Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Cultures and Vegetable Juice Fermentation
2.2. Comprehensive Metabolic Profiling Using GC-MS
2.3. Comprehensive Lipid Profiling Using DI-MS
2.4. Carotenoid Analysis Using LC-MS
2.5. Statistical Analysis
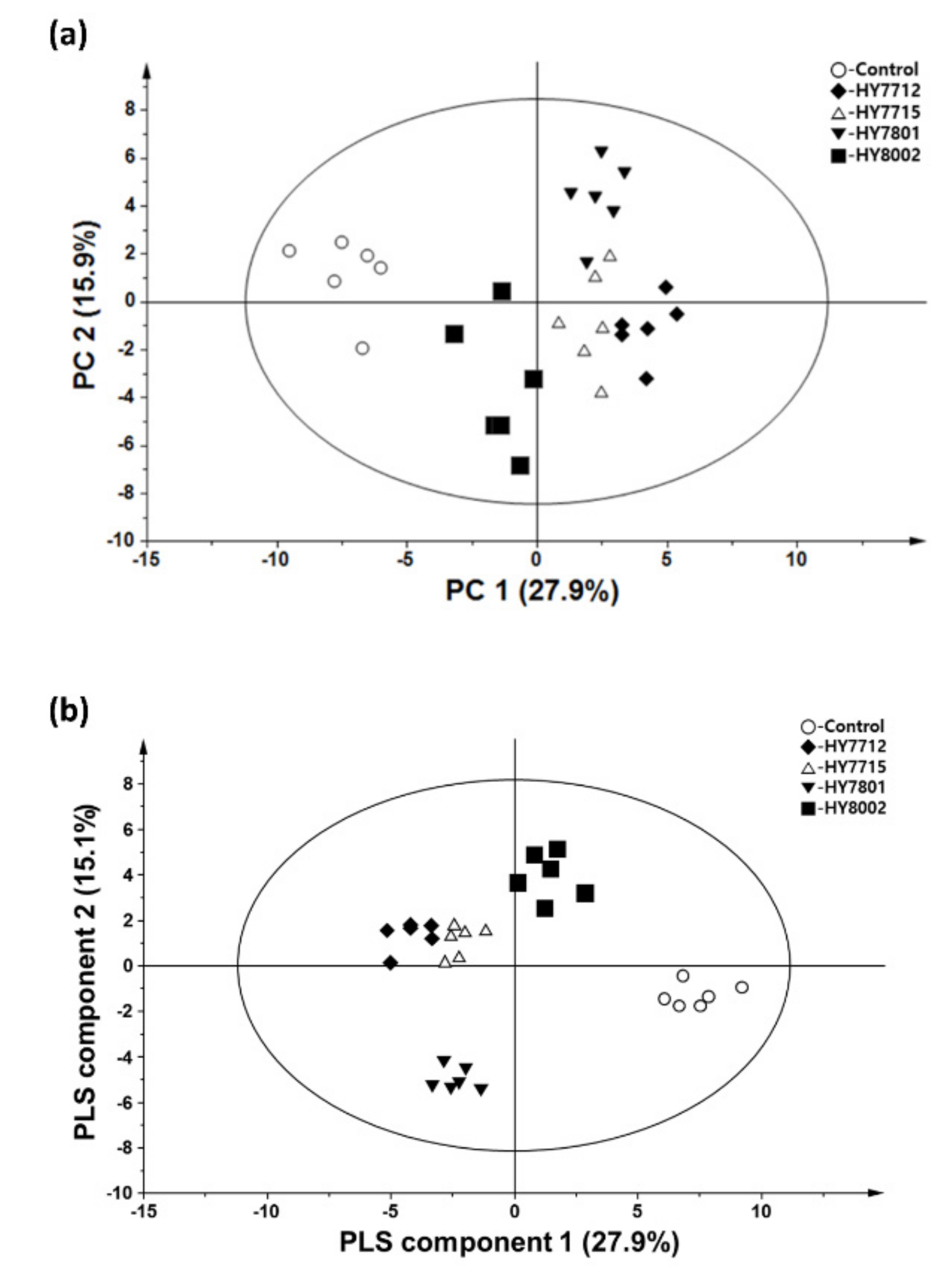
3. Results
3.1. Identification and Quantification of Metabolites and Lipids in Fermented and Non-Fermented VJs Using GC-MS, DI-MS, and LC-MS
3.2. Probiotic Fermentation of VJ Alters its Metabolic and Lipidomic Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. In Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligne, B.; Ganzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Shao, P.; Lee, R.P.; Huang, J.; Ly, A.; Hsu, M.; Lu, Q.Y.; Thames, G.; Heber, D.; et al. Health benefit of vegetable/fruit juice-based diet: Role of microbiome. Sci. Rep. 2017, 7, 2167. [Google Scholar] [CrossRef] [PubMed]
- Havas, P.; Kun, S.; Styevkó, G.; Slačanac, V.; Hardi, J.; Rezessy-Szabó, J. Fruit and vegetable juice fermentation with bifidobacteria. Acta Alimentaria 2014, 43, 64–72. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef]
- Kosewski, G.; Gorna, I.; Boleslawska, I.; Kowalowka, M.; Wieckowska, B.; Glowka, A.K.; Morawska, A.; Jakubowski, K.; Dobrzynska, M.; Miszczuk, P.; et al. Comparison of antioxidative properties of raw vegetables and thermally processed ones using the conventional and sous-vide methods. Food Chem. 2018, 240, 1092–1096. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014. [Google Scholar] [CrossRef]
- Corona, O.; Randazzo, W.; Miceli, A.; Guarcello, R.; Francesca, N.; Erten, H.; Moschetti, G.; Settanni, L. Characterization of kefir-like beverages produced from vegetable juices. LWT Food Sci. Technol. 2016, 66, 572–581. [Google Scholar] [CrossRef]
- Kim, S.Y. Production of fermented kale juices with Lactobacillus strains and nutritional composition. Prev. Nutr. Food Sci. 2017, 22, 231–236. [Google Scholar] [PubMed]
- Tomita, S.; Saito, K.; Nakamura, T.; Sekiyama, Y.; Kikuchi, J. Rapid discrimination of strain-dependent fermentation characteristics among Lactobacillus strains by NMR-based metabolomics of fermented vegetable juice. PLoS ONE 2017, 12, e0182229. [Google Scholar] [CrossRef]
- Yang, K.; Xu, M.; Zhong, F.; Zhu, J. Rapid differentiation of Lactobacillus species via metabolic profiling. J. Microbiol. Methods 2018, 154, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Nakamura, T.; Okada, S. NMR- and GC/MS-based metabolomic characterization of sunki, an unsalted fermented pickle of turnip leaves. Food Chem. 2018, 258, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Seo, S.H.; Lee, K.I.; Na, C.S.; Son, H.S. Metabolite profiling of fermented ginseng extracts by gas chromatography mass spectrometry. J. Ginseng Res. 2018, 42, 57–67. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.Y.; Jeon, J.Y.; Kim, D.M.; Zhou, Y.; Lee, J.S.; Lee, H.; Choi, H.K. Effects of coronatine elicitation on growth and metabolic profiles of Lemna paucicostata culture. PLoS ONE 2017, 12, e0187622. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, S.R.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J. Agric. Food Chem. 2016, 64, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Liu, K.H.; Lee, D.Y.; DeFelice, B.; Meissen, J.K.; Fiehn, O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 2013, 10, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.lipidmaps.org/ (accessed on 24 October 2018).
