Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace
Abstract
1. A Brief Summary of Acinetobacter baumannii as a Pathogen
2. Mechanisms of Resistance to Carbapenems in A. baumannii
3. Carbapenemases in A. baumannii: OXA β-Lactamases
3.1. OXA-23-Like Group
3.2. OXA-24/40-Like Group
3.3. OXA-51-Like Group
3.4. OXA-58-Like Group
3.5. OXA-143-Like Group
3.6. OXA-235-Like Group
4. Carbapenemases in A. baumannii: Metallo-β-Lactamases
4.1. NDM Group
4.2. VIM Group
4.3. IMP Group
5. Carbapenemases in A. baumannii: KPC β-Lactamases
6. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Giamarellou, H.; Antoniadou, A.; Kanellakopoulou, K. Acinetobacter baumannii: A universal threat to public health? Int. J. Antimicrob. Agents 2008, 32, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Richet, H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 2006, 42, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Actis, L.A.; Tolmasky, M.E.; Crosa, L.M.; Crosa, J.H. Effect of iron-limiting conditions on growth of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 1993, 31, 2812–2815. [Google Scholar] [CrossRef]
- Hartstein, A.I.; Rashad, A.L.; Liebler, J.M.; Actis, L.A.; Freeman, J.; Rourke, J.W., Jr.; Stibolt, T.B.; Tolmasky, M.E.; Ellis, G.R.; Crosa, J.H. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am. J. Med. 1988, 85, 624–631. [Google Scholar] [CrossRef]
- Hartstein, A.I.; Morthland, V.H.; Rourke, J.W., Jr.; Freeman, J.; Garber, S.; Sykes, R.; Rashad, A.L. Plasmid DNA fingerprinting of Acinetobacter calcoaceticus subspecies anitratus from intubated and mechanically ventilated patients. Infect. Control. Hosp. Epidemiol. 1990, 11, 531–538. [Google Scholar] [CrossRef]
- Larson, E. A decade of nosocomial Acinetobacter. Am. J. Infect. Control 1984, 12, 14–18. [Google Scholar] [CrossRef]
- Bergogne-Berezin, E.; Towner, K.J. Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 1996, 9, 148–165. [Google Scholar] [CrossRef]
- CDC. Antibiotic resistance threats in the United States; Centers for Disease Control: Atlanta, GA, USA, 2019.
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2019, 63, e01110-18. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachon, J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, A.P.; Flagler, M.J.; Dorsey, C.W.; Gaddy, J.A.; Actis, L.A. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 2008, 154, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Nemec, A.; Krizova, L.; Maixnerova, M.; van der Reijden, T.J.; Deschaght, P.; Passet, V.; Vaneechoutte, M.; Brisse, S.; Dijkshoorn, L. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res. Microbiol. 2011, 162, 393–404. [Google Scholar] [PubMed]
- Traglia, G.; Chiem, K.; Quinn, B.; Fernandez, J.S.; Montana, S.; Almuzara, M.; Mussi, M.A.; Tolmasky, M.E.; Iriarte, A.; Centron, D.; et al. Genome sequence analysis of an extensively drug-resistant Acinetobacter baumannii indigo-pigmented strain depicts evidence of increase genome plasticity. Sci. Rep. 2018, 8, 16961. [Google Scholar] [CrossRef]
- Xu, A.; Zhu, H.; Gao, B.; Weng, H.; Ding, Z.; Li, M.; Weng, X.; He, G. Diagnosis of severe community-acquired pneumonia caused by Acinetobacter baumannii through next-generation sequencing: A case report. BMC Infect. Dis. 2020, 20, 45. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, Y.C.; Kuo, S.C.; Shih, F.H.; Chen, T.L.; How, C.K.; Yang, Y.S.; Lee, Y.T. Community-acquired bloodstream infections caused by Acinetobacter baumannii: A matched case-control study. J. Microbiol. Immunol. Infect. 2018, 51, 629–635. [Google Scholar] [CrossRef]
- Leung, W.S.; Chu, C.M.; Tsang, K.Y.; Lo, F.H.; Lo, K.F.; Ho, P.L. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest 2006, 129, 102–109. [Google Scholar] [CrossRef]
- Chen, M.Z.; Hsueh, P.R.; Lee, L.N.; Yu, C.J.; Yang, P.C.; Luh, K.T. Severe community-acquired pneumonia due to Acinetobacter baumannii. Chest 2001, 120, 1072–1077. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef]
- Abbo, A.; Carmeli, Y.; Navon-Venezia, S.; Siegman-Igra, Y.; Schwaber, M.J. Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Rafailidis, P.I. Attributable mortality of Acinetobacter baumannii: No longer a controversial issue. Crit. Care 2007, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Garnacho, J.; Sole-Violan, J.; Sa-Borges, M.; Diaz, E.; Rello, J. Clinical impact of pneumonia caused by Acinetobacter baumannii in intubated patients: A matched cohort study. Crit. Care Med. 2003, 31, 2478–2482. [Google Scholar] [CrossRef] [PubMed]
- Ciginskiene, A.; Dambrauskiene, A.; Rello, J.; Adukauskiene, D. Ventilator-associated pneumonia due to drug-resistant Acinetobacter baumannii: Risk factors and mortality relation with resistance profiles, and independent predictors of in-hospital mortality. Medicina (Kaunas) 2019, 55, 49. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Paulus, T.; Lugenheim, M.; Stefanik, D.; Higgins, P.G.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J. Infect. 2012, 64, 282–290. [Google Scholar] [CrossRef]
- Zurawski, D.V.; Banerjee, J.; Alamneh, Y.A.; Shearer, J.P.; Demons, S.T. Skin and soft tissue models for Acinetobacter baumannii infection. Methods Mol. Biol. 2019, 1946, 271–287. [Google Scholar]
- Matthews, L.; Goodrich, J.S.; Weber, D.J.; Bergman, N.H.; Miller, M.B. The brief case: A fatal case of necrotizing fasciitis due to multidrug-resistant Acinetobacter baumannii. J. Clin. Microbiol. 2019, 57, e01751-18. [Google Scholar]
- Guerrero, D.M.; Perez, F.; Conger, N.G.; Solomkin, J.S.; Adams, M.D.; Rather, P.N.; Bonomo, R.A. Acinetobacter baumannii-associated skin and soft tissue infections: Recognizing a broadening spectrum of disease. Surg. Infect. (Larchmt.) 2010, 11, 49–57. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Ye, Y.Z.; Yu, H. Postoperative multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intravenous doxycycline and intraventricular gentamicin: A case report. World J. Clin Cases 2019, 7, 4342–4348. [Google Scholar] [CrossRef]
- Zurawski, D.V.; Thompson, M.G.; McQueary, C.N.; Matalka, M.N.; Sahl, J.W.; Craft, D.W.; Rasko, D.A. Genome sequences of four divergent multidrug-resistant Acinetobacter baumannii strains isolated from patients with sepsis or osteomyelitis. J. Bacteriol. 2012, 194, 1619–1620. [Google Scholar] [CrossRef] [PubMed]
- Charnot-Katsikas, A.; Dorafshar, A.H.; Aycock, J.K.; David, M.Z.; Weber, S.G.; Frank, K.M. Two cases of necrotizing fasciitis due to Acinetobacter baumannii. J. Clin. Microbiol. 2009, 47, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, M.; Corpina, C.; Sturiale, L. Endocarditis due to Acinetobacter baumannii. Int. J. Cardiol. 2016, 209, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Kunhi, M.; Sanagar, S.; Jagadeesh, N.; Shankar, B.; Abraham, A. Emergency cardiac double valve surgery in active infective endocarditis due to Acinetobacter baumannii with aortic root abscess in a patient with dialysis-dependent end-stage renal failure: A rare case report. J. Surg Case Rep. 2016, 2016, rjw168. [Google Scholar] [CrossRef][Green Version]
- Patel, G.; Perez, F.; Hujer, A.M.; Rudin, S.D.; Augustine, J.J.; Jacobs, G.H.; Jacobs, M.R.; Bonomo, R.A. Fulminant endocarditis and disseminated infection caused by carbapenem-resistant Acinetobacter baumannii in a renal-pancreas transplant recipient. Transpl. Infect. Dis. 2015, 17, 289–296. [Google Scholar] [CrossRef]
- Borer, A.; Gilad, J.; Smolyakov, R.; Eskira, S.; Peled, N.; Porat, N.; Hyam, E.; Trefler, R.; Riesenberg, K.; Schlaeffer, F. Cell phones and Acinetobacter transmission. Emerg. Infect. Dis. 2005, 11, 1160–1161. [Google Scholar] [CrossRef]
- Cohen, R.; Shimoni, Z.; Ghara, R.; Ram, R.; Ben-Ami, R. Effect of a ventilator-focused intervention on the rate of Acinetobacter baumannii infection among ventilated patients. Am. J. Infect. Control 2014, 42, 996–1001. [Google Scholar] [CrossRef]
- Villegas, M.V.; Hartstein, A.I. Acinetobacter outbreaks, 1977-2000. Infect. Control. Hosp. Epidemiol. 2003, 24, 284–295. [Google Scholar] [CrossRef]
- Weernink, A.; Severin, W.P.; Tjernberg, I.; Dijkshoorn, L. Pillows, an unexpected source of Acinetobacter. J. Hosp. Infect. 1995, 29, 189–199. [Google Scholar] [CrossRef]
- Farrow, J.M., 3rd; Wells, G.; Pesci, E.C. Desiccation tolerance in Acinetobacter baumannii is mediated by the two-component response regulator BfmR. PLoS ONE 2018, 13, e0205638. [Google Scholar] [CrossRef]
- Aranda, J.; Bardina, C.; Beceiro, A.; Rumbo, S.; Cabral, M.P.; Barbe, J.; Bou, G. Acinetobacter baumannii RecA protein in repair of DNA damage, antimicrobial resistance, general stress response, and virulence. J. Bacteriol. 2011, 193, 3740–3747. [Google Scholar] [CrossRef] [PubMed]
- Chapartegui-Gonzalez, I.; Lazaro-Diez, M.; Bravo, Z.; Navas, J.; Icardo, J.M.; Ramos-Vivas, J. Acinetobacter baumannii maintains its virulence after long-time starvation. PLoS ONE 2018, 13, e0201961. [Google Scholar] [CrossRef] [PubMed]
- Boll, J.M.; Tucker, A.T.; Klein, D.R.; Beltran, A.M.; Brodbelt, J.S.; Davies, B.W.; Trent, M.S. Reinforcing lipid A acylation on the cell surface of Acinetobacter baumannii promotes cationic antimicrobial peptide resistance and desiccation survival. mBio 2015, 6, e00478-15. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Espinal, P.; Vila-Farres, X.; Vila, J. The Acinetobacter baumannii oxymoron: Commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 2012, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Actis, L.A. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009, 4, 273–278. [Google Scholar] [CrossRef]
- Thompson, M.G.; Black, C.C.; Pavlicek, R.L.; Honnold, C.L.; Wise, M.C.; Alamneh, Y.A.; Moon, J.K.; Kessler, J.L.; Si, Y.; Williams, R.; et al. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2014, 58, 1332–1342. [Google Scholar] [CrossRef]
- Greene, C.; Vadlamudi, G.; Newton, D.; Foxman, B.; Xi, C. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am. J. Infect. Control 2016, 44, e65–e71. [Google Scholar] [CrossRef]
- Wood, C.R.; Ohneck, E.J.; Edelmann, R.E.; Actis, L.A. A light-regulated type I pilus contributes to Acinetobacter baumannii biofilm, motility, and virulence functions. Infect. Immun. 2018, 86, e00442-18. [Google Scholar] [CrossRef]
