biobehav. Rev. 2016, 65, 264–275. [Google Scholar] [CrossRef]
- Zoons, E.; Tijssen, M.A.; Dreissen, Y.E.; Speelman, J.D.; Smit, M.; Booij, J. The relationship between the dopaminergic system and depressive symptoms in cervical dystonia. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1375–1382. [Google Scholar] [CrossRef]
- Zoons, E.; Booij, J.; Speelman, J.D.; Dreissen, Y.E.M.; Smit, M.; Tijssen, M.A.J. Lower serotonin transporter binding in patients with cervical dystonia is associated with psychiatric symptoms. EJNMMI Res. 2017, 7, 87. [Google Scholar] [CrossRef]
- Vaswani, M.; Linda, F.K.; Ramesh, S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 85–102. [Google Scholar] [CrossRef]
- Catafau, A.M.; Perez, V.; Plaza, P.; Pascual, J.C.; Bullich, S.; Suarez, M.; Penengo, M.M.; Corripio, I.; Puigdemont, D.; Danus, M.; et al. Serotonin transporter occupancy induced by paroxetine in patients with major depression disorder: A 123I-ADAM SPECT study. Psychopharmacology (Berlin) 2006, 189, 145–153. [Google Scholar] [CrossRef]
- Rominger, A.; Cumming, P.; Brendel, M.; Xiong, G.; Zach, C.; Karch, S.; Tatsch, K.; Bartenstein, P.; la Fougere, C.; Koch, W.; et al. Altered serotonin and dopamine transporter availabilities in brain of depressed patients upon treatment with escitalopram: A [123 I]beta-CIT SPECT study. Eur. Neuropsychopharmacol. 2015, 25, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, M.; Holm-Hansen, S.; Thomsen, G.; Wagner, A.; Jensen, P.; Pinborg, L.H.; Knudsen, G.M. Serotonin transporters in dopamine transporter imaging: A head-to-head comparison of dopamine transporter SPECT radioligands 123I-FP-CIT and 123I-PE2I. J. Nucl. Med. 2010, 51, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Baldinger, P.; Kranz, G.S.; Haeusler, D.; Savli, M.; Spies, M.; Philippe, C.; Hahn, A.; Hoflich, A.; Wadsak, W.; Mitterhauser, M.; et al. Regional differences in SERT occupancy after acute and prolonged SSRI intake investigated by brain PET. Neuroimage 2014, 88, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Sacher, J.; Geiss-Granadia, T.; Mossaheb, N.; Attarbaschi, T.; Lanzenberger, R.; Spindelegger, C.; Holik, A.; Asenbaum, S.; Dudczak, R.; et al. Higher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: An [123I]ADAM SPECT study. Psychopharmacology (Berlin) 2007, 191, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, H.G.; Booij, J.; Reitsma, J.B.; Schene, A.H. Serotonin transporter binding with [123I]beta-CIT SPECT in major depressive disorder versus controls: Effect of season and gender. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 841–849. [Google Scholar] [CrossRef]