- Bohoyo-Gil, D.; Dominguez-Valhondo, D.; Garcia-Parra, J.; González-Gómez, D. UHPLC as a suitable methodology for the analysis of carotenoids in food matrix. Eur. Food Res. Technol. 2012, 235, 1055–1061. [Google Scholar] [CrossRef]
- Kim, S.H.; Liu, K.H.; Lee, S.Y.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE 2013, 8, e72415. [Google Scholar] [CrossRef]
- Available online: http://www.metaboanalyst.ca (accessed on 24 October 2018).
- Desjardins, M.-L.; Roy, D.; Goulet, J. Growth of bifidobacteria and their enzyme profiles. J. Dairy Sci. 1990, 73, 299–307. [Google Scholar] [CrossRef]
- Premi, L.; Sandine, W.E.; Elliker, P.R. Lactose-hydrolyzing enzymes of Lactobacillus species. Appl. Microbiol. 1972, 24, 51–57. [Google Scholar] [CrossRef][Green Version]
- Caspritz, G.; Radler, F. Malolactic enzyme of Lactobacillus plantarum. Purification, properties, and distribution among bacteria. J. Biol. Chem. 1983, 258, 4907–4910. [Google Scholar]
- Pham, P.L.; Dupont, I.; Roy, D.; Lapointe, G.; Cerning, J. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl. Environ. Microbiol. 2000, 66, 2302–2310. [Google Scholar] [CrossRef]
- Degeest, B.; Janssens, B.; De Vuyst, L. Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0–1: Production kinetics, enzyme activities and EPS yields. J. Appl. Microbiol. 2001, 91, 470–477. [Google Scholar] [CrossRef]
- Palasz, A.; Ciez, D. In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef]
- Elli, M.; Zink, R.; Rytz, A.; Reniero, R.; Morelli, L. Iron requirement of Lactobacillus spp. in completely chemically defined growth media. J. Appl. Microbiol. 2000, 88, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Patra, J.K.; Lee, S.Y.; Kim, C.; Park, J.G.; Baek, K.H. Analysis of metabolomic profile of fermented Orostachys japonicus A. Berger by capillary electrophoresis time of flight mass spectrometry. PLoS ONE 2017, 12, e0181280. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.M. Escherichia coli-derived uracil increases the antibacterial activity and growth rate of Lactobacillus plantarum. J. Microbiol. Biotechnol. 2016, 26, 975–987. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zeng, S.Y.; Leu, Y.L.; Tsai, T.Y. Antihypertensive effect of a combination of uracil and glycerol derived from Lactobacillus plantarum strain TWK10-fermented soy milk. J. Agric. Food Chem. 2015, 63, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.C.; Sun, D.; Chen, G.; Sharma, A.K.; Amin, S.; Ropson, I.J.; Spratt, T.E.; Lazarus, P. Characterization of dibenzo[a,l]pyrene-trans-11,12-diol(dibenzo[def,p]chrysene) glucuronidation by UDP-glucuronosyltransferases. Chem. Res. Toxicol. 2011, 24, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-kB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar]
- Zimin, Y.S.; Borisova, N.; Timerbaeva, G.; Gimadieva, A.; Mustafin, A. Preparation, toxicity, and anti-inflammatory activity of complexes of uracil derivatives with polyfunctional acids. Pharm. Chem. J. 2017, 50, 649–653. [Google Scholar] [CrossRef]
- Kim, J.-E.; Chae, C.S.; Kim, G.-C.; Hwang, W.; Hwang, J.-S.; Hwang, S.-M.; Kim, Y.; Ahn, Y.-T.; Park, S.-G.; Jun, C.-D. Lactobacillus helveticus suppresses experimental rheumatoid arthritis by reducing inflammatory T cell responses. J. Funct. Foods 2015, 13, 350–362. [Google Scholar] [CrossRef]