- Hassan, K.A.; Jackson, S.M.; Penesyan, A.; Patching, S.G.; Tetu, S.G.; Eijkelkamp, B.A.; Brown, M.H.; Henderson, P.J.; Paulsen, I.T. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 20254–20259. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.S.; Harding, C.M.; Feldman, M.F. Pathogenic Acinetobacter: From the cell surface to infinity and beyond. J. Bacteriol. 2015, 198, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Luke, N.R.; Beanan, J.M.; Olson, R.; Sauberan, S.L.; MacDonald, U.; Schultz, L.W.; Umland, T.C.; Campagnari, A.A. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 2010, 78, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Adams, F.G.; Brown, M.H. Diversity and function of capsular polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2018, 9, 3301. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Guo, L.; Luo, Q.; Zhou, K.; Yu, W.; Chen, Y.; Huang, C.; Xiao, Y. Wza gene knockout decreases Acinetobacter baumannii virulence and affects Wzy-dependent capsular polysaccharide synthesis. Virulence 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.Y.; Tipton, K.A.; Farokhyfar, M.; Burd, E.M.; Weiss, D.S.; Rather, P.N. A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii. Nat. Microbiol. 2018, 3, 563–569. [Google Scholar] [CrossRef]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A. Masquerading microbial pathogens: Capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef]
- Tolmasky, M.E.; Staneloni, R.J.; Leloir, L.F. Lipid-bound saccharides in Rhizobium meliloti. J. Biol. Chem. 1982, 257, 6751–6757. [Google Scholar]
- Tolmasky, M.E.; Staneloni, R.J.; Ugalde, R.A.; Leloir, L.F. Lipid-bound sugars in Rhizobium meliloti. Arch. Biochem. Biophys. 1980, 203, 358–364. [Google Scholar] [CrossRef]
- Gonzalez, J.E.; Semino, C.E.; Wang, L.X.; Castellano-Torres, L.E.; Walker, G.C. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 1998, 95, 13477–13482. [Google Scholar] [CrossRef]
- Ielpi, L.; Couso, R.O.; Dankert, M.A. Sequential assembly and polymerization of the polyprenol-linked pentasaccharide repeating unit of the xanthan polysaccharide in Xanthomonas campestris. J. Bacteriol. 1993, 175, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Hug, I.; Feldman, M.F. Analogies and homologies in lipopolysaccharide and glycoprotein biosynthesis in bacteria. Glycobiology 2011, 21, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Lees-Miller, R.G.; Iwashkiw, J.A.; Scott, N.E.; Seper, A.; Vinogradov, E.; Schild, S.; Feldman, M.F. A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol. Microbiol. 2013, 89, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol 2015, 6, 496. [Google Scholar] [CrossRef]
- Staneloni, R.J.; Tolmasky, M.E.; Leloir, L.F. Lipid-bound saccharides containing glucose and galactose in Agrobacterium tumefaciens. J. Gen. Microbiol. 1984, 130, 869–879. [Google Scholar] [CrossRef][Green Version]
- Low, K.E.; Howell, P.L. Gram-negative synthase-dependent exopolysaccharide biosynthetic machines. Curr. Opin. Struct. Biol. 2018, 53, 32–44. [Google Scholar] [CrossRef]
- Choi, A.H.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litran, T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef]
- Kenyon, J.J.; Nigro, S.J.; Hall, R.M. Variation in the OC locus of Acinetobacter baumannii genomes predicts extensive structural diversity in the lipooligosaccharide. PLoS ONE 2014, 9, e107833. [Google Scholar] [CrossRef]
- Geisinger, E.; Huo, W.; Hernandez-Bird, J.; Isberg, R.R. Acinetobacter baumannii: Envelope determinants that control drug resistance, virulence, and surface variability. Annu. Rev. Microbiol. 2019, 73, 481–506. [Google Scholar] [CrossRef]
- Pelletier, M.R.; Casella, L.G.; Jones, J.W.; Adams, M.D.; Zurawski, D.V.; Hazlett, K.R.; Doi, Y.; Ernst, R.K. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 4831–4840. [Google Scholar] [CrossRef]
- Arroyo, L.A.; Herrera, C.M.; Fernandez, L.; Hankins, J.V.; Trent, M.S.; Hancock, R.E. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob. Agents Chemother. 2011, 55, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Snitkin, E.S.; Zelazny, A.M.; Gupta, J.; Program, N.C.S.; Palmore, T.N.; Murray, P.R.; Segre, J.A. Genomic insights into the fate of colistin resistance and Acinetobacter baumannii during patient treatment. Genome Res. 2013, 23, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Llobet, E.; Aranda, J.; Bengoechea, J.A.; Doumith, M.; Hornsey, M.; Dhanji, H.; Chart, H.; Bou, G.; Livermore, D.M.; et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 2011, 55, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Iwashkiw, J.A.; Seper, A.; Weber, B.S.; Scott, N.E.; Vinogradov, E.; Stratilo, C.; Reiz, B.; Cordwell, S.J.; Whittal, R.; Schild, S.; et al. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog. 2012, 8, e1002758. [Google Scholar] [CrossRef] [PubMed]
- May, H.C.; Yu, J.J.; Zhang, H.; Wang, Y.; Cap, A.P.; Chambers, J.P.; Guentzel, M.N.; Arulanandam, B.P. Thioredoxin-A is a virulence factor and mediator of the type IV pilus system in Acinetobacter baumannii. PLoS ONE 2019, 14, e0218505. [Google Scholar] [CrossRef]
- Elhosseiny, N.M.; Elhezawy, N.B.; Attia, A.S. Comparative proteomics analyses of Acinetobacter baumannii strains ATCC 17978 and AB5075 reveal the differential role of type II secretion system secretomes in lung colonization and ciprofloxacin resistance. Microb. Pathog. 2019, 128, 20–27. [Google Scholar] [CrossRef]
- Harding, C.M.; Kinsella, R.L.; Palmer, L.D.; Skaar, E.P.; Feldman, M.F. Medically relevant Acinetobacter species require a type II secretion system and specific membrane-associated chaperones for the export of multiple substrates and full virulence. PLoS Pathog. 2016, 12, e1005391. [Google Scholar] [CrossRef]
- Lopez, J.; Ly, P.M.; Feldman, M.F. The tip of the VgrG spike Is essential to functional type VI secretion system assembly in Acinetobacter baumannii. mBio 2020, 11, e02761-19. [Google Scholar] [CrossRef]
- Repizo, G.D.; Gagne, S.; Foucault-Grunenwald, M.L.; Borges, V.; Charpentier, X.; Limansky, A.S.; Gomes, J.P.; Viale, A.M.; Salcedo, S.P. Differential role of the T6SS in Acinetobacter baumannii virulence. PLoS ONE 2015, 10, e0138265. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; He, F.; Ruan, Z.; Jiang, Y.; Hua, X.; Yu, Y. The role of the type VI secretion system vgrG gene in the virulence and antimicrobial resistance of Acinetobacter baumannii ATCC 19606. PLoS ONE 2018, 13, e0192288. [Google Scholar] [CrossRef]
- Hesse, L.E.; Lonergan, Z.R.; Beavers, W.N.; Skaar, E.P. The Acinetobacter baumannii Znu system overcomes host-imposed nutrient zinc limitation. Infect. Immun. 2019, 87, e00746-19. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, B.L.; Skaar, E.P. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front. Cell Infect. Microbiol. 2013, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Juttukonda, L.J.; Skaar, E.P. The manganese-responsive transcriptional regulator MumR protects Acinetobacter baumannii from oxidative stress. Infect. Immun. 2019, 88, e00762-19. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Penwell, W.F.; Traglia, G.M.; Zimbler, D.L.; Gaddy, J.A.; Nikolaidis, N.; Arivett, B.A.; Adams, M.D.; Bonomo, R.A.; Actis, L.A.; et al. Identification of potential virulence factors in the model strain Acinetobacter baumannii A118. Front. Microbiol. 2019, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Penwell, W.F.; Actis, L.A. Isolation and characterization of the acinetobactin and baumannoferrin siderophores produced by Acinetobacter baumannii. Methods Mol. Biol. 2019, 1946, 259–270. [Google Scholar] [PubMed]
- Penwell, W.F.; Arivett, B.A.; Actis, L.A. The Acinetobacter baumannii entA gene located outside the acinetobactin cluster is critical for siderophore production, iron acquisition and virulence. PLoS ONE 2012, 7, e36493. [Google Scholar] [CrossRef]
- Antunes, L.C.; Imperi, F.; Towner, K.J.; Visca, P. Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res. Microbiol. 2011, 162, 279–284. [Google Scholar] [CrossRef]
- Proschak, A.; Lubuta, P.; Grun, P.; Lohr, F.; Wilharm, G.; De Berardinis, V.; Bode, H.B. Structure and biosynthesis of fimsbactins A-F, siderophores from Acinetobacter baumannii and Acinetobacter baylyi. Chembiochem 2013, 14, 633–638. [Google Scholar] [CrossRef]
- Juttukonda, L.J.; Chazin, W.J.; Skaar, E.P. Acinetobacter baumannii coordinates urea metabolism with metal import to resist host-mediated metal limitation. mBio 2016, 7, e01475–e01486. [Google Scholar] [CrossRef]
- Crosa, J.H.; Walsh, C.T. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 223–249. [Google Scholar] [CrossRef]
- Wooldridge, K.G.; Williams, P.H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol. Rev. 1993, 12, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Tolmasky, M.E.; Crosa, J.H. Regulation of plasmid-mediated iron transport and virulence in Vibrio anguillarum. Biol. Met. 1991, 4, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Stork, M. Plasmid-encoded iron uptake systems. Microbiol. Spectr. 2014, 2, PLAS–0030–2014. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Fleming, R.E.; Minnick, M.F. Sequestration and scavenging of iron in infection. Infect. Immun. 2013, 81, 3503–3514. [Google Scholar] [CrossRef] [PubMed]
- Porcheron, G.; Garenaux, A.; Proulx, J.; Sabri, M.; Dozois, C.M. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: Correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front. Cell Infect. Microbiol. 2013, 3, 90. [Google Scholar] [CrossRef]
- Lau, C.K.; Krewulak, K.D.; Vogel, H.J. Bacterial ferrous iron transport: The Feo system. FEMS Microbiol. Rev. 2016, 40, 273–298. [Google Scholar] [CrossRef]
- Alvarez-Fraga, L.; Vazquez-Ucha, J.C.; Martinez-Guitian, M.; Vallejo, J.A.; Bou, G.; Beceiro, A.; Poza, M. Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii. Virulence 2018, 9, 496–509. [Google Scholar] [CrossRef]
- de Leseleuc, L.; Harris, G.; KuoLee, R.; Xu, H.H.; Chen, W. Serum resistance, gallium nitrate tolerance and extrapulmonary dissemination are linked to heme consumption in a bacteremic strain of Acinetobacter baumannii. Int. J. Med. Microbiol. 2014, 304, 360–369. [Google Scholar] [CrossRef]
- Zimbler, D.L.; Penwell, W.F.; Gaddy, J.A.; Menke, S.M.; Tomaras, A.P.; Connerly, P.L.; Actis, L.A. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals 2009, 22, 23–32. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Adams, M.D.; Bonomo, R.A.; Centron, D.; Tolmasky, M.E. Genomic analysis of Acinetobacter baumannii A118 by comparison of optical maps: Identification of structures related to its susceptibility phenotype. Antimicrob. Agents Chemother. 2011, 55, 1520–1526. [Google Scholar] [CrossRef]
- Dorsey, C.W.; Tomaras, A.P.; Connerly, P.L.; Tolmasky, M.E.; Crosa, J.H.; Actis, L.A. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 2004, 150, 3657–3667. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.C.; Bohac, T.J.; Shapiro, J.A.; Giblin, D.E.; Wencewicz, T.A.; Gulick, A.M. Crystal structure of the siderophore binding protein BauB bound to an unusual 2:1 complex between acinetobactin and ferric iron. Biochemistry 2018, 57, 6653–6661. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Poppelaars, S.; Stork, M.; Nagasawa, M.; Tolmasky, M.E.; Crosa, J.H. A nonribosomal peptide synthetase with a novel domain organization is essential for siderophore biosynthesis in Vibrio anguillarum. J. Bacteriol. 2004, 186, 7327–7336. [Google Scholar] [CrossRef] [PubMed]