- Ruhe, H.G.; Booij, J.; v Weert, H.C.; Reitsma, J.B.; Franssen, E.J.; Michel, M.C.; Schene, A.H. Evidence why paroxetine dose escalation is not effective in major depressive disorder: A randomized controlled trial with assessment of serotonin transporter occupancy. Neuropsychopharmacology 2009, 34, 999–1010. [Google Scholar] [CrossRef]
- Zoons, E.; Booij, J.; Delnooz, C.C.S.; Dijk, J.M.; Dreissen, Y.E.M.; Koelman, J.; van der Salm, S.M.A.; Skorvanek, M.; Smit, M.; Aramideh, M.; et al. Randomised controlled trial of escitalopram for cervical dystonia with dystonic jerks/tremor. J. Neurol. Neurosurg Psychiatry 2018. [Google Scholar] [CrossRef]
- Booij, J.; Kemp, P. Dopamine transporter imaging with [(123)I]FP-CIT SPECT: Potential effects of drugs. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 424–438. [Google Scholar] [CrossRef]
- Unceta, N.; Goicolea, M.A.; Barrio, R.J. Analytical procedures for the determination of the selective serotonin reuptake inhibitor antidepressant citalopram and its metabolites. Biomed. Chromatogr. 2011, 25, 238–257. [Google Scholar] [CrossRef]
- Guy, W.; Cleary, P.A. Pretreatment status and its relationship to the length of drying-out period. Psychopharmacol. Bull. 1976, 12, 20–22. [Google Scholar]
- Booij, J.; Hemelaar, T.G.; Speelman, J.D.; de Bruin, K.; Janssen, A.G.; van Royen, E.A. One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT. J. Nucl. Med. 1999, 40, 753–761. [Google Scholar]
- Koopman, K.E.; la Fleur, S.E.; Fliers, E.; Serlie, M.J.; Booij, J. Assessing the optimal time point for the measurement of extrastriatal serotonin transporter binding with 123I-FP-CIT SPECT in healthy, male subjects. J. Nucl. Med. 2012, 53, 1087–1090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Booij, J.; Habraken, J.B.; Bergmans, P.; Tissingh, G.; Winogrodzka, A.; Wolters, E.C.; Janssen, A.G.; Stoof, J.C.; van Royen, E.A. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J. Nucl. Med. 1998, 39, 1879–1884. [Google Scholar] [PubMed]
- Booij, J.; Knol, R.J. SPECT imaging of the dopaminergic system in (premotor) Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13 (Suppl. 3), S425–S428. [Google Scholar] [CrossRef]
- Booij, J.; Korn, P.; Linszen, D.H.; van Royen, E.A. Assessment of endogenous dopamine release by methylphenidate challenge using iodine-123 iodobenzamide single-photon emission tomography. Eur. J. Nucl. Med. 1997, 24, 674–677. [Google Scholar] [PubMed]
- Laruelle, M.; Abi-Dargham, A.; Van Dyck, C.H.; Rosenblatt, W.; Zea-Ponce, Y.; Zoghbi, S.S.; Baldwin, R.M.; Charney, D.S.; Hoffer, P.B.; Kung, H.F.; et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J. Nucl. Med. 1995, 36, 1182–1190. [Google Scholar] [PubMed]