- Joo, H.M.; Kim, K.A.; Myoung, K.S.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus helveticus HY7801 ameliorates vulvovaginal candidiasis in mice by inhibiting fungal growth and NF-κB activation. Int. Immunopharmacol. 2012, 14, 39–46. [Google Scholar] [CrossRef]
- Hong, Y.S.; Ahn, Y.T.; Park, J.C.; Lee, J.H.; Lee, H.; Huh, C.S.; Kim, D.H.; Ryu, D.H.; Hwang, G.S. 1H NMR-based metabonomic assessment of probiotic effects in a colitis mouse model. Arch. Pharm. Res. 2010, 33, 1091–1101. [Google Scholar] [CrossRef]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Zeikus, J.; Jain, M.; Elankovan, P. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 1999, 51, 545–552. [Google Scholar] [CrossRef]
- Dudley, E.G.; Steele, J.L. Succinate production and citrate catabolism by Cheddar cheese nonstarter lactobacilli. J. Appl. Microbiol. 2005, 98, 14–23. [Google Scholar] [CrossRef]
- Hillier, A.J. The metabolism of [14C]bicarbonate by Streptococcus lactis: The synthesis of succinic acid. J. Dairy Res. 1978, 45, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, R.; Adriany, T.; Verbrugghe, K.; De Vuyst, L. Kinetic analysis of bifidobacterial metabolism reveals a minor role for succinic acid in the regeneration of NAD+ through its growth-associated production. Appl. Environ. Microbiol. 2006, 72, 5204–5210. [Google Scholar] [CrossRef]
- Giorgi-Coll, S.; Amaral, A.I.; Hutchinson, P.J.A.; Kotter, M.R.; Carpenter, K.L.H. Succinate supplementation improves metabolic performance of mixed glial cell cultures with mitochondrial dysfunction. Sci. Rep. 2017, 7, 1003. [Google Scholar] [CrossRef]
- Jalloh, I.; Helmy, A.; Howe, D.J.; Shannon, R.J.; Grice, P.; Mason, A.; Gallagher, C.N.; Stovell, M.G.; van der Heide, S.; Murphy, M.P.; et al. Focally perfused succinate potentiates brain metabolism in head injury patients. J. Cereb. Blood Flow Metab. 2017, 37, 2626–2638. [Google Scholar] [CrossRef]
- Iplik, E.S.; Catmakas, T.; Cakmakoglu, B. A new target for the treatment of endometrium cancer by succinic acid. Cell Mol. Biol. 2018, 64, 60–63. [Google Scholar] [CrossRef]
- Zarubina, I.V.; Lukk, M.V.; Shabanov, P.D. Antihypoxic and antioxidant effects of exogenous succinic acid and aminothiol succinate-containing antihypoxants. Bull. Exp. Biol. Med. 2012, 153, 336–339. [Google Scholar] [CrossRef]
- Carman, G.M.; Henry, S.A. Phosphatidic acid plays a central role in the transcriptional regulation of glycerophospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 37293–37297. [Google Scholar] [CrossRef]
- Henry, S.A.; Kohlwein, S.D.; Carman, G.M. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 2012, 190, 317–349. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Kohlwein, S.; Paltauf, F. Role of phosphatidylinositol (PI) in ethanol production and ethanol tolerance by a high ethanol producing yeast. J. Ind. Microbiol. Biotechnol. 1999, 22, 58–63. [Google Scholar] [CrossRef]
- Holub, B.J. The nutritional significance, metabolism, and function of myo-inositol and phosphatidylinositol in health and disease. Adv. Nutr. Res. 1982, 4, 107–141. [Google Scholar] [PubMed]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.W.; Neville, T.A.; Rouillard, P.; Harder, Z.; Beanlands, D.S.; Sparks, D.L. Phosphatidylinositol increases HDL-C levels in humans. J. Lipid Res. 2005, 46, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Brunton, J.A.; Baldwin, M.P.; Hanna, R.A.; Bertolo, R.F. Proline supplementation to parenteral nutrition results in greater rates of protein synthesis in the muscle, skin, and small intestine in neonatal Yucatan miniature piglets. J. Nutr. 2012, 142, 1004–1008. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Barbul, A. Proline precursors to sustain mammalian collagen synthesis. J. Nutr. 2008, 138, 2021s–2024s. [Google Scholar] [CrossRef]