- Actis, L.A.; Fish, W.; Crosa, J.H.; Kellerman, K.; Ellenberger, S.R.; Hauser, F.M.; Sanders-Loehr, J. Characterization of anguibactin, a novel siderophore from Vibrio anguillarum 775(pJM1). J. Bacteriol. 1986, 167, 57–65. [Google Scholar] [CrossRef]
- Actis, L.; Tolmasky, M.E.; Crosa, J.H. Vibriosis. In Fish Diseases and Disorders; Woo, P.T., Bruno, D.W., Eds.; CAB International: New York, NY, USA, 1999; Volume 3, pp. 523–557. [Google Scholar]
- Bohac, T.J.; Fang, L.; Giblin, D.E.; Wencewicz, T.A. Fimsbactin and acinetobactin compete for the periplasmic siderophore binding protein BauB in pathogenic Acinetobacter baumannii. ACS Chem. Biol. 2019, 14, 674–687. [Google Scholar] [CrossRef]
- Penwell, W.F.; DeGrace, N.; Tentarelli, S.; Gauthier, L.; Gilbert, C.M.; Arivett, B.A.; Miller, A.A.; Durand-Reville, T.F.; Joubran, C.; Actis, L.A. Discovery and characterization of new hydroxamate siderophores, baumannoferrin A and B, produced by Acinetobacter baumannii. Chembiochem 2015, 16, 1896–1904. [Google Scholar] [CrossRef]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The mechanisms of disease caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef]
- Li, F.J.; Starrs, L.; Burgio, G. Tug of war between Acinetobacter baumannii and host immune responses. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef]
- Kroger, C.; Kary, S.C.; Schauer, K.; Cameron, A.D. Genetic regulation of virulence and antibiotic resistance in Acinetobacter baumannii. Genes (Basel) 2016, 8, 12. [Google Scholar] [CrossRef]
- Kareem, S.M.; Al-Kadmy, I.M.S.; Al-Kaabi, M.H.; Aziz, S.N.; Ahmad, M. Acinetobacter baumannii virulence is enhanced by the combined presence of virulence factors genes phospholipase C (plcN) and elastase (lasB). Microb. Pathog. 2017, 110, 568–572. [Google Scholar] [CrossRef]
- Fiester, S.E.; Arivett, B.A.; Schmidt, R.E.; Beckett, A.C.; Ticak, T.; Carrier, M.V.; Ghosh, R.; Ohneck, E.J.; Metz, M.L.; Sellin Jeffries, M.K.; et al. Iron-regulated phospholipase C activity contributes to the cytolytic activity and virulence of Acinetobacter baumannii. PLoS ONE 2016, 11, e0167068. [Google Scholar] [CrossRef] [PubMed]
- Bentancor, L.V.; Routray, A.; Bozkurt-Guzel, C.; Camacho-Peiro, A.; Pier, G.B.; Maira-Litran, T. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect. Immun. 2012, 80, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mondal, A.; Mitra, S.; Basu, S. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. J. Antimicrob. Chemother. 2017, 72, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, M.H.; Kim, S.I.; Son, J.H.; Kim, S.; Lee, Y.C.; Shin, M.; Oh, M.H.; Lee, J.C. The sensor kinase BfmS controls production of outer membrane vesicles in Acinetobacter baumannii. BMC Microbiol. 2019, 19, 301. [Google Scholar] [CrossRef]
- Koenigs, A.; Stahl, J.; Averhoff, B.; Gottig, S.; Wichelhaus, T.A.; Wallich, R.; Zipfel, P.F.; Kraiczy, P. CipA of Acinetobacter baumannii is a novel plasminogen binding and complement inhibitory protein. J. Infect. Dis. 2016, 213, 1388–1399. [Google Scholar] [CrossRef]
- Koenigs, A.; Zipfel, P.F.; Kraiczy, P. Translation elongation factor Tuf of Acinetobacter baumannii is a plasminogen-binding protein. PLoS ONE 2015, 10, e0134418. [Google Scholar]
- Gebhardt, M.J.; Shuman, H.A. GigA and GigB are master regulators of antibiotic resistance, stress responses, and virulence in Acinetobacter baumannii. J. Bacteriol. 2017, 199, e00066-17. [Google Scholar] [CrossRef]
- Gebhardt, M.J.; Gallagher, L.A.; Jacobson, R.K.; Usacheva, E.A.; Peterson, L.R.; Zurawski, D.V.; Shuman, H.A. Joint transcriptional control of virulence and resistance to antibiotic and environmental stress in Acinetobacter baumannii. mBio 2015, 6, e01660-15. [Google Scholar] [CrossRef]
- Cerqueira, G.M.; Kostoulias, X.; Khoo, C.; Aibinu, I.; Qu, Y.; Traven, A.; Peleg, A.Y. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J. Infect. Dis. 2014, 210, 46–55. [Google Scholar] [CrossRef]
- Lin, F.; Xu, Y.; Chang, Y.; Liu, C.; Jia, X.; Ling, B. Molecular characterization of reduced susceptibility to biocides in clinical isolates of Acinetobacter baumannii. Front. Microbiol. 2017, 8, 1836. [Google Scholar] [CrossRef]
- Darwish Alipour Astaneh, S.; Rasooli, I.; Mousavi Gargari, S.L. Filamentous hemagglutinin adhesin FhaB limits A.baumannii biofilm formation. Front. Biosci. 2017, 9, 266–275. [Google Scholar]
- Tipton, K.A.; Rather, P.N. An ompR-envZ two-component system ortholog regulates phase variation, osmotic tolerance, motility, and virulence in Acinetobacter baumannii strain AB5075. J. Bacteriol. 2017, 199, e00705–e00716. [Google Scholar] [CrossRef] [PubMed]
- Camarena, L.; Bruno, V.; Euskirchen, G.; Poggio, S.; Snyder, M. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog. 2010, 6, e1000834. [Google Scholar] [CrossRef] [PubMed]
- Nwugo, C.C.; Arivett, B.A.; Zimbler, D.L.; Gaddy, J.A.; Richards, A.M.; Actis, L.A. Effect of ethanol on differential protein production and expression of potential virulence functions in the opportunistic pathogen Acinetobacter baumannii. PLoS ONE 2012, 7, e51936. [Google Scholar] [CrossRef]
- Szabo, G.; Verma, B.; Catalano, D. Selective inhibition of antigen-specific T lymphocyte proliferation by acute ethanol exposure: The role of impaired monocyte antigen presentation capacity and mediator production. J. Leukoc. Biol. 1993, 54, 534–544. [Google Scholar] [CrossRef]
- Szabo, G.; Dolganiuc, A.; Mandrekar, P.; White, B. Inhibition of antigen-presenting cell functions by alcohol: Implications for hepatitis C virus infection. Alcohol 2004, 33, 241–249. [Google Scholar] [CrossRef]
- Achur, R.N.; Freeman, W.M.; Vrana, K.E. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J. Neuroimmune Pharmacol. 2010, 5, 83–91. [Google Scholar] [CrossRef]
- Gallucci, R.M.; Pfister, L.J.; Meadows, G.G. Effects of ethanol consumption on enriched natural killer cells from C57BL/6 mice. Alcohol. Clin. Exp. Res. 1994, 18, 625–631. [Google Scholar] [CrossRef]
- Spinozzi, F.; Agea, E.; Bassotti, G.; Belia, S.; Rondoni, F.; Broccucci, L.; Solinas, A.; Gerli, R.; Bertotto, A. Ethanol-specific impairment of T-lymphocyte activation is caused by a transitory block in signal-transduction pathways. Gastroenterology 1993, 105, 1490–1501. [Google Scholar] [CrossRef]
- Gandhi, J.A.; Ekhar, V.V.; Asplund, M.B.; Abdulkareem, A.F.; Ahmadi, M.; Coelho, C.; Martinez, L.R. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS ONE 2014, 9, e95707. [Google Scholar] [CrossRef]
- Asplund, M.B.; Coelho, C.; Cordero, R.J.; Martinez, L.R. Alcohol impairs J774.16 macrophage-like cell antimicrobial functions in Acinetobacter baumannii infection. Virulence 2013, 4, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.G.; Des Etages, S.G.; Snyder, M. Microbial synergy via an ethanol-triggered pathway. Mol. Cell. Biol. 2004, 24, 3874–3884. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.M.; Bonomo, R.A. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: Beta-lactams in peril! Curr. Opin. Microbiol. 2005, 8, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sazlly Lim, S.; Zainal Abidin, A.; Liew, S.M.; Roberts, J.A.; Sime, F.B. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J. Infect. 2019, 79, 593–600. [Google Scholar] [CrossRef]
- Butler, D.A.; Biagi, M.; Tan, X.; Qasmieh, S.; Bulman, Z.P.; Wenzler, E. Multidrug resistant Acinetobacter baumannii: Resistance by any other name would still be hard to treat. Curr. Infect. Dis. Rep. 2019, 21, 46. [Google Scholar] [CrossRef]
- Nowak, P.; Paluchowska, P. Acinetobacter baumannii: Biology and drug resistance - role of carbapenemases. Folia Histochem. Cytobiol. 2016, 54, 61–74. [Google Scholar] [CrossRef][Green Version]
- Paterson, D.L.; Bonomo, R.A. Multidrug-resistant gram-negative pathogens: The urgent need for ‘old’ polymyxins. Adv. Exp. Med. Biol. 2019, 1145, 9–13. [Google Scholar]
- Perez, F.; Hujer, A.M.; Hujer, K.M.; Decker, B.K.; Rather, P.N.; Bonomo, R.A. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3471–3484. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Nikolaidis, N.; Tolmasky, M.E. Rise and dissemination of aminoglycoside resistance: The aac(6’)-Ib paradigm. Front. Microbiol. 2013, 4, 121. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Szabo, D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 43 (Suppl. 2), S49–S56. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, V.; Sanchaita, S.; Singh, N. Multidrug resistant Acinetobacter. J. Glob. Infect. Dis. 2010, 2, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; El Chakhtoura, N.G.; Yasmin, M.; Bonomo, R.A. Polymyxins: To combine or not to combine? Antibiotics (Basel) 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Park, W. Antibiotic resistance of pathogenic Acinetobacter species and emerging combination therapy. J. Microbiol 2017, 55, 837–849. [Google Scholar] [CrossRef]
- Nasr, P. Genetics, epidemiology, and clinical manifestations of multidrug-resistant Acinetobacter baumannii. J. Hosp. Infect. 2020, 104, 4–11. [Google Scholar] [CrossRef]
- Hou, P.F.; Chen, X.Y.; Yan, G.F.; Wang, Y.P.; Ying, C.M. Study of the correlation of imipenem resistance with efflux pumps AdeABC, AdeIJK, AdeDE and AbeM in clinical isolates of Acinetobacter baumannii. Chemotherapy 2012, 58, 152–158. [Google Scholar] [CrossRef]
- Xu, C.; Bilya, S.R.; Xu, W. adeABC efflux gene in Acinetobacter baumannii. New Microbes New Infect. 2019, 30, 100549. [Google Scholar] [CrossRef]
- Hamidian, M.; Nigro, S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom 2019, 5, e000306. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; He, X.; Ding, F.; Wu, W.; Luo, Y.; Fan, B.; Cao, H. Overproduction of efflux pumps caused reduced susceptibility to carbapenem under consecutive imipenem-selected stress in Acinetobacter baumannii. Infect Drug Resist 2017, 11, 457–467. [Google Scholar] [CrossRef]
- Rumbo, C.; Gato, E.; Lopez, M.; Ruiz de Alegria, C.; Fernandez-Cuenca, F.; Martinez-Martinez, L.; Vila, J.; Pachon, J.; Cisneros, J.M.; Rodriguez-Bano, J.; et al. Contribution of efflux pumps, porins, and beta-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 5247–5257. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Mussi, M.A.; Limansky, A.S.; Viale, A.M. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: Natural insertional inactivation of a gene encoding a member of a novel family of beta-barrel outer membrane proteins. Antimicrob. Agents Chemother. 2005, 49, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- del Mar Tomas, M.; Beceiro, A.; Perez, A.; Velasco, D.; Moure, R.; Villanueva, R.; Martinez-Beltran, J.; Bou, G. Cloning and functional analysis of the gene encoding the 33- to 36-kilodalton outer membrane protein associated with carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 5172–5175. [Google Scholar] [CrossRef] [PubMed]