- Boot, E.; Booij, J.; Hasler, G.; Zinkstok, J.R.; de Haan, L.; Linszen, D.H.; van Amelsvoort, T.A. AMPT-induced monoamine depletion in humans: Evaluation of two alternative [123I]IBZM SPECT procedures. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1350–1356. [Google Scholar] [CrossRef][Green Version]
- Booij, J.; Tissingh, G.; Boer, G.J.; Speelman, J.D.; Stoof, J.C.; Janssen, A.G.; Wolters, E.C.; van Royen, E.A. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J. Neurol. Neurosurg Psychiatry 1997, 62, 133–140. [Google Scholar] [CrossRef]
- Figee, M.; de Koning, P.; Klaassen, S.; Vulink, N.; Mantione, M.; van den Munckhof, P.; Schuurman, R.; van Wingen, G.; van Amelsvoort, T.; Booij, J.; et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol. Psychiatry 2014, 75, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Innis, R.B.; Cunningham, V.J.; Delforge, J.; Fujita, M.; Gjedde, A.; Gunn, R.N.; Holden, J.; Houle, S.; Huang, S.C.; Ichise, M.; et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007, 27, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Booij, J.; de Jong, J.; de Bruin, K.; Knol, R.; de Win, M.M.; van Eck-Smit, B.L. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: A double-blind, placebo-controlled, crossover study in healthy control subjects. J. Nucl. Med. 2007, 48, 359–366. [Google Scholar]
- Koch, W.; Unterrainer, M.; Xiong, G.; Bartenstein, P.; Diemling, M.; Varrone, A.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; et al. Extrastriatal binding of [(1)(2)(3)I]FP-CIT in the thalamus and pons: Gender and age dependencies assessed in a European multicentre database of healthy controls. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; Gandelman, M.S.; DeErausquin, G.A.; Zea-Ponce, Y.; Zoghbi, S.S.; Baldwin, R.M.; Laruelle, M.; Charney, D.S.; Hoffer, P.B.; Neumeyer, J.L.; et al. SPECT imaging of dopamine transporters in human brain with iodine-123-fluoroalkyl analogs of beta-CIT. J. Nucl. Med. 1996, 37, 1129–1133. [Google Scholar]
- Zitterl, W.; Aigner, M.; Stompe, T.; Zitterl-Eglseer, K.; Gutierrez-Lobos, K.; Schmidl-Mohl, B.; Wenzel, T.; Demal, U.; Zettinig, G.; Hornik, K.; et al. [123I]-beta-CIT SPECT imaging shows reduced thalamus-hypothalamus serotonin transporter availability in 24 drug-free obsessive-compulsive checkers. Neuropsychopharmacology 2007, 32, 1661–1668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, J.H. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J. Psychiatry Neurosci. 2007, 32, 86–102. [Google Scholar] [PubMed]