- Liang, X.; Dickman, M.B.; Becker, D.F. Proline biosynthesis is required for endoplasmic reticulum stress tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 2014, 289, 27794–27806. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Kato, J.; Horie, S.; Komatsubara, S.; Kisumi, M.; Chibata, I. Production of L-proline by Kurthia catenaforma. Appl. Microbiol. 1968, 16, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Juva, K. Synthesis of hydroxyproline in vitro by the hydroxylation of proline in a precursor of collagen. Proc. Natl. Acad. Sci. USA 1965, 53, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Guo, Q.; Yin, Y.; Blachier, F.; Kong, X. Dietary proline supplementation alters colonic luminal microbiota and bacterial metabolite composition between days 45 and 70 of pregnancy in Huanjiang mini-pigs. J. Anim. Sci. Biotechnol. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.; Stoimenova, A.; Obreshkova, D.; Saso, L. Biotechnology in the production of pharmaceutical industry ingredients: Amino acids. Biotechnol. Biotechnol. Equip. 2013, 27, 3620–3626. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.-J.; Park, S.-K. Amino acids analysis during lactic acid fermentation by single strain cultures of lactobacilli and mixed culture starter made from them. Afr. J. Biotechnol. 2014, 13, 2867–2873. [Google Scholar]
- Bannai, M.; Kawai, N.; Ono, K.; Nakahara, K.; Murakami, N. The effects of glycine on subjective daytime performance in partially sleep-restricted healthy volunteers. Front Neurol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Rose, M.L.; Madren, J.; Bunzendahl, H.; Thurman, R.G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 1999, 20, 793–798. [Google Scholar] [CrossRef]
- Garrido-Fernandez, J.; Maldonado-Barragan, A.; Caballero-Guerrero, B.; Hornero-Mendez, D.; Ruiz-Barba, J.L. Carotenoid production in Lactobacillus plantarum. Int. J. Food Microbiol. 2010, 140, 34–39. [Google Scholar] [CrossRef]
- Arab, L.; Steck, S. Lycopene and cardiovascular disease. Am. J. Clin. Nutr. 2000, 71, 1691S–1695S. [Google Scholar] [CrossRef]
- Bartkiene, E.; Vidmantiene, D.; Juodeikiene, G.; Viskelis, P.; Urbonaviciene, D. Lactic acid fermentation of tomato: Effects on cis/trans lycopene isomer ratio, β-carotene mass fraction and formation of L (+)-and D (–)-lactic acid. Food Technol. Biotech. 2013, 51, 471. [Google Scholar]
- Breithaupt, D.E.; Schwack, W.; Wolf, G.; Hammes, W.P. Characterization of the triterpenoid 4, 4′-diaponeurosporene and its isomers in food-associated bacteria. Eur. Food Res. Technol. 2001, 213, 231–233. [Google Scholar] [CrossRef]
- Sanchez-Contreras, A.; Jimenez, M.; Sanchez, S. Bioconversion of lutein to products with aroma. Appl. Microbiol. Biotechnol. 2000, 54, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, Y.T.; Park, S.H.; Park, D.Y.; Jin, Y.W.; Kim, C.S.; Sung, S.H.; Huh, C.S.; Kim, D.H. Lactobacillus plantarum HY7712 protects against the impairment of NK-cell activity caused by whole-body γ-irradiation in mice. J. Microbiol. Biotechnol. 2014, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Joh, E.H.; Lee, H.Y.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus plantarum HY7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J. Microbiol. Biotechnol. 2013, 23, 414–421. [Google Scholar] [CrossRef]