- Vashist, J.; Tiwari, V.; Das, R.; Kapil, A.; Rajeswari, M.R. Analysis of penicillin-binding proteins (PBPs) in carbapenem resistant Acinetobacter baumannii. Indian J. Med. Res. 2011, 133, 332–338. [Google Scholar]
- Fernandez-Cuenca, F.; Martinez-Martinez, L.; Conejo, M.C.; Ayala, J.A.; Perea, E.J.; Pascual, A. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 2003, 51, 565–574. [Google Scholar] [CrossRef]
- Bush, K. Past and present perspectives on beta-lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef]
- Bonomo, R.A. beta-lactamases: A focus on current challenges. Cold Spring Harb. Perspect. Med. 2017, 7, a025239. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of beta-lactamase-producing pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar]
- Evans, B.A.; Amyes, S.G. OXA beta-lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Sykes, R.B.; Matthew, M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J. Antimicrob. Chemother. 1976, 2, 115–157. [Google Scholar] [CrossRef]
- Medeiros, A.A.; Cohenford, M.; Jacoby, G.A. Five novel plasmid-determined beta-lactamases. Antimicrob. Agents Chemother. 1985, 27, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Tolmasky, M.E. Sequencing and expression of aadA, bla, and tnpR from the multiresistance transposon Tn1331. Plasmid 1990, 24, 218–226. [Google Scholar] [CrossRef]
- Tolmasky, M.E.; Crosa, J.H. Genetic organization of antibiotic resistance genes (aac(6’)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid 1993, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.M.; Livermore, D.M.; Gur, D.; Akova, M.; Akalin, H.E. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1993, 37, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Donald, H.M.; Scaife, W.; Amyes, S.G.; Young, H.K. Sequence analysis of ARI-1, a novel OXA beta-lactamase, responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 2000, 44, 196–199. [Google Scholar] [CrossRef]
- Afzal-Shah, M.; Woodford, N.; Livermore, D.M. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D beta-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2001, 45, 583–588. [Google Scholar] [CrossRef]
- Kaitany, K.C.; Klinger, N.V.; June, C.M.; Ramey, M.E.; Bonomo, R.A.; Powers, R.A.; Leonard, D.A. Structures of the class D carbapenemases OXA-23 and OXA-146: Mechanistic basis of activity against carbapenems, extended-spectrum cephalosporins, and aztreonam. Antimicrob. Agents Chemother. 2013, 57, 4848–4855. [Google Scholar] [CrossRef]
- Smith, C.A.; Antunes, N.T.; Stewart, N.K.; Toth, M.; Kumarasiri, M.; Chang, M.; Mobashery, S.; Vakulenko, S.B. Structural basis for carbapenemase activity of the OXA-23 beta-lactamase from Acinetobacter baumannii. Chem. Biol. 2013, 20, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Mugnier, P.D.; Poirel, L.; Naas, T.; Nordmann, P. Worldwide dissemination of the blaOXA-23 carbapenemase gene of Acinetobacter baumannii. Emerg. Infect. Dis. 2010, 16, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Figueiredo, S.; Cattoir, V.; Carattoli, A.; Nordmann, P. Acinetobacter radioresistens as a silent source of carbapenem resistance for Acinetobacter spp. Antimicrob. Agents Chemother. 2008, 52, 1252–1256. [Google Scholar] [CrossRef]
- Nigro, S.J.; Hall, R.M. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J. Antimicrob. Chemother. 2016, 71, 1135–1147. [Google Scholar] [CrossRef]
- Mugnier, P.D.; Poirel, L.; Nordmann, P. Functional analysis of insertion sequence ISAba1, responsible for genomic plasticity of Acinetobacter baumannii. J. Bacteriol. 2009, 191, 2414–2418. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Zhang, H.; Ying, C. Spread of the blaOXA-23-containing Tn2008 in carbapenem-resistant Acinetobacter baumannii isolates grouped in CC92 from China. Front. Microbiol. 2017, 8, 163. [Google Scholar] [CrossRef]
- Hua, X.; Xu, Q.; Zhou, Z.; Ji, S.; Yu, Y. Relocation of Tn2009 and characterization of an ABGRI3-2 from re-sequenced genome sequence of Acinetobacter baumannii MDR-ZJ06. J. Antimicrob. Chemother. 2019, 74, 1153–1155. [Google Scholar] [CrossRef]
- Linder, P.; Lasko, P.F.; Ashburner, M.; Leroy, P.; Nielsen, P.J.; Nishi, K.; Schnier, J.; Slonimski, P.P. Birth of the D-E-A-D box. Nature 1989, 337, 121–122. [Google Scholar] [CrossRef]
- Bou, G.; Oliver, A.; Martinez-Beltran, J. OXA-24, a novel class D beta-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 2000, 44, 1556–1561. [Google Scholar] [CrossRef]
- D’Andrea, M.M.; Giani, T.; D’Arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P.; Luzzaro, F.; Rossolini, G.M. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.B.; Adams-Haduch, J.M.; Bogdanovich, T.; Pasculle, A.W.; Quinn, J.P.; Wang, H.N.; Doi, Y. Identification of diverse OXA-40 group carbapenemases, including a novel variant, OXA-160, from Acinetobacter baumannii in Pennsylvania. Antimicrob. Agents Chemother. 2011, 55, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.; Acosta, J.; Poza, M.; Sanz, F.; Beceiro, A.; Chaves, F.; Bou, G. OXA-24 carbapenemase gene flanked by XerC/XerD-like recombination sites in different plasmids from different Acinetobacter species isolated during a nosocomial outbreak. Antimicrob. Agents Chemother. 2010, 54, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Andres, P.; Petroni, A.; Soler-Bistue, A.; Albornoz, E.; Zorreguieta, A.; Reyes-Lamothe, R.; Sherratt, D.J.; Corso, A.; Tolmasky, M.E. Small plasmids harboring qnrB19: A model for plasmid evolution mediated by site-specific recombination at oriT and Xer sites. Antimicrob. Agents Chemother. 2012, 56, 1821–1827. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Beletskaya, I.V.; Denjmukhametov, M.M.; Yurkova, T.V.; Semenova, L.M.; Shlyapnikov, M.G.; Solonin, A.S. Characterization of pECL18 and pKPN2: A proposed pathway for the evolution of two plasmids that carry identical genes for a Type II restriction-modification system. Mol Genet. Genomics 2002, 267, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Traglia, G.M.; Lin, D.L.; Tran, T.; Tolmasky, M.E. Plasmid-mediated antibiotic resistance and virulence in Gram-negatives: The Klebsiella pneumoniae paradigm. Microbiology Spectr. 2014, 2, 1–15. [Google Scholar] [CrossRef]
- Santillana, E.; Beceiro, A.; Bou, G.; Romero, A. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc. Natl. Acad. Sci. USA 2007, 104, 5354–5359. [Google Scholar] [CrossRef]
- Schneider, K.D.; Ortega, C.J.; Renck, N.A.; Bonomo, R.A.; Powers, R.A.; Leonard, D.A. Structures of the class D carbapenemase OXA-24 from Acinetobacter baumannii in complex with doripenem. J. Mol. Biol. 2011, 406, 583–594. [Google Scholar] [CrossRef]
- Bou, G.; Santillana, E.; Sheri, A.; Beceiro, A.; Sampson, J.M.; Kalp, M.; Bethel, C.R.; Distler, A.M.; Drawz, S.M.; Pagadala, S.R.; et al. Design, synthesis, and crystal structures of 6-alkylidene-2’-substituted penicillanic acid sulfones as potent inhibitors of Acinetobacter baumannii OXA-24 carbapenemase. J. Am. Chem. Soc. 2010, 132, 13320–13331. [Google Scholar] [CrossRef]
- Che, T.; Bonomo, R.A.; Shanmugam, S.; Bethel, C.R.; Pusztai-Carey, M.; Buynak, J.D.; Carey, P.R. Carboxylation and decarboxylation of active site Lys 84 controls the activity of OXA-24 beta-lactamase of Acinetobacter baumannii: Raman crystallographic and solution evidence. J. Am. Chem. Soc. 2012, 134, 11206–11215. [Google Scholar] [CrossRef]
- Staude, M.W.; Leonard, D.A.; Peng, J.W. Expanded substrate activity of OXA-24/40 in carbapenem-resistant Acinetobacter baumannii involves enhanced binding loop flexibility. Biochemistry 2016, 55, 6535–6544. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Young, H.K.; Amyes, S.G. Characterisation of OXA-51, a novel class D carbapenemase found in genetically unrelated clinical strains of Acinetobacter baumannii from Argentina. Clin. Microbiol. Infect. 2005, 11, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Merkier, A.K.; Centron, D. bla(OXA-51)-type beta-lactamase genes are ubiquitous and vary within a strain in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2006, 28, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Woodford, N.; Glover, J.; Yarde, S.; Kaufmann, M.E.; Pitt, T.L. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 2006, 44, 2974–2976. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Biswas, I.; Veeraraghavan, B. Accurate identification of clinically important Acinetobacter spp.: An update. Future Sci. OA 2019, 5, FSO395. [Google Scholar] [CrossRef]
- Chen, T.L.; Lee, Y.T.; Kuo, S.C.; Hsueh, P.R.; Chang, F.Y.; Siu, L.K.; Ko, W.C.; Fung, C.P. Emergence and distribution of plasmids bearing the blaOXA-51-like gene with an upstream ISAba1 in carbapenem-resistant Acinetobacter baumannii isolates in Taiwan. Antimicrob. Agents Chemother. 2010, 54, 4575–4581. [Google Scholar] [CrossRef]
- Lee, Y.T.; Kuo, S.C.; Chiang, M.C.; Yang, S.P.; Chen, C.P.; Chen, T.L.; Fung, C.P. Emergence of carbapenem-resistant non-baumannii species of Acinetobacter harboring a blaOXA-51-like gene that is intrinsic to A. baumannii. Antimicrob. Agents Chemother. 2012, 56, 1124–1127. [Google Scholar] [CrossRef]
- Ahmadi, A.; Salimizand, H. Delayed identification of Acinetobacter baumannii during an outbreak owing to disrupted blaOXA-51-like by ISAba19. Int. J. Antimicrob. Agents 2017, 50, 119–122. [Google Scholar] [CrossRef]
- Tiwari, V.; Moganty, R.R. Conformational stability of OXA-51 beta-lactamase explains its role in carbapenem resistance of Acinetobacter baumannii. J. Biomol. Struct. Dyn. 2014, 32, 1406–1420. [Google Scholar] [CrossRef]
- Wong, M.H.; Chan, B.K.; Chan, E.W.; Chen, S. Over-expression of ISAba1-linked intrinsic and exogenously acquired OXA type carbapenem-hydrolyzing-class D-beta-lactamase-encoding genes is key mechanism underlying carbapenem resistance in Acinetobacter baumannii. Front. Microbiol. 2019, 10, 2809. [Google Scholar] [CrossRef]
- Nigro, S.J.; Hall, R.M. Does the intrinsic oxaAb (blaOXA-51-like) gene of Acinetobacter baumannii confer resistance to carbapenems when activated by ISAba1? J. Antimicrob. Chemother. 2018, 73, 3518–3520. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, S.; Poirel, L.; Croize, J.; Recule, C.; Nordmann, P. In vivo selection of reduced susceptibility to carbapenems in Acinetobacter baumannii related to ISAba1-mediated overexpression of the natural bla(OXA-66) oxacillinase gene. Antimicrob. Agents Chemother. 2009, 53, 2657–2659. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Ward, M.E.; Woodford, N.; Kaufmann, M.E.; Pike, R.; Livermore, D.M.; Pitt, T.L. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 2006, 258, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.S.; Yao, S.M.; Fung, C.P.; Hsieh, Y.P.; Liu, C.P.; Lin, J.F. An OXA-66/OXA-51-like carbapenemase and possibly an efflux pump are associated with resistance to imipenem in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3844–3852. [Google Scholar] [CrossRef] [PubMed]