- Smith, G.S.; Dewey, S.L.; Brodie, J.D.; Logan, J.; Vitkun, S.A.; Simkowitz, P.; Schloesser, R.; Alexoff, D.A.; Hurley, A.; Cooper, T.; et al. Serotonergic modulation of dopamine measured with [11C]raclopride and PET in normal human subjects. Am. J. Psychiatry 1997, 154, 490–496. [Google Scholar] [CrossRef]
- Klimke, A.; Larisch, R.; Janz, A.; Vosberg, H.; Muller-Gartner, H.W.; Gaebel, W. Dopamine D2 receptor binding before and after treatment of major depression measured by [123I]IBZM SPECT. Psychiatry Res. 1999, 90, 91–101. [Google Scholar] [CrossRef]
- Sokoloff, P.; Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 2017, 45, 2–19. [Google Scholar] [CrossRef]
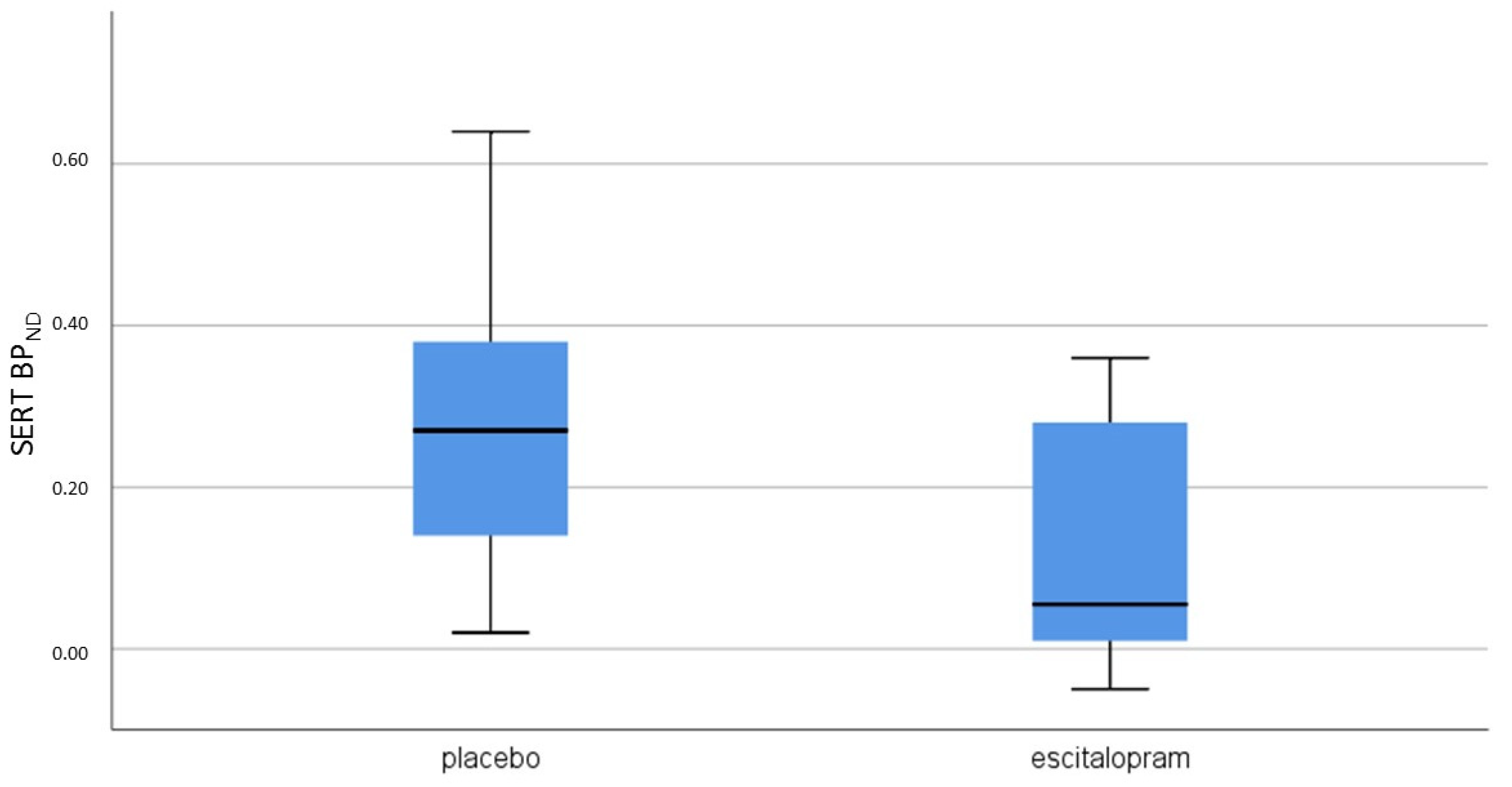
| Variables | Escitalopram (n = 8) | Placebo (n = 8) | p-Value |
|---|---|---|---|
| Age, mean (SD) | 56.4 (9.1) | 56.8 (10.3) | 0.94 |
| Male, n (%) | 4 (50%) | 6 (75%) | 0.61 |
| Baseline | After Treatment | p-Value | |
|---|---|---|---|
| Escitalopram (n = 8) | |||
| SERT | 0.18 (0.12−0.31) | 0.05 ((−0.02)−0.28) | 0.26 |
| DAT | 3.31 (2.47−4.10) | 2.90 (2.65−3.97) | 0.40 |
| D2/3R | 0.71 (0.60−0.92) | 0.89 (0.50−0.92) | 0.60 |
| Placebo (n = 8) | |||
| SERT | 0.28 (0.16−0.38) | 0.20 (0.08−0.39) | 0.40 |
| DAT | 3.54 (3.40−3.62) | 3.71 (3.34−4.56) | 0.61 |
| D2/3R | 0.92 (0.62−1.13) | 0.88 (0.84−1.03) | 0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoons, E.; Tijssen, M.A.J.; Dreissen, Y.E.M.; Smit, M.; Booij, J. The Effect of Escitalopram on Central Serotonergic and Dopaminergic Systems in Patients with Cervical Dystonia, and Its Relationship with Clinical Treatment Effects: A Double-Blind Placebo-Controlled Trial. Biomolecules 2020, 10, 880. https://doi.org/10.3390/biom10060880
Zoons E, Tijssen MAJ, Dreissen YEM, Smit M, Booij J. The Effect of Escitalopram on Central Serotonergic and Dopaminergic Systems in Patients with Cervical Dystonia, and Its Relationship with Clinical Treatment Effects: A Double-Blind Placebo-Controlled Trial. Biomolecules. 2020; 10(6):880. https://doi.org/10.3390/biom10060880
Chicago/Turabian StyleZoons, Evelien, Marina A.J. Tijssen, Yasmine E.M. Dreissen, Marenka Smit, and Jan Booij. 2020. "The Effect of Escitalopram on Central Serotonergic and Dopaminergic Systems in Patients with Cervical Dystonia, and Its Relationship with Clinical Treatment Effects: A Double-Blind Placebo-Controlled Trial" Biomolecules 10, no. 6: 880. https://doi.org/10.3390/biom10060880
APA StyleZoons, E., Tijssen, M. A. J., Dreissen, Y. E. M., Smit, M., & Booij, J. (2020). The Effect of Escitalopram on Central Serotonergic and Dopaminergic Systems in Patients with Cervical Dystonia, and Its Relationship with Clinical Treatment Effects: A Double-Blind Placebo-Controlled Trial. Biomolecules, 10(6), 880. https://doi.org/10.3390/biom10060880