| No. | Compound | m/z | RT (min) | Fragmentation Ions (m/z) | TMS | Control | L. plantarum (HY7712) | L. plantarum (HY7715) | L. helveticus (HY7801) | B. lactis (HY8002) |
|---|---|---|---|---|---|---|---|---|---|---|
| Amino acids | ||||||||||
| 1 | β-alanine | 174 | 17.37 | 100, 174, 248, 290 | 3 | 0.352 ± 0.032 | ND | ND | 0.302 ± 0.011 + | 0.328 ± 0.032 |
| 2 | γ-aminobutanoic acid | 174 | 19.85 | 174, 216, 246, 304 | 3 | 83.630 ± 2.806 | 66.176 ± 8.063 * | 84.661 ± 1.777 | 77.351 ± 2.740 + | 81.219 ± 6.131 |
| 3 | Alanine | 116 | 8.80 | 100, 116, 190, 218 | 2 | 50.422 ± 1.816 | 50.480 ± 2.053 | 50.026 ± 5.861 | 53.394 ± 3.054 | 40.845 ± 24.358 |
| 4 | Asparagine | 116 | 23.13 | 116, 132, 188, 231 | 3 | 20.862 ± 1.038 | 17.253 ± 1.558 * | 18.952 ± 0.744 # | 13.289 ± 1.015 + | 16.524 ± 2.890 ^ |
| 5 | Aspartic acid | 232 | 19.68 | 100, 202, 218, 232 | 3 | 190.073 ± 8.232 | 142.789 ± 2.909 * | 159.894 ± 1.455 # | 157.900 ± 5.864 + | 122.123 ± 15.734 ^ |
| 6 | Glutamic acid | 246 | 22.06 | 128, 156, 246, 348 | 3 | 175.620 ± 4.918 | 152.488 ± 7.547 * | 154.917 ± 3.394 # | 134.961 ± 5.251 + | 163.626 ± 19.308 |
| 7 | Glycine | 174 | 14.19 | 86, 174, 248, 276 | 2 | 3.261 ± 0.147 | 3.118 ± 0.448 | 2.453 ± 0.061 # | 2.163 ± 0.071 + | 5.763 ± 0.237 ^ |
| 8 | Isoleucine | 158 | 13.88 | 100, 158, 218, 232 | 2 | 6.115 ± 0.241 | 2.297 ± 0.237 * | 1.711 ± 0.171 # | 2.861 ± 0.080 + | 4.462 ± 1.543 |
| 9 | Proline | 142 | 13.96 | 100, 142, 144, 216 | 2 | 4.192 ± 0.234 | 4.804 ± 0.729 | 6.941 ± 0.784 # | 4.736 ± 0.149 + | 3.890 ± 3.297 |
| 10 | Pyroglutamic acid | 156 | 19.62 | 133, 156, 230, 258 | 2 | 562.497 ± 15.928 | 483.337 ± 18.371 * | 508.725 ± 11.686 # | 521.194 ± 20.262 + | 526.650 ± 16.360 ^ |
| 11 | Serine | 204 | 15.68 | 100, 188, 204, 218 | 3 | 14.700 ± 0.692 | 1.474 ± 0.232 * | 7.357 ± 0.522 # | 13.816 ± 0.457 + | 10.529 ± 3.635 ^ |
| 12 | Threonine | 218 | 16.32 | 101, 117, 218, 291 | 3 | 4.661 ± 0.119 | 1.712 ± 0.157 * | 2.217 ± 0.037 # | 2.617 ± 0.156 + | 4.863 ± 1.161 |
| 13 | Valine | 144 | 11.80 | 100, 133, 144, 218 | 2 | 10.732 ± 0.785 | 5.977 ± 0.323 * | 5.387 ± 0.257 # | 7.584 ± 0.239 + | 7.687 ± 3.507 |
| Fatty acids | ||||||||||
| 14 | 1-Monopalmitin | 371 | 45.26 | 103, 129, 205, 371 | 2 | 4.647 ± 0.403 | 3.781 ± 1.278 | 3.705 ± 0.421 # | 2.835 ± 0.433 + | 4.372 ± 1.145 |
| 15 | Linoleic acid | 75 | 37.82 | 67, 75, 81, 337 | 1 | 0.528 ± 0.074 | 0.394 ± 0.089 * | 0.268 ± 0.059 # | 0.357 ± 0.044 + | 0.337 ± 0.044 ^ |
| 16 | Palmitic acid | 117 | 33.33 | 117, 132, 145, 313 | 1 | 0.673 ± 0.049 | 0.636 ± 0.057 | 0.649 ± 0.053 | 0.617 ± 0.090 | 0.707 ± 0.080 |
| 17 | Stearic acid | 117 | 38.66 | 117, 132, 145, 341 | 1 | 0.240 ± 0.039 | 0.367 ± 0.083 * | 0.286 ± 0.103 | 0.267 ± 0.081 | 0.323 ± 0.076 |
| Organic acids | ||||||||||
| 18 | Acetic acid | 177 | 8.12 | 133, 161, 177, 205 | 2 | 0.143 ± 0.012 | 0.124 ± 0.008 * | 0.137 ± 0.020 | 0.143 ± 0.016 | 0.150 ± 0.017 |
| 19 | Citric acid | 273 | 26.52 | 273, 347, 363, 375 | 4 | 120.814 ± 4.491 | 34.142 ± 1.741 * | 50.563 ± 1.841 # | 9.052 ± 4.326 + | 114.971 ± 4.669 |
| 20 | Fumaric acid | 245 | 15.46 | 115, 132, 143, 245 | 2 | 1.181 ± 0.074 | 0.043 ± 0.005 * | 0.045 ± 0.004 # | 0.287 ± 0.033 + | 0.189 ± 0.036 ^ |