- June, C.M.; Muckenthaler, T.J.; Schroder, E.C.; Klamer, Z.L.; Wawrzak, Z.; Powers, R.A.; Szarecka, A.; Leonard, D.A. The structure of a doripenem-bound OXA-51 class D beta-lactamase variant with enhanced carbapenemase activity. Protein Sci. 2016, 25, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Leonard, D.A. Common clinical substitutions enhance the carbapenemase activity of OXA-51-like class D beta-lactamases from Acinetobacter spp. Antimicrob. Agents Chemother. 2014, 58, 7015–7016. [Google Scholar] [CrossRef]
- Schroder, E.C.; Klamer, Z.L.; Saral, A.; Sugg, K.A.; June, C.M.; Wymore, T.; Szarecka, A.; Leonard, D.A. Clinical variants of the native class D beta-lactamase of Acinetobacter baumannii pose an emerging threat through Increased hydrolytic activity against carbapenems. Antimicrob. Agents Chemother. 2016, 60, 6155–6164. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Zhang, Y.; Wang, X.; Zhao, C.; Chen, H.; Zhang, F.; Zhu, B.; Hu, Y.; Wang, H. Evolution of carbapenem-resistant Acinetobacter baumannii revealed through whole-genome sequencing and comparative genomic analysis. Antimicrob. Agents Chemother. 2015, 59, 1168–1176. [Google Scholar] [CrossRef]
- Evans, B.A.; Brown, S.; Hamouda, A.; Findlay, J.; Amyes, S.G. Eleven novel OXA-51-like enzymes from clinical isolates of Acinetobacter baumannii. Clin. Microbiol. Infect. 2007, 13, 1137–1138. [Google Scholar] [CrossRef]
- Tsakris, A.; Ikonomidis, A.; Spanakis, N.; Pournaras, S.; Bethimouti, K. Identification of a novel bla(OXA-51) variant, bla(OXA-92), from a clinical isolate of Acinetobacter baumannii. Clin. Microbiol. Infect. 2007, 13, 348–349. [Google Scholar] [CrossRef]
- Smith, C.A.; Antunes, N.T.; Stewart, N.K.; Frase, H.; Toth, M.; Kantardjieff, K.A.; Vakulenko, S. Structural basis for enhancement of carbapenemase activity in the OXA-51 family of class D beta-lactamases. ACS Chem. Biol. 2015, 10, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Marque, S.; Heritier, C.; Segonds, C.; Chabanon, G.; Nordmann, P. OXA-58, a novel class D {beta}-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Heritier, C.; Dubouix, A.; Poirel, L.; Marty, N.; Nordmann, P. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA-58. J. Antimicrob. Chemother. 2005, 55, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Marque, S.; Poirel, L.; Heritier, C.; Brisse, S.; Blasco, M.D.; Filip, R.; Coman, G.; Naas, T.; Nordmann, P. Regional occurrence of plasmid-mediated carbapenem-hydrolyzing oxacillinase OXA-58 in Acinetobacter spp. in Europe. J. Clin. Microbiol. 2005, 43, 4885–4888. [Google Scholar] [CrossRef]
- Matos, A.P.; Cayo, R.; Almeida, L.G.P.; Streling, A.P.; Nodari, C.S.; Martins, W.; Narciso, A.C.; Silva, R.M.; Vasconcelos, A.T.R.; Gales, A.C. Genetic characterization of plasmid-borne blaOXA-58 in distinct Acinetobacter species. mSphere 2019, 4, e00376-19. [Google Scholar] [CrossRef]
- Bertini, A.; Poirel, L.; Bernabeu, S.; Fortini, D.; Villa, L.; Nordmann, P.; Carattoli, A. Multicopy blaOXA-58 gene as a source of high-level resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 2324–2328. [Google Scholar] [CrossRef]
- Reams, A.B.; Roth, J.R. Mechanisms of gene duplication and amplification. Cold Spring Harb. Perspect. Biol. 2015, 7, a016592. [Google Scholar] [CrossRef]
- Peterson, B.C.; Rownd, R.H. Drug resistance gene amplification of plasmid NR1 derivatives with various amounts of resistance determinant DNA. J. Bacteriol. 1985, 161, 1042–1048. [Google Scholar] [CrossRef]
- Tolmasky, M.E.; Chamorro, R.M.; Crosa, J.H.; Marini, P.M. Transposon-mediated amikacin resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 1988, 32, 1416–1420. [Google Scholar] [CrossRef]
- Peterson, B.C.; Rownd, R.H. Recombination sites in plasmid drug resistance gene amplification. J. Bacteriol. 1985, 164, 1359–1361. [Google Scholar] [CrossRef]
- Schechter, L.M.; Creely, D.P.; Garner, C.D.; Shortridge, D.; Nguyen, H.; Chen, L.; Hanson, B.M.; Sodergren, E.; Weinstock, G.M.; Dunne, W.M., Jr.; et al. Extensive gene amplification as a mechanism for piperacillin-tazobactam resistance in Escherichia coli. mBio 2018, 9, e00583-18. [Google Scholar] [CrossRef]
- Higgins, P.G.; Poirel, L.; Lehmann, M.; Nordmann, P.; Seifert, H. OXA-143, a novel carbapenem-hydrolyzing class D beta-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 5035–5038. [Google Scholar] [CrossRef] [PubMed]
- Levy-Blitchtein, S.; Roca, I.; Plasencia-Rebata, S.; Vicente-Taboada, W.; Velasquez-Pomar, J.; Munoz, L.; Moreno-Morales, J.; Pons, M.J.; Del Valle-Mendoza, J.; Vila, J. Emergence and spread of carbapenem-resistant Acinetobacter baumannii international clones II and III in Lima, Peru. Emerg. Microbes Infect. 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Gionco, B.; Pelayo, J.S.; Venancio, E.J.; Cayo, R.; Gales, A.C.; Carrara-Marroni, F.E. Detection of OXA-231, a new variant of blaOXA-143, in Acinetobacter baumannii from Brazil: A case report. J. Antimicrob. Chemother. 2012, 67, 2531–2532. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Lee, Y.; Lee, H.; Woo, G.J.; Song, W.; Kim, M.N.; Lee, W.G.; Jeong, S.H.; Lee, K.; Chong, Y. Prevalence and diversity of carbapenemases among imipenem-nonsusceptible Acinetobacter isolates in Korea: Emergence of a novel OXA-182. Diagn. Microbiol. Infect. Dis. 2010, 68, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Mostachio, A.K.; Levin, A.S.; Rizek, C.; Rossi, F.; Zerbini, J.; Costa, S.F. High prevalence of OXA-143 and alteration of outer membrane proteins in carbapenem-resistant Acinetobacter spp. isolates in Brazil. Int. J. Antimicrob. Agents 2012, 39, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Zander, E.; Bonnin, R.A.; Seifert, H.; Higgins, P.G. Characterization of blaOXA-143 variants in Acinetobacter baumannii and Acinetobacter pittii. Antimicrob. Agents Chemother. 2014, 58, 2704–2708. [Google Scholar] [CrossRef]
- Girlich, D.; Damaceno, Q.S.; Oliveira, A.C.; Nordmann, P. OXA-253, a variant of the carbapenem-hydrolyzing class D beta-lactamase OXA-143 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 2976–2978. [Google Scholar] [CrossRef]
- Higgins, P.G.; Perez-Llarena, F.J.; Zander, E.; Fernandez, A.; Bou, G.; Seifert, H. OXA-235, a novel class D beta-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 2121–2126. [Google Scholar] [CrossRef]
- Boyd, D.A.; Mataseje, L.F.; Pelude, L.; Mitchell, R.; Bryce, E.; Roscoe, D.; Embree, J.; Katz, K.; Kibsey, P.; Lavallee, C.; et al. Results from the Canadian Nosocomial Infection Surveillance Program for detection of carbapenemase-producing Acinetobacter spp. in Canadian hospitals, 2010–2016. J. Antimicrob. Chemother. 2019, 74, 315–320. [Google Scholar] [CrossRef]
- Buser, G.L.; Cassidy, P.M.; Cunningham, M.C.; Rudin, S.; Hujer, A.M.; Vega, R.; Furuno, J.P.; Marshall, S.H.; Higgins, P.G.; Jacobs, M.R.; et al. Failure to communicate: Transmission of extensively drug-resistant blaOXA-237-containing Acinetobacter baumannii - multiple facilities in Oregon, 2012-2014. Infect. Control. Hosp. Epidemiol. 2017, 38, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Hujer, A.M.; Higgins, P.G.; Rudin, S.D.; Buser, G.L.; Marshall, S.H.; Xanthopoulou, K.; Seifert, H.; Rojas, L.J.; Domitrovic, T.N.; Cassidy, P.M.; et al. Nosocomial outbreak of extensively drug-resistant Acinetobacter baumannii isolates containing blaOXA-237 carried on a plasmid. Antimicrob. Agents Chemother. 2017, 61, e00797-17. [Google Scholar] [CrossRef] [PubMed]
- Palzkill, T. Metallo-beta-lactamase structure and function. Ann. N. Y. Acad. Sci. 2013, 1277, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; Bonomo, R.A.; Fast, W. B1-Metallo-beta-Lactamases: Where Do We Stand? Curr. Drug Targets 2016, 17, 1029–1050. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-beta-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Nelson, K.; Rubio-Aparicio, D.; Tsivkovski, R.; Sun, D.; Dudley, M.N. The impact of intrinsic resistance mechanisms on potency of QPX7728, a new ultra-broad-spectrum beta-lactamase inhibitor of serine and metallo beta-lactamases in Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2020, AAC.00552-20. [Google Scholar] [CrossRef]
- Hamrick, J.C.; Docquier, J.D.; Uehara, T.; Myers, C.L.; Six, D.A.; Chatwin, C.L.; John, K.J.; Vernacchio, S.F.; Cusick, S.M.; Trout, R.E.L.; et al. VNRX-5133 (Taniborbactam), a broad-spectrum inhibitor of serine- and metallo-beta-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, e01963-19. [Google Scholar] [CrossRef]
- Krajnc, A.; Brem, J.; Hinchliffe, P.; Calvopina, K.; Panduwawala, T.D.; Lang, P.A.; Kamps, J.; Tyrrell, J.M.; Widlake, E.; Saward, B.G.; et al. Bicyclic boronate VNRX-5133 inhibits metallo- and serine-beta-lactamases. J. Med. Chem. 2019, 62, 8544–8556. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Khan, A.U.; Maryam, L.; Zarrilli, R. Structure, genetics and worldwide spread of New Delhi metallo-beta-lactamase (NDM): A threat to public health. BMC Microbiol. 2017, 17, 101. [Google Scholar] [CrossRef]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM metallo-beta-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019, 32, e00115–e00118. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Thirunarayan, M.A.; Krishnan, P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J. Antimicrob. Chemother. 2010, 65, 2253–2254. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Pasteran, F.; Traglia, G.M.; Martinez, J.; Huang, F.; Liu, C.; Fernandez, J.S.; Lopez, C.; Gonzalez, L.J.; Albornoz, E.; et al. Distinct mechanisms of dissemination of NDM-1 metallo-beta-lactamase in Acinetobacter species in Argentina. Antimicrob. Agents Chemother. 2020, 64, e00324-20. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Poirel, L.; Nordmann, P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed. Res. Int. 2014, 2014, 249856. [Google Scholar] [CrossRef]
- Groundwater, P.W.; Xu, S.; Lai, F.; Varadi, L.; Tan, J.; Perry, J.D.; Hibbs, D.E. New Delhi metallo-beta-lactamase-1: Structure, inhibitors and detection of producers. Future Med. Chem. 2016, 8, 993–1012. [Google Scholar] [CrossRef]
- Khalid, S.; Ahmad, N.; Ali, S.M.; Khan, A.U. Outbreak of efficiently transferred carbapenem-resistant blaNDM-producing gram-negative bacilli isolated from neonatal intensive care unit of an indian hospital. Microb Drug Resist. 2020, 26, 284–289. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Singh, K.; Pathak, A.; Prasad, K.N. Acinetobacter baumannii strain ASKPNAB1 Subclass B1 Metallo-Beta-Lactamase NDM-5 (blaNDM) Gene, blaNDM-5 Allele, Complete Cds. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MK682761.1 (accessed on 30 April 2020).