| 21 | Lactic acid | 117 | 7.73 | 117, 133, 191, 219 | 2 | 21.595 ± 1.210 | 602.047 ± 19.653 * | 563.702 ± 33.204 # | 547.899 ± 14.857 + | 420.494 ± 27.212 ^ |
| 22 | Malic acid | 233 | 18.93 | 133, 189, 233, 245 | 3 | 89.641 ± 5.199 | ND | ND | 41.678 ± 2.598 + | ND |
| 23 | Malonic acid | 75 | 11.51 | 66, 75, 133, 233 | 2 | 0.441 ± 0.035 | 0.429 ± 0.023 | 0.425 ± 0.028 | 0.419 ± 0.029 | 0.533 ± 0.115 |
| 24 | Succinic acid | 247 | 14.48 | 75, 129, 172, 247 | 2 | 1.544 ± 0.071 | 1.768 ± 0.071 * | 2.462 ± 0.108 # | 56.616 ± 3.446 + | 3.044 ± 0.131 ^ |
| 25 | Tartaric acid | 292 | 22.43 | 189, 219, 292, 423 | 4 | 0.776 ± 0.028 | 0.602 ± 0.084 * | 0.638 ± 0.037 # | 0.686 ± 0.019 + | 0.683 ± 0.034 ^ |
| Sugars | ||||||||||
| 26 | Fructose | 217 | 26.34 | 204, 217, 319, 437 | 5 | 526.553 ± 32.602 | 553.891 ± 8.751 | 520.415 ± 26.583 | 512.824 ± 9.722 | 521.351 ± 26.811 |
| 103 | 28.01 | 103, 133, 217, 307 | 5(MeOX) | |||||||
| 28.32 | ||||||||||
| 27 | Galactose | 204 | 28.89 | 129, 191, 204, 217 | 5 | 6.029 ± 0.373 | 5.561 ± 0.336 | 6.183 ± 0.352 | 4.300 ± 0.139 + | 5.980 ± 0.365 |
| 28 | Glucose | 204 | 28.61 | 129, 191, 204, 217 | 5 | 2905.897 ± 138.257 | 1486.807 ± 39.829 * | 2156.601 ± 58.234 # | 805.171 ± 102.179 + | 2385.734 ± 64.071 ^ |
| 31.40 | ||||||||||
| 319 | 28.72 | 160, 205, 217, 319 | 5(MeOX) | |||||||
| 29 | Glucose-6-phosphate | 204 | 40.26 | 204, 217, 299, 387 | 6 | 0.329 ± 0.032 | 0.288 ± 0.025 * | 0.316 ± 0.021 | 0.251 ± 0.026 + | 0.618 ± 0.058 ^ |
| 41.74 | ||||||||||
| 30 | Sedoheptulose | 319 | 35.74 | 205, 217, 262, 319 | 6(MeOX) | 50.748 ± 3.714 | 42.976 ± 1.234 * | 47.967 ± 1.442 | 49.610 ± 0.918 | 44.614 ± 3.511 ^ |
| 35.89 | ||||||||||
| 31 | Sucrose | 361 | 46.06 | 103, 217, 361, 437 | 8 | 437.362 ± 192.453 | 556.267 ± 20.170 | 535.635 ± 35.941 | 509.509 ± 14.353 | 507.215 ± 24.494 |
| 32 | Xylose | 103 | 22.85 | 103, 189, 217, 307 | 4(MeOX) | 0.700 ± 0.042 | 0.686 ± 0.104 | 0.708 ± 0.051 | 0.715 ± 0.047 | 0.783 ± 0.022 ^ |
| Sugar acids | ||||||||||
| 33 | Glyceric acid | 189 | 14.88 | 103, 189, 205, 292 | 3 | 0.268 ± 0.024 | ND | ND | ND | 0.148 ± 0.016 ^ |
| 34 | Threonic acid | 292 | 20.66 | 117, 205, 220, 292 | 4 | 0.569 ± 0.030 | 0.455 ± 0.031 * | 0.502 ± 0.026 # | 0.494 ± 0.026 + | 0.554 ± 0.029 |
| Sugar alcohols | ||||||||||
| 35 | Erythritol | 217 | 19.22 | 103, 117, 205, 217 | 4 | 13.869 ± 0.680 | 1.057 ± 0.045 * | 1.119 ± 0.054 # | 6.792 ± 0.327 + | 1.647 ± 0.436 ^ |
| 19.39 | ||||||||||
| 36 | Glycerol | 205 | 13.41 | 103, 117, 133, 205 | 3 | 16.011 ± 0.751 | 18.614 ± 0.157 * | 18.262 ± 0.573 # | 16.246 ± 0.480 | 20.278 ± 0.708 ^ |
| 37 | Mannitol | 319 | 29.66 | 103, 205, 217, 319 | 6 | 11.188 ± 0.638 | 9.783 ± 0.434 * | 10.961 ± 0.551 | 10.554 ± 0.242 + | 18.955 ± 12.144 |
| 38 | Myo-Inositol | 305 | 34.45 | 191, 217, 305, 318 | 6 | 50.657 ± 1.580 | 45.353 ± 1.026 * | 48.372 ± 2.080 # | 50.395 ± 1.749 | 49.615 ± 1.095 |
| 39 | Xylitol | 217 | 24.25 | 103, 205, 217, 307 | 5 | 1.149 ± 0.043 | 1.050 ± 0.050 * | 1.122 ± 0.047 | 1.096 ± 0.051 | 1.185 ± 0.082 |
| Others | ||||||||||
| 40 | Phosphoric acid | 299 | 13.30 | 133, 211, 299, 314 | 3 | 262.434 ± 9.607 | 186.249 ± 12.682 * | 201.558 ± 3.812 # | 184.653 ± 4.847 + | 217.457 ± 7.081 ^ |
| 41 | Uracil | 241 | 15.03 | 99, 113, 241, 255 | 2 | 0.075 ± 0.011 | 0.057 ± 0.005 * | 0.054 ± 0.006# | 0.835 ± 0.046 + | 0.099 ± 0.064 |
| No. | Lipid Species | Ion Species | m/z | Control | L. plantarum (HY7712) | L. plantarum (HY7715) | L. helveticus (HY7801) | B. lactis (HY8002) |
|---|---|---|---|---|---|---|---|---|
| Positive ion mode | ||||||||
| Monogalactosyldiacylglycerol (MGDG) | ||||||||
| 1 | MGDG 18:2/18:3 | [M + Na]+ | 799 | 1.93 ± 0.62 | 1.74 ± 0.43 | 1.67 ± 0.47 | 1.88 ± 0.31 | 1.55 ± 0.52 |
| 2 | MGDG 18:2/18:2 | [M + Na]+ | 801 | 14.42 ± 4.00 | 13.24 ± 3.19 | 12.68 ± 3.14 | 13.64 ± 1.71 | 11.89 ± 3.22 |
| 3 | MGDG 18:1/18:2 | [M + Na]+ | 803 | 11.51 ± 3.39 | 10.38 ± 1.90 | 9.52 ± 1.93 | 10.37 ± 0.93 | 9.28 ± 2.10 |
| Lysophosphatidylcholine (Lyso-PC) | ||||||||
| 4 | Lyso-PC 18:2 | [M + H]+ | 520 | 5.30 ± 1.04 | 4.18 ± 1.05 | 4.36 ± 0.96 | 4.56 ± 0.70 | 3.73 ± 0.70 ^ |
| 5 | Lyso-PC 18:1 | [M + H]+ | 522 | 6.56 ± 1.60 | 6.53 ± 1.16 | 6.87 ± 1.16 | 6.38 ± 0.64 | 6.37 ± 1.01 |
| 6 | Lyso-PC 22:5 | [M + Na]+ | 592 | 2.81 ± 0.61 | 2.55 ± 0.52 | 2.31 ± 0.52 | 2.80 ± 0.38 | 2.39 ± 0.56 |
| Phosphatidylcholine (PC) | ||||||||
| 7 | PC 18:2/18:2 | [M + H]+ | 782 | 4.29 ± 0.50 | 4.16 ± 0.23 | 4.08 ± 0.28 | 4.06 ± 0.30 | 4.10 ± 0.30 |
| Phosphatidylethanolamine (PE) | ||||||||
| 8 | PE 16:0/20:0 | [M + H]+ | 748 | 1.17 ± 0.30 | 1.05 ± 0.11 | 0.98 ± 0.14 | 1.08 ± 0.11 | 1.08 ± 0.22 |
| Triacylglycerol (TG) | ||||||||
| 9 | TG 16:0/18:2/18:2 | [M + NH4]+ | 872 | 3.88 ± 0.26 | 4.19 ± 0.42 | 4.13 ± 0.49 | 4.17 ± 0.59 | 4.09 ± 0.31 |
| 10 | TG 18:2/18:2/18:3 | [M + NH4]+ | 894 | 2.85 ± 0.19 | 3.03 ± 0.32 | 3.01 ± 0.30 | 3.03 ± 0.39 | 2.98 ± 0.28 |
| 11 | TG 18:2/18:2/18:2 | [M + NH4]+ | 896 | 9.76 ± 0.61 | 10.66 ± 1.03 | 10.45 ± 1.07 | 10.49 ± 1.31 | 10.47 ± 0.83 |
| 12 | TG 18:1/18:2/18:2 | [M + NH4]+ | 898 | 3.33 ± 0.18 | 3.73 ± 0.35 | 3.68 ± 0.46 | 3.64 ± 0.56 | 3.64 ± 0.28 |
| 13 | TG 18:1/18:1/18:2 | [M + NH4]+ | 900 | 1.30 ± 0.04 | 1.46 ± 0.16 | 1.46 ± 0.21 | 1.41 ± 0.20 | 1.42 ± 0.13 |
| Negative ion mode | ||||||||
| Phosphatic acid (PA) | ||||||||
| 14 | PA 16:0/18:2 | [M − H]- | 671 | 1.81 ± 0.27 | 1.76 ± 0.21 | 1.81 ± 0.43 | 1.84 ± 0.30 | 1.67 ± 0.13 |
| 15 | PA 18:2/18:2 | [M − H]- | 695 | 1.74 ± 0.27 | 1.56 ± 0.35 | 1.48 ± 0.35 | 1.69 ± 0.32 | 1.44 ± 0.19 |
| Phosphatidylethanolamine (PE) | ||||||||
| 16 | PE 16:0/18:2 | [M − H]- | 714 | 1.46 ± 0.06 | 1.35 ± 0.08 | 1.35 ± 0.04 # | 1.41 ± 0.09 | 1.41 ± 0.10 |
| 17 | PE 18:2/18:2 | [M − H]- | 738 | 1.23 ± 0.05 | 3.75 ± 2.19 * | 2.72 ± 0.69 # | 4.43 ± 2.06 + | 1.96 ± 0.31 ^ |
| 18 | PE 18:1/18:2 | [M − H]- | 740 | 0.73 ± 0.05 | 1.18 ± 0.83 | 0.76 ± 0.17 | 1.15 ± 0.70 + | 0.62 ± 0.09 ^ |
| 19 | PE 18:0/18:2 | [M − H]- | 742 | 4.50 ± 0.84 | 1.51 ± 0.85 * | 1.22 ± 0.49 # | 1.01 ± 0.51 + | 2.29 ± 0.75 ^ |
| Phosphatidylglycerol (PG) | ||||||||
| 20 | PG 16:0/18:2 | [M − H]- | 745 | 1.28 ± 0.12 | 1.57 ± 0.25 * | 1.49 ± 0.30 | 1.66 ± 0.21 + | 1.47 ± 0.20 |
| Phosphatidylserine (PS) | ||||||||
| 21 | PS 18:2/20:0 | [M − H]- | 814 | 0.23 ± 0.04 | 0.33 ± 0.21 | 0.21 ± 0.05 | 0.15 ± 0.08 + | 0.27 ± 0.04 |
| 22 | PS 18:2/22:0 | [M − H]- | 842 | 0.75 ± 0.12 | 0.98 ± 0.18 * | 0.92 ± 0.09 # | 0.91 ± 0.11 | 0.98 ± 0.14 ^ |
| Phosphatidylinositol (PI) | ||||||||
| 23 | PI 16:0/18:2 | [M − H]- | 833 | 6.34 ± 0.86 | 7.59 ± 1.00 | 7.44 ± 1.13 | 8.04 ± 1.21 + | 7.50 ± 1.05 |
| 24 | PI 16:0/18:1 | [M − H]- | 835 | 1.01 ± 0.15 | 1.32 ± 0.21 * | 1.34 ± 0.23 # | 3.58 ± 0.21 + | 1.24 ± 0.15 ^ |
| Compound | Formula | RT (min) | m/z [M + H] + | Control | L. plantarum (HY7712) | L. plantarum (HY7715) | L. helveticus (HY7801) | B. lactis (HY8002) |