- Rogers, B.A.; Sidjabat, H.E.; Silvey, A.; Anderson, T.L.; Perera, S.; Li, J.; Paterson, D.L. Treatment options for New Delhi metallo-beta-lactamase-harboring enterobacteriaceae. Microb Drug Resist. 2013, 19, 100–103. [Google Scholar] [CrossRef]
- Abdul Rahim, N.; Cheah, S.E.; Johnson, M.D.; Yu, H.; Sidjabat, H.E.; Boyce, J.; Butler, M.S.; Cooper, M.A.; Fu, J.; Paterson, D.L.; et al. Synergistic killing of NDM-producing MDR Klebsiella pneumoniae by two ’old’ antibiotics-polymyxin B and chloramphenicol. J. Antimicrob. Chemother. 2015, 70, 2589–2597. [Google Scholar] [CrossRef]
- Linciano, P.; Cendron, L.; Gianquinto, E.; Spyrakis, F.; Tondi, D. Ten years with New Delhi metallo-beta-lactamase-1 (NDM-1): From structural insights to inhibitor design. ACS Infect. Dis 2019, 5, 9–34. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Analysis of the resistome of a multidrug-resistant NDM-1-producing Escherichia coli strain by high-throughput genome sequencing. Antimicrob. Agents Chemother. 2011, 55, 4224–4229. [Google Scholar] [CrossRef]
- Huang, T.W.; Wang, J.T.; Lauderdale, T.L.; Liao, T.L.; Lai, J.F.; Tan, M.C.; Lin, A.C.; Chen, Y.T.; Tsai, S.F.; Chang, S.C. Complete sequences of two plasmids in a blaNDM-1-positive Klebsiella oxytoca isolate from Taiwan. Antimicrob. Agents Chemother. 2013, 57, 4072–4076. [Google Scholar] [CrossRef] [PubMed]
- Wailan, A.M.; Paterson, D.L.; Kennedy, K.; Ingram, P.R.; Bursle, E.; Sidjabat, H.E. Genomic characteristics of NDM-producing Enterobacteriaceae isolates in Australia and their blaNDM genetic contexts. Antimicrob. Agents Chemother. 2016, 60, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Toleman, M.A.; Spencer, J.; Jones, L.; Walsh, T.R. blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 2773–2776. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Boulanger, A.; Schrenzel, J.; Kaase, M.; Nordmann, P. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Bontron, S.; Nordmann, P.; Poirel, L. Transposition of Tn125 encoding the NDM-1 carbapenemase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 7245–7251. [Google Scholar]
- Jones, L.S.; Toleman, M.A.; Weeks, J.L.; Howe, R.A.; Walsh, T.R.; Kumarasamy, K.K. Plasmid carriage of blaNDM-1 in clinical Acinetobacter baumannii isolates from India. Antimicrob. Agents Chemother. 2014, 58, 4211–4213. [Google Scholar] [CrossRef]
- McGann, P.; Hang, J.; Clifford, R.J.; Yang, Y.; Kwak, Y.I.; Kuschner, R.A.; Lesho, E.P.; Waterman, P.E. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob. Agents Chemother. 2012, 56, 1673–1679. [Google Scholar] [CrossRef]
- Sekizuka, T.; Matsui, M.; Yamane, K.; Takeuchi, F.; Ohnishi, M.; Hishinuma, A.; Arakawa, Y.; Kuroda, M. Complete sequencing of the bla(NDM-1)-positive IncA/C plasmid from Escherichia coli ST38 isolate suggests a possible origin from plant pathogens. PLoS ONE 2011, 6, e25334. [Google Scholar] [CrossRef]
- Wailan, A.M.; Sartor, A.L.; Zowawi, H.M.; Perry, J.D.; Paterson, D.L.; Sidjabat, H.E. Genetic contexts of blaNDM-1 in patients carrying multiple NDM-producing Strains. Antimicrob. Agents Chemother. 2015, 59, 7405–7410. [Google Scholar] [CrossRef]
- Hudson, C.M.; Bent, Z.W.; Meagher, R.J.; Williams, K.P. Resistance determinants and mobile genetic elements of an NDM-1-encoding Klebsiella pneumoniae strain. PLoS ONE 2014, 9, e99209. [Google Scholar] [CrossRef]
- Partridge, S.R.; Iredell, J.R. Genetic contexts of blaNDM-1. Antimicrob. Agents Chemother. 2012, 56, 6065–6067. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Jullien, E.; Courvalin, P. Nucleotide sequence of Acinetobacter baumannii aphA-6 gene: Evolutionary and functional implications of sequence homologies with nucleotide-binding proteins, kinases and other aminoglycoside-modifying enzymes. Mol. Microbiol. 1988, 2, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hao, Q. Crystal structure of NDM-1 reveals a common beta-lactam hydrolysis mechanism. FASEB J. 2011, 25, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Strynadka, N. Crystal structure of New Delhi metallo-beta-lactamase reveals molecular basis for antibiotic resistance. Protein Sci. 2011, 20, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Tesar, C.; Mire, J.; Jedrzejczak, R.; Binkowski, A.; Babnigg, G.; Sacchettini, J.; Joachimiak, A. Structure of apo- and monometalated forms of NDM-1--a highly potent carbapenem-hydrolyzing metallo-beta-lactamase. PLoS ONE 2011, 6, e24621. [Google Scholar]
- Lisa, M.N.; Palacios, A.R.; Aitha, M.; Gonzalez, M.M.; Moreno, D.M.; Crowder, M.W.; Bonomo, R.A.; Spencer, J.; Tierney, D.L.; Llarrull, L.I.; et al. A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-beta-lactamases. Nat. Commun. 2017, 8, 538. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, D. New Delhi metallo-beta-lactamase I: Substrate binding and catalytic mechanism. J. Phys. Chem. B 2013, 117, 11596–11607. [Google Scholar] [CrossRef]
- Kim, Y.; Cunningham, M.A.; Mire, J.; Tesar, C.; Sacchettini, J.; Joachimiak, A. NDM-1, the ultimate promiscuous enzyme: Substrate recognition and catalytic mechanism. FASEB J. 2013, 27, 1917–1927. [Google Scholar] [CrossRef]
- Kozlyuk, N.; Monteith, A.J.; Garcia, V.; Damo, S.M.; Skaar, E.P.; Chazin, W.J. S100 Proteins in the Innate Immune Response to Pathogens. Methods Mol. Biol. 2019, 1929, 275–290. [Google Scholar]
- Diaz-Ochoa, V.E.; Jellbauer, S.; Klaus, S.; Raffatellu, M. Transition metal ions at the crossroads of mucosal immunity and microbial pathogenesis. Front. Cell Infect. Microbiol. 2014, 4, 2. [Google Scholar] [CrossRef]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Meini, M.R.; Tomatis, P.E.; Medrano Martin, F.J.; Cricco, J.A.; Vila, A.J. Metallo-beta-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions. Nat. Chem. Biol. 2012, 8, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.J.; Bahr, G.; Nakashige, T.G.; Nolan, E.M.; Bonomo, R.A.; Vila, A.J. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-beta-lactamase. Nat. Chem. Biol. 2016, 12, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Ayala, J.A.; Bonomo, R.A.; Gonzalez, L.J.; Vila, A.J. Protein determinants of dissemination and host specificity of metallo-beta-lactamases. Nat. Commun. 2019, 10, 3617. [Google Scholar] [CrossRef]
- Lauretti, L.; Riccio, M.L.; Mazzariol, A.; Cornaglia, G.; Amicosante, G.; Fontana, R.; Rossolini, G.M. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999, 43, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Naas, T.; Nicolas, D.; Collet, L.; Bellais, S.; Cavallo, J.D.; Nordmann, P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 2000, 44, 891–897. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Rabine, S.; Hackel, M.; McLaughlin, R.E.; Biedenbach, D.J.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Multiyear, multinational survey of the incidence and global distribution of metallo-beta-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 1067–1078. [Google Scholar] [CrossRef]
- Matsumura, Y.; Peirano, G.; Devinney, R.; Bradford, P.A.; Motyl, M.R.; Adams, M.D.; Chen, L.; Kreiswirth, B.; Pitout, J.D.D. Genomic epidemiology of global VIM-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2017, 72, 2249–2258. [Google Scholar] [CrossRef]
- Lee, K.; Lee, W.G.; Uh, Y.; Ha, G.Y.; Cho, J.; Chong, Y.; Korean Nationwide Surveillance of Antimicrobial Resistance Group. VIM- and IMP-type metallo-beta-lactamase-producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg. Infect. Dis. 2003, 9, 868–871. [Google Scholar] [CrossRef]
- Yum, J.H.; Yi, K.; Lee, H.; Yong, D.; Lee, K.; Kim, J.M.; Rossolini, G.M.; Chong, Y. Molecular characterization of metallo-beta-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: Identification of two new integrons carrying the bla(VIM-2) gene cassettes. J. Antimicrob. Chemother. 2002, 49, 837–840. [Google Scholar] [CrossRef]
- Tsakris, A.; Ikonomidis, A.; Pournaras, S.; Tzouvelekis, L.S.; Sofianou, D.; Legakis, N.J.; Maniatis, A.N. VIM-1 metallo-beta-lactamase in Acinetobacter baumannii. Emerg. Infect. Dis. 2006, 12, 981–983. [Google Scholar] [CrossRef] [PubMed]
- El-Ageery, S.M.; Al-Hazmi, S.S. Microbiological and molecular detection of VIM-1 metallo beta lactamase-producing Acinetobacter baumannii. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 965–970. [Google Scholar] [PubMed]
- Beigverdi, R.; Sattari-Maraji, A.; Emaneini, M.; Jabalameli, F. Status of carbapenem-resistant Acinetobacter baumannii harboring carbapenemase: First systematic review and meta-analysis from Iran. Infect. Genet. Evol. 2019, 73, 433–443. [Google Scholar] [CrossRef]
- Davoodi, S.; Boroumand, M.A.; Sepehriseresht, S.; Pourgholi, L. Detection of VIM- and IMP-type metallo-beta-lactamase genes in Acinetobacter baumannii isolates from patients in two hospitals in Tehran. Iran. J. Biotechnol. 2015, 13, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Fazeli, H.; Halaji, M.; Moghadampour, M.; Faghri, J. Prevalence of metallo-beta-lactamase producing Acinetobacter baumannii isolated from intensive care unit in tertiary care hospitals. Ann. Ig. 2018, 30, 330–336. [Google Scholar] [PubMed]
- Lee, M.F.; Peng, C.F.; Hsu, H.J.; Chen, Y.H. Molecular characterisation of the metallo-beta-lactamase genes in imipenem-resistant Gram-negative bacteria from a university hospital in southern Taiwan. Int. J. Antimicrob. Agents 2008, 32, 475–480. [Google Scholar] [CrossRef]