|---|---|---|---|---|---|---|---|---|
| LUT | C40H56O2 | 5.61 | 569.4 | 4.0 ± 0.2 | 4.2 ± 0.3 | 4.2 ± 0.1 | 2.6 ± 0.5 + | 4.4 ± 0.5 |
| LYC | C40H56 | 9.32 | 537.4 | 30.8 ± 0.9 | 36.3 ± 0.6 * | 45.1 ± 3.0 # | 34.0 ± 9.7 | 38.2 ± 2.1 ^ |
| α-CAR | C40H56 | 10.95 | 537.4 | 50.9 ± 3.5 | 60.9 ± 5.7 * | 69.0 ± 5.9 # | 61.8 ± 6.2 + | 54.8 ± 4.0 |
| β-CAR | C40H56 | 11.12 | 537.4 | 113.1 ± 7.6 | 134.4 ± 13.4 * | 147.7 ± 13.5 # | 111.3 ± 12.6 | 120.2 ± 8.7 |
| No. | Pathway Name | Compound a | Total b | Hits c | p d | Impact e |
|---|---|---|---|---|---|---|
| 1 | Alanine, aspartate and glutamate metabolism | alanine, aspartic acid, glutamic acid, asparagine, succinic acid, γ-aminobutanoic acid, fumaric acid | 20 | 7 | 8.18 × 10−6 | 0.60 |
| 2 | Glycine, serine and threonine metabolism | glycine, serine, threonine, glyceric acid, aspartic acid | 28 | 5 | 5.40× 10−3 | 0.42 |
| 3 | Citrate cycle (TCA cycle) | citric acid, fumaric acid, malic acid, succinic acid | 20 | 4 | 8.64 × 10−3 | 0.20 |
| 4 | Aminoacyl-tRNA biosynthesis | asparagine, glycine, aspartic acid, serine, valine, alanine, threonine, proline, glutamic acid, isoleucine | 66 | 10 | 2.52 × 10−4 | 0.18 |
| 5 | Starch and sucrose metabolism | fructose, glucose, glucose-6-phosphate, sucrose, xylose | 30 | 5 | 7.35 × 10−3 | 0.15 |
| 6 | Arginine and proline metabolism | aspartic acid, fumaric acid, proline, glutamic acid, γ-aminobutanoic acid | 40 | 5 | 2.47 × 10−2 | 0.10 |
| No. | Compound | VIP Value |
|---|---|---|
| 1 | γ-aminobutanoic acid | 1.71 |
| 2 | Glycine | 1.51 |
| 3 | Glucose-6-phosphate | 1.43 |
| 4 | Uracil | 1.42 |
| 5 | β-alanine | 1.42 |
| 6 | Succinic acid | 1.41 |
| 7 | Linoleic acid | 1.41 |
| 8 | Aspartic acid | 1.39 |
| 9 | Galactose | 1.38 |
| 10 | Phosphatidylinositol (PI) 16:0/18:1 | 1.36 |
| 11 | Proline | 1.35 |
| 12 | Asparagine | 1.33 |
| 13 | Serine | 1.29 |
| 14 | Glycerol | 1.28 |
| 15 | Sedoheptulose | 1.23 |
| 16 | Glucose | 1.23 |
| 17 | Myo-inositol | 1.20 |
| 18 | Malic acid | 1.16 |
| 19 | Erythritol | 1.14 |
| 20 | Isoleucine | 1.13 |
| 21 | Threonine | 1.13 |
| 22 | Fructose | 1.12 |
| 23 | Fumaric acid | 1.08 |
| 24 | Citric acid | 1.08 |
| 25 | Glyceric acid | 1.03 |
| 26 | Malonic acid | 1.03 |
| 27 | Xylitol | 1.01 |
| 28 | Phosphatidylethanolamine (PE) 18:0/18:2 | 1.00 |
| 29 | Glutamic acid | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-J.; Lee, H.; Na, G.; Jung, H.; Kim, D.-G.; Shin, S.-I.; Jung, S.-E.; Choi, I.-d.; Lee, J.-H.; Sim, J.-H.; et al. Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics. Biomolecules 2020, 10, 725. https://doi.org/10.3390/biom10050725
Chung H-J, Lee H, Na G, Jung H, Kim D-G, Shin S-I, Jung S-E, Choi I-d, Lee J-H, Sim J-H, et al. Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics. Biomolecules. 2020; 10(5):725. https://doi.org/10.3390/biom10050725
Chicago/Turabian StyleChung, Hyuk-Jin, Hwanhui Lee, Guknam Na, Heechul Jung, Dong-Gun Kim, Sang-Ick Shin, Seong-Eun Jung, Il-dong Choi, Jae-Hwan Lee, Jae-Hun Sim, and et al. 2020. "Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics" Biomolecules 10, no. 5: 725. https://doi.org/10.3390/biom10050725
APA StyleChung, H.-J., Lee, H., Na, G., Jung, H., Kim, D.-G., Shin, S.-I., Jung, S.-E., Choi, I.-d., Lee, J.-H., Sim, J.-H., & Choi, H.-K. (2020). Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics. Biomolecules, 10(5), 725. https://doi.org/10.3390/biom10050725