- Peymani, A.; Nahaei, M.R.; Farajnia, S.; Hasani, A.; Mirsalehian, A.; Sohrabi, N.; Abbasi, L. High prevalence of metallo-beta-lactamase-producing Acinetobacter baumannii in a teaching hospital in Tabriz, Iran. Jpn. J. Infect. Dis. 2011, 64, 69–71. [Google Scholar]
- El-Badawy, M.F.; Abdelwahab, S.F.; Alghamdi, S.A.; Shohayeb, M.M. Characterization of phenotypic and genotypic traits of carbapenem-resistant Acinetobacter baumannii clinical isolates recovered from a tertiary care hospital in Taif, Saudi Arabia. Infect. Drug Resist. 2019, 12, 3113–3124. [Google Scholar] [CrossRef]
- Benmahmod, A.B.; Said, H.S.; Ibrahim, R.H. Prevalence and mechanisms of carbapenem resistance among Acinetobacter baumannii clinical isolates in Egypt. Microb. Drug Resist. 2019, 25, 480–488. [Google Scholar] [CrossRef]
- Azimi, L.; Talebi, M.; Pourshafie, M.R.; Owlia, P.; Rastegar Lari, A. Characterization of carbapenemases in extensively drug resistant Acinetobacter baumannii in a burn care center in Iran. Int. J. Mol Cell Med. 2015, 4, 46–53. [Google Scholar]
- AlAmri, A.M.; AlQurayan, A.M.; Sebastian, T.; AlNimr, A.M. Molecular surveillance of multidrug-resistant Acinetobacter baumannii. Curr. Microbiol. 2020, 77, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Girija, S.A.; Jayaseelan, V.P.; Arumugam, P. Prevalence of VIM- and GIM-producing Acinetobacter baumannii from patients with severe urinary tract infection. Acta Microbiol. Immunol. Hung. 2018, 65, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Navidifar, T.; Saleh Shooshtari, F.; Goodarzi, H. Association of the genes encoding metallo-beta-lactamase with the presence of integrons among multidrug-resistant clinical isolates of Acinetobacter baumannii. Infect. Drug Resist. 2019, 12, 1171–1180. [Google Scholar] [CrossRef]
- Watanabe, M.; Iyobe, S.; Inoue, M.; Mitsuhashi, S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1991, 35, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Osano, E.; Arakawa, Y.; Wacharotayankun, R.; Ohta, M.; Horii, T.; Ito, H.; Yoshimura, F.; Kato, N. Molecular characterization of an enterobacterial metallo beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 1994, 38, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y.; Murakami, M.; Suzuki, K.; Ito, H.; Wacharotayankun, R.; Ohsuka, S.; Kato, N.; Ohta, M. A novel integron-like element carrying the metallo-beta-lactamase gene blaIMP. Antimicrob. Agents Chemother. 1995, 39, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Arakawa, Y.; Ohsuka, S.; Wacharotayankun, R.; Kato, N.; Ohta, M. Plasmid-mediated dissemination of the metallo-beta-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob. Agents Chemother. 1995, 39, 824–829. [Google Scholar] [CrossRef]
- Hirakata, Y.; Izumikawa, K.; Yamaguchi, T.; Takemura, H.; Tanaka, H.; Yoshida, R.; Matsuda, J.; Nakano, M.; Tomono, K.; Maesaki, S.; et al. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-beta-lactamase gene blaIMP. Antimicrob. Agents Chemother. 1998, 42, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.H.; Babini, G.S.; Woodford, N.; Sng, L.H.; Hall, L.M.; Livermore, D.M. Carbapenem-hydrolysing IMP-1 beta-lactamase in Klebsiella pneumoniae from Singapore. Lancet 1999, 353, 2162. [Google Scholar] [CrossRef]
- Senda, K.; Arakawa, Y.; Nakashima, K.; Ito, H.; Ichiyama, S.; Shimokata, K.; Kato, N.; Ohta, M. Multifocal outbreaks of metallo-beta-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum beta-lactams, including carbapenems. Antimicrob. Agents Chemother. 1996, 40, 349–353. [Google Scholar] [CrossRef]
- Riccio, M.L.; Franceschini, N.; Boschi, L.; Caravelli, B.; Cornaglia, G.; Fontana, R.; Amicosante, G.; Rossolini, G.M. Characterization of the metallo-beta-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of bla(IMP) allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 2000, 44, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Cornaglia, G.; Riccio, M.L.; Mazzariol, A.; Lauretti, L.; Fontana, R.; Rossolini, G.M. Appearance of IMP-1 metallo-beta-lactamase in Europe. Lancet 1999, 353, 899–900. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Parenteau, T.R.; Centron, D.; Tolmasky, M.E. Functional characterization of Tn1331 gene cassettes. J. Antimicrob. Chemother. 2008, 62, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Sarno, R.; McGillivary, G.; Sherratt, D.J.; Actis, L.A.; Tolmasky, M.E. Complete nucleotide sequence of Klebsiella pneumoniae multiresistance plasmid pJHCMW1. Antimicrob. Agents Chemother. 2002, 46, 3422–3427. [Google Scholar] [CrossRef]
- Tolmasky, M.E.; Crosa, J.H. Tn1331, a novel multiresistance transposon encoding resistance to amikacin and ampicillin in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 1987, 31, 1955–1960. [Google Scholar] [CrossRef]
- Casin, I.; Hanau-Bercot, B.; Podglajen, I.; Vahaboglu, H.; Collatz, E. Salmonella enterica serovar Typhimurium bla(PER-1)-carrying plasmid pSTI1 encodes an extended-spectrum aminoglycoside 6’-N-acetyltransferase of type Ib. Antimicrob. Agents Chemother. 2003, 47, 697–703. [Google Scholar] [CrossRef]
- Dery, K.J.; Soballe, B.; Witherspoon, M.S.; Bui, D.; Koch, R.; Sherratt, D.J.; Tolmasky, M.E. The aminoglycoside 6’-N-acetyltransferase type Ib encoded by Tn1331 is evenly distributed within the cell’s cytoplasm. Antimicrob. Agents Chemother. 2003, 47, 2897–2902. [Google Scholar] [CrossRef]
- Nobuta, K.; Tolmasky, M.E.; Crosa, L.M.; Crosa, J.H. Sequencing and expression of the 6’-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J. Bacteriol. 1988, 170, 3769–3773. [Google Scholar] [CrossRef]
- Da Silva, G.J.; Correia, M.; Vital, C.; Ribeiro, G.; Sousa, J.C.; Leitao, R.; Peixe, L.; Duarte, A. Molecular characterization of bla(IMP-5), a new integron-borne metallo-beta-lactamase gene from an Acinetobacter baumannii nosocomial isolate in Portugal. FEMS Microbiol. Lett. 2002, 215, 33–39. [Google Scholar]
- Iyobe, S.; Kusadokoro, H.; Ozaki, J.; Matsumura, N.; Minami, S.; Haruta, S.; Sawai, T.; O’Hara, K. Amino acid substitutions in a variant of IMP-1 metallo-beta-lactamase. Antimicrob. Agents Chemother. 2000, 44, 2023–2027. [Google Scholar] [CrossRef]
- Chu, Y.W.; Afzal-Shah, M.; Houang, E.T.; Palepou, M.I.; Lyon, D.J.; Woodford, N.; Livermore, D.M. IMP-4, a novel metallo-beta-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 2001, 45, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M.; Xiong, J.; Ye, H.; Li, H.; M’Zali, F.H. Occurrence of a new metallo-beta-lactamase IMP-4 carried on a conjugative plasmid in Citrobacter youngae from the People’s Republic of China. FEMS Microbiol. Lett. 2001, 194, 53–57. [Google Scholar] [PubMed]
- Koh, T.H.; Sng, L.H.; Wang, G.C.; Hsu, L.Y.; Zhao, Y. IMP-4 and OXA beta-lactamases in Acinetobacter baumannii from Singapore. J. Antimicrob. Chemother. 2007, 59, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Ginn, A.N.; Paulsen, I.T.; Iredell, J.R. pEl1573 Carrying blaIMP-4, from Sydney, Australia, is closely related to other IncL/M plasmids. Antimicrob. Agents Chemother. 2012, 56, 6029–6032. [Google Scholar] [CrossRef]
- Sidjabat, H.E.; Heney, C.; George, N.M.; Nimmo, G.R.; Paterson, D.L. Interspecies transfer of blaIMP-4 in a patient with prolonged colonization by IMP-4-producing Enterobacteriaceae. J. Clin. Microbiol. 2014, 52, 3816–3818. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Franklin, C.; Walters, L.J.; Bell, J.M.; Spelman, D.W. OXA-58 and IMP-4 carbapenem-hydrolyzing beta-lactamases in an Acinetobacter junii blood culture isolate from Australia. Antimicrob. Agents Chemother. 2006, 50, 399–400. [Google Scholar] [CrossRef]
- Azizi, O.; Shakibaie, M.R.; Badmasti, F.; Modarresi, F.; Ramazanzadeh, R.; Mansouri, S.; Shahcheraghi, F. Class 1 integrons in non-clonal multidrug-resistant Acinetobacter baumannii from Iran, description of the new blaIMP-55 allele in In1243. J. Med. Microbiol. 2016, 65, 928–936. [Google Scholar] [CrossRef]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef]
- Miriagou, V.; Tzouvelekis, L.S.; Rossiter, S.; Tzelepi, E.; Angulo, F.J.; Whichard, J.M. Imipenem resistance in a Salmonella clinical strain due to plasmid-mediated class A carbapenemase KPC-2. Antimicrob. Agents Chemother. 2003, 47, 1297–1300. [Google Scholar] [CrossRef]
- Smith Moland, E.; Hanson, N.D.; Herrera, V.L.; Black, J.A.; Lockhart, T.J.; Hossain, A.; Johnson, J.A.; Goering, R.V.; Thomson, K.S. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 2003, 51, 711–714. [Google Scholar] [CrossRef]
- Woodford, N.; Tierno, P.M., Jr.; Young, K.; Tysall, L.; Palepou, M.F.; Ward, E.; Painter, R.E.; Suber, D.F.; Shungu, D.; Silver, L.L.; et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob. Agents Chemother. 2004, 48, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Landman, D.; Ghitan, M.; Collins, B.; Bratu, S.; Quale, J. Rise and fall of KPC-producing Klebsiella pneumoniae in New York City. J. Antimicrob. Chemother. 2016, 71, 2945–2948. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, K.M.; Biedenbach, D.J.; Hackel, M.; Rabine, S.; de Jonge, B.L.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob. Agents Chemother. 2016, 60, 4490–4500. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Nordmann, P.; Vedel, G.; Poyart, C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob. Agents Chemother. 2005, 49, 4423–4424. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Xie, G.; Marshall, S.H.; Hujer, K.M.; Chain, P.S.; Bonomo, R.A.; Tolmasky, M.E. Multidrug-resistant (MDR) Klebsiella pneumoniae clinical isolates: A zone of high heterogeneity (HHZ) as a tool for epidemiological studies. Clin. Microbiol. Infect. 2012, 18, E254–E258. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Xie, G.; Traglia, G.M.; Johnson, S.L.; Davenport, K.W.; van Duin, D.; Ramazani, A.; Perez, F.; Jacobs, M.R.; Sherratt, D.J.; et al. Whole-genome comparative analysis of two carbapenem-resistant ST-258 Klebsiella pneumoniae strains Isolated during a North-eastern Ohio outbreak: Differences within the high heterogeneity zones. Genome Biol. Evol. 2016, 8, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Park, S.O.; Liu, J.; Furuya, E.Y.; Larson, E.L. Carbapenem-resistant Klebsiella pneumoniae infection in three New York City hospitals trended downwards from 2006 to 2014. Open Forum Infect. Dis 2016, 3, ofw222. [Google Scholar] [CrossRef]
- Naas, T.; Cuzon, G.; Villegas, M.V.; Lartigue, M.F.; Quinn, J.P.; Nordmann, P. Genetic structures at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob. Agents Chemother. 2008, 52, 1257–1263. [Google Scholar] [CrossRef]
- Leavitt, A.; Chmelnitsky, I.; Ofek, I.; Carmeli, Y.; Navon-Venezia, S. Plasmid pKpQIL encoding KPC-3 and TEM-1 confers carbapenem resistance in an extremely drug-resistant epidemic Klebsiella pneumoniae strain. J. Antimicrob. Chemother. 2010, 65, 243–248. [Google Scholar] [CrossRef]
- Gootz, T.D.; Lescoe, M.K.; Dib-Hajj, F.; Dougherty, B.A.; He, W.; Della-Latta, P.; Huard, R.C. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 2009, 53, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chavda, K.D.; Al Laham, N.; Melano, R.G.; Jacobs, M.R.; Bonomo, R.A.; Kreiswirth, B.N. Complete nucleotide sequence of a blaKPC-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 2013, 57, 5019–5025. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.L.; Cheung, Y.Y.; Lo, W.U.; Li, Z.; Chow, K.H.; Lin, C.H.; Chan, J.F.; Cheng, V.C. Molecular characterization of an atypical IncX3 plasmid pKPC-NY79 carrying blaKPC-2 in a Klebsiella pneumoniae. Curr. Microbiol. 2013, 67, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R. Tn4401 carrying blaKPC is inserted within another insertion in pKpQIL and related plasmids. J. Clin. Microbiol. 2014, 52, 4448–4449. [Google Scholar] [CrossRef]
- Rice, L.B.; Carias, L.L.; Hutton, R.A.; Rudin, S.D.; Endimiani, A.; Bonomo, R.A. The KQ element, a complex genetic region conferring transferable resistance to carbapenems, aminoglycosides, and fluoroquinolones in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2008, 52, 3427–3429. [Google Scholar] [CrossRef]
- Souza, R.C.; Dabul, A.N.G.; Boralli, C.; Zuvanov, L.; Camargo, I. Dissemination of blaKPC-2 in an NTEKPC by an IncX5 plasmid. Plasmid 2019, 106, 102446. [Google Scholar] [CrossRef]
- Chen, L.; Mathema, B.; Chavda, K.D.; DeLeo, F.R.; Bonomo, R.A.; Kreiswirth, B.N. Carbapenemase-producing Klebsiella pneumoniae: Molecular and genetic decoding. Trends Microbiol. 2014, 22, 686–696. [Google Scholar] [CrossRef]
- Wang, L.; Fang, H.; Feng, J.; Yin, Z.; Xie, X.; Zhu, X.; Wang, J.; Chen, W.; Yang, R.; Du, H.; et al. Complete sequences of KPC-2-encoding plasmid p628-KPC and CTX-M-55-encoding p628-CTXM coexisted in Klebsiella pneumoniae. Front. Microbiol. 2015, 6, 838. [Google Scholar] [CrossRef]
- Azimi, L.; Talebi, M.; Khodaei, F.; Najafi, M.; Lari, A.R. Comparison of multiple-locus variable-number tandem-repeat analysis with pulsed-field gel electrophoresis typing of carbapenemases producing Acinetobacter baumannii isolated from burn patients. Burns 2016, 42, 441–445. [Google Scholar] [CrossRef]
- Lima, W.G.; Silva Alves, G.C.; Sanches, C.; Antunes Fernandes, S.O.; de Paiva, M.C. Carbapenem-resistant Acinetobacter baumannii in patients with burn injury: A systematic review and meta-analysis. Burns 2019, 45, 1495–1508. [Google Scholar] [CrossRef]
- Ribeiro, P.C.; Monteiro, A.S.; Marques, S.G.; Monteiro, S.G.; Monteiro-Neto, V.; Coqueiro, M.M.; Marques, A.C.; de Jesus Gomes Turri, R.; Santos, S.G.; Bomfim, M.R. Phenotypic and molecular detection of the blaKPC gene in clinical isolates from inpatients at hospitals in Sao Luis, MA, Brazil. BMC Infect. Dis. 2016, 16, 737. [Google Scholar] [CrossRef] [PubMed]
- Robledo, I.E.; Aquino, E.E.; Sante, M.I.; Santana, J.L.; Otero, D.M.; Leon, C.F.; Vazquez, G.J. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob. Agents Chemother. 2010, 54, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.C.; Cheng, Z.; Fast, W.; Bonomo, R.A.; Crowder, M.W. The continuing challenge of metallo-beta-lactamase inhibition: Mechanism matters. Trends Pharmacol. Sci. 2018, 39, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Tolmasky, M.E. Strategies to prolong the useful life of existing antibiotics and help overcoming the antibiotic resistance crisis. In Frontiers in Clinical Drug Research-Anti Infectives; Atta-ur-Rhaman, Ed.; Bentham Books: Sharjah, UAE, 2017; Volume 1, pp. 1–27. [Google Scholar]
- Papp-Wallace, K.M.; Bonomo, R.A. New beta-lactamase inhibitors in the clinic. Infect. Dis. Clin. North. Am. 2016, 30, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Noval, M.; Banoub, M.; Claeys, K.C.; Heil, E. The battle is on: New beta-lactams for the treatment of multidrug-resistant gram-negative organisms. Curr. Infect. Dis. Rep. 2020, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.; Neidig, N.; Campbell, A.; Thornsberry, T.; Truex, T.; Fortney, T.; Zhang, Y.; Bush, K. Activity of imipenem/relebactam against carbapenemase-producing Enterobacteriaceae with high colistin resistance. J. Antimicrob. Chemother. 2019, 74, 3260–3263. [Google Scholar] [CrossRef]
- Haidar, G.; Clancy, C.J.; Shields, R.K.; Hao, B.; Cheng, S.; Nguyen, M.H. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 2017, 61, e02534-16. [Google Scholar] [CrossRef]
- Porreca, A.M.; Sullivan, K.V.; Gallagher, J.C. The epidemiology, evolution, and treatment of KPC-producing organisms. Curr. Infect. Dis. Rep. 2018, 20, 13. [Google Scholar] [CrossRef]
- Doi, Y.; Murray, G.L.; Peleg, A.Y. Acinetobacter baumannii: Evolution of antimicrobial resistance-treatment options. Semin. Respir. Crit. Care Med. 2015, 36, 85–98. [Google Scholar]
- Traglia, G.M.; Chua, K.; Centron, D.; Tolmasky, M.E.; Ramirez, M.S. Whole-genome sequence analysis of the naturally competent Acinetobacter baumannii clinical isolate A118. Genome Biol. Evol. 2014, 6, 2235–2239. [Google Scholar] [CrossRef]
- Imperi, F.; Antunes, L.C.; Blom, J.; Villa, L.; Iacono, M.; Visca, P.; Carattoli, A. The genomics of Acinetobacter baumannii: Insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life 2011, 63, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Traglia, G.M.; Place, K.; Dotto, C.; Fernandez, J.S.; Montana, S.; Bahiense, C.D.S.; Soler-Bistue, A.; Iriarte, A.; Perez, F.; Tolmasky, M.E.; et al. Interspecies DNA acquisition by a naturally competent Acinetobacter baumannii strain. Int. J. Antimicrob. Agents 2019, 53, 483–490. [Google Scholar] [CrossRef] [PubMed]
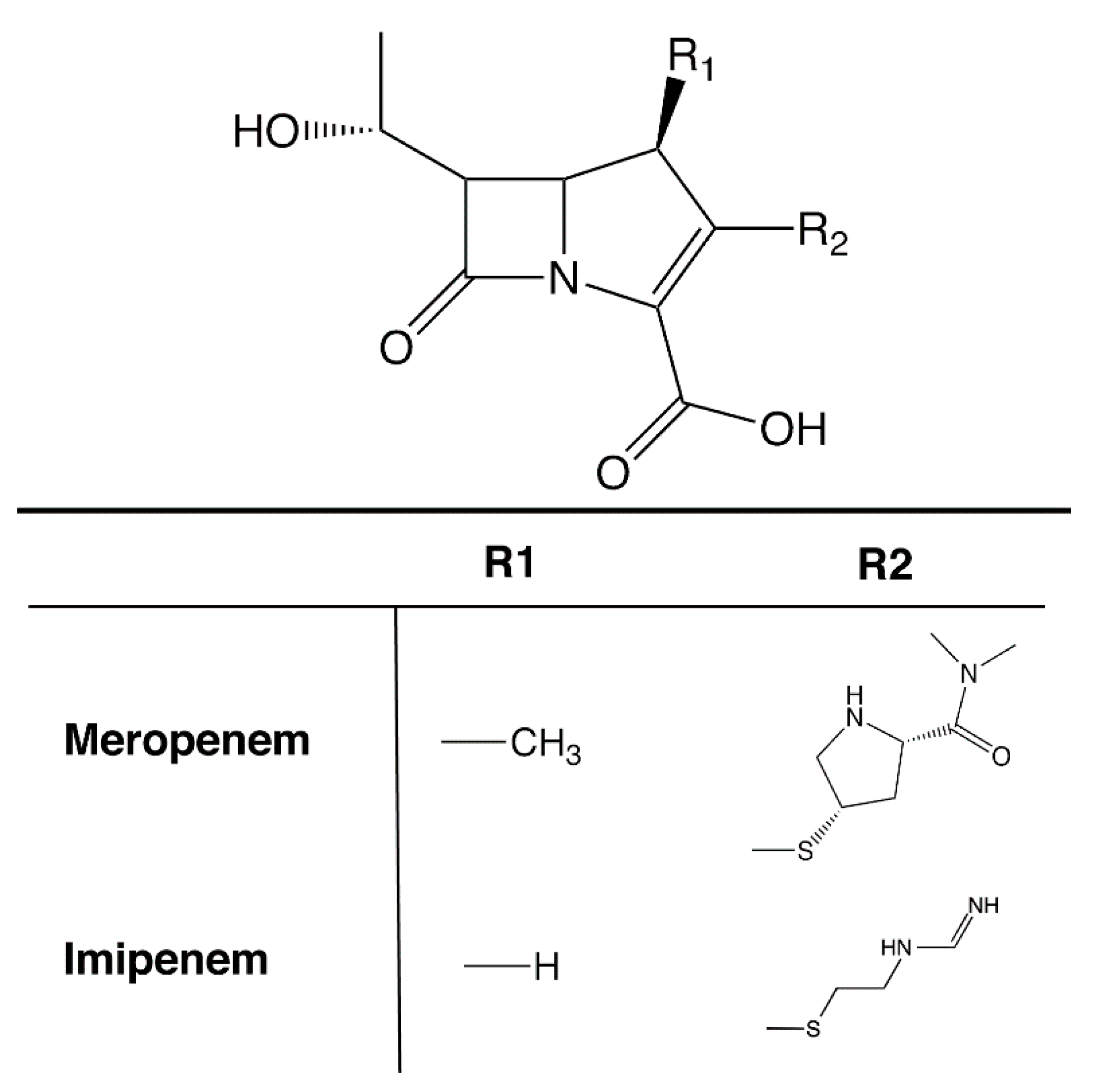
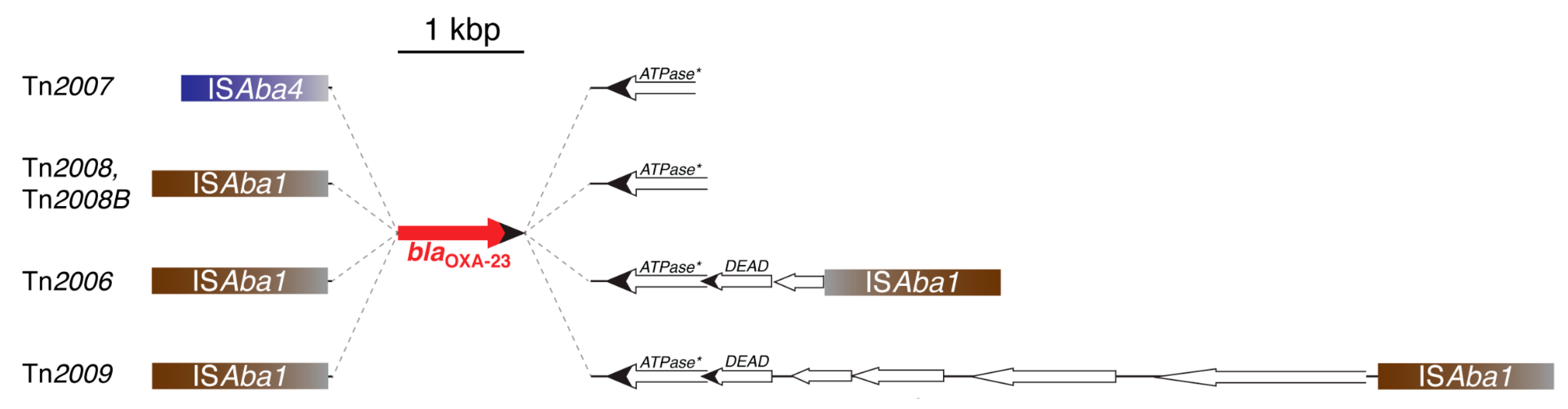
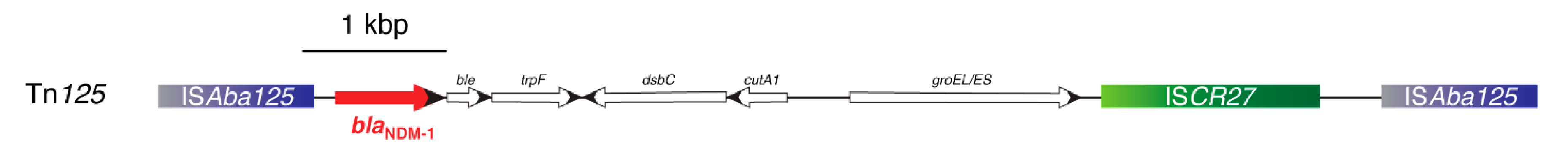
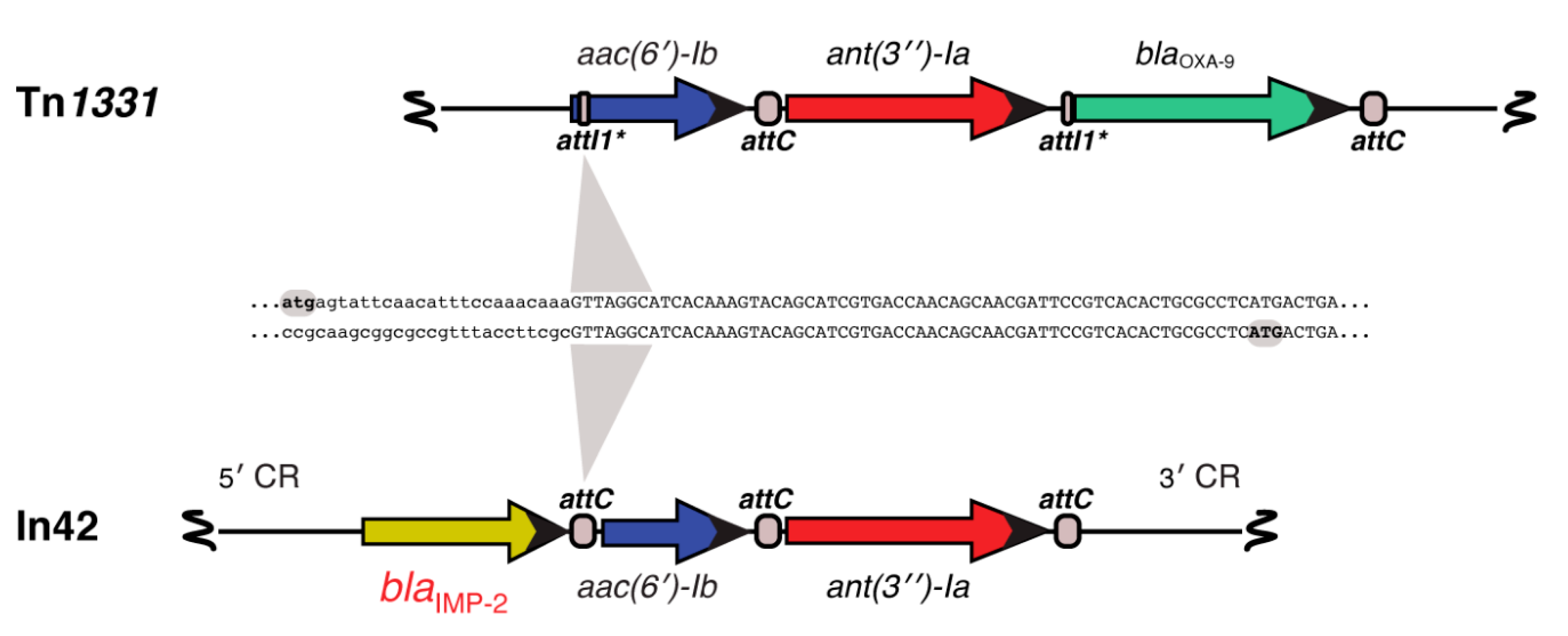
| Enzyme Group | Genetic Location | Predominant Isolation Countries * | Isolation Source | Other Reported Species | Total Reported |
|---|---|---|---|---|---|
| OXA-23-like | Plasmid, chromosome | USA (564), India (125), South Korea (122) | Clinical (2,830) Environmental/other (1128) | Providencia alcalifaciens, Proteus mirabilis, Klebsiella pneumoniae, Citrobacter freundii, E. coli/Shigella, Serratia marcescens, Acinetobacter non-baumannii, Pseudomonas aeruginosa | 4048 |
| OXA-24/40-like | USA (100), Spain (5) | Clinical (124) Environmental/other (21) | Acinetobacter non-baumannii, Klebsiella pneumoniae, Providencia rettgeri, Staphylococcus aureus | 162 | |
| OXA-51-like | Germany (8), Brazil (8), Japan (6) | Clinical (39) Environmental/other (20) | Acinetobacter non-baumannii, Klebsiella pneumoniae | 88 | |
| OXA-58-like | Plasmid, Chromosome | USA (84), Spain (12), Thailand (8) | Clinical (90) Environmental/other (177) | Providencia alcalifaciens, Klebsiella pneumoniae, E. coli/Shigella, Proteus mirabilis, Enterobacter sp., Acinetobacter non-baumannii. | 284 |
| OXA-143-like | Brazil (3) | Clinical (3) | Acinetobacter non-baumannii | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, M.S.; Bonomo, R.A.; Tolmasky, M.E. Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace. Biomolecules 2020, 10, 720. https://doi.org/10.3390/biom10050720
Ramirez MS, Bonomo RA, Tolmasky ME. Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace. Biomolecules. 2020; 10(5):720. https://doi.org/10.3390/biom10050720
Chicago/Turabian StyleRamirez, Maria Soledad, Robert A. Bonomo, and Marcelo E. Tolmasky. 2020. "Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace" Biomolecules 10, no. 5: 720. https://doi.org/10.3390/biom10050720
APA StyleRamirez, M. S., Bonomo, R. A., & Tolmasky, M. E. (2020). Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace. Biomolecules, 10(5), 720. https://doi.org/10.3390/biom10050720







