Tailoring Uptake Efficacy of HSV-1 gD Tailoring Uptake Efficacy of Hsv-1 GD Derived Carrier Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Peptide Synthesis
2.3. Stability Studies of Synthetic Peptides
2.4. Fluorescence Spectroscopy of the 5(6)-Carboxyfluorescein Labelled Peptide Derivatives
2.5. Secondary Structure Prediction
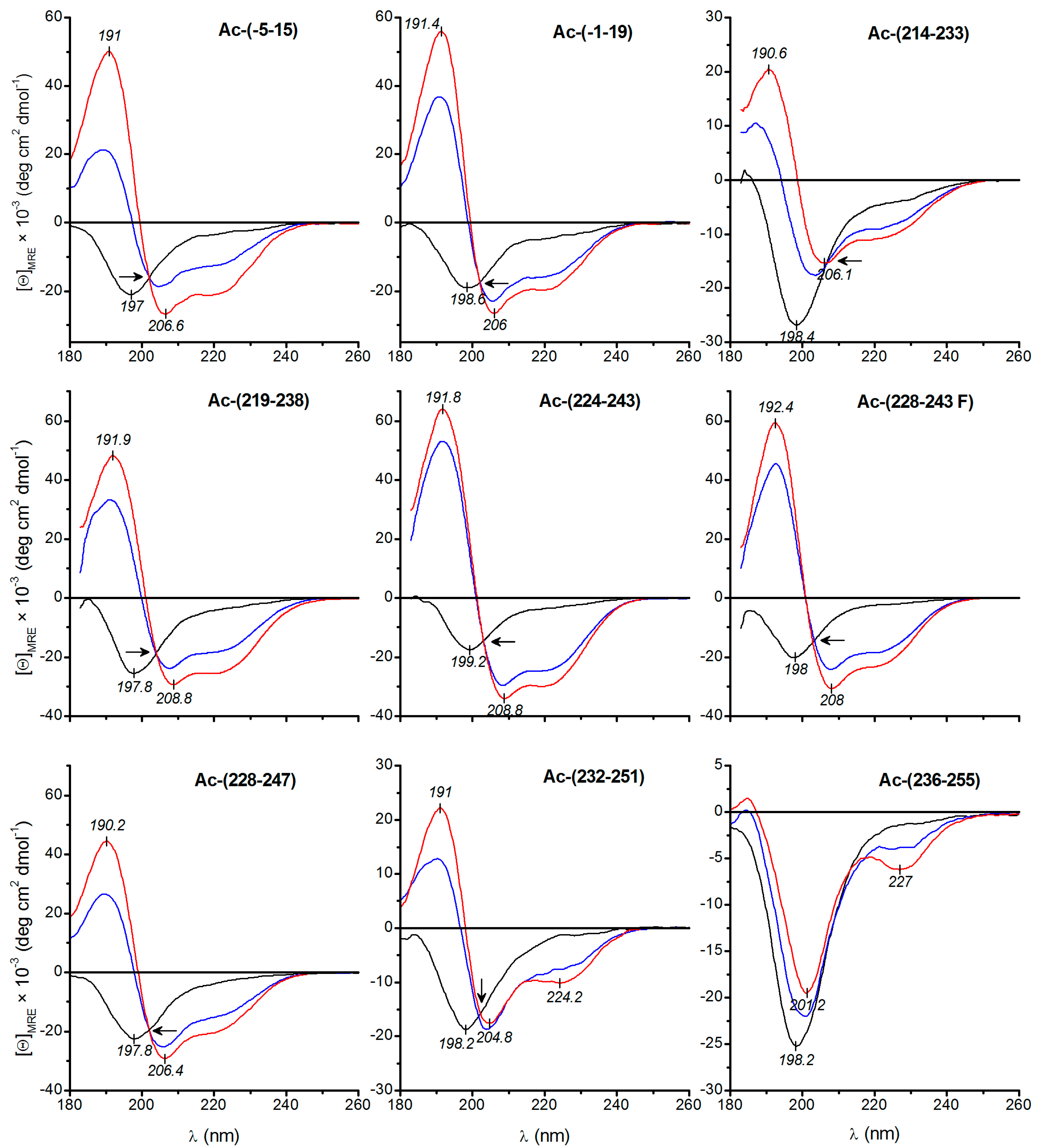
2.6. Electronic Circular Dichroism (ECD) Spectroscopy
2.7. Cell Culturing and Cellular Uptake Studies
2.8. Intracellular Localisation Studies using Confocal Laser Scanning Microscopy
3. Results
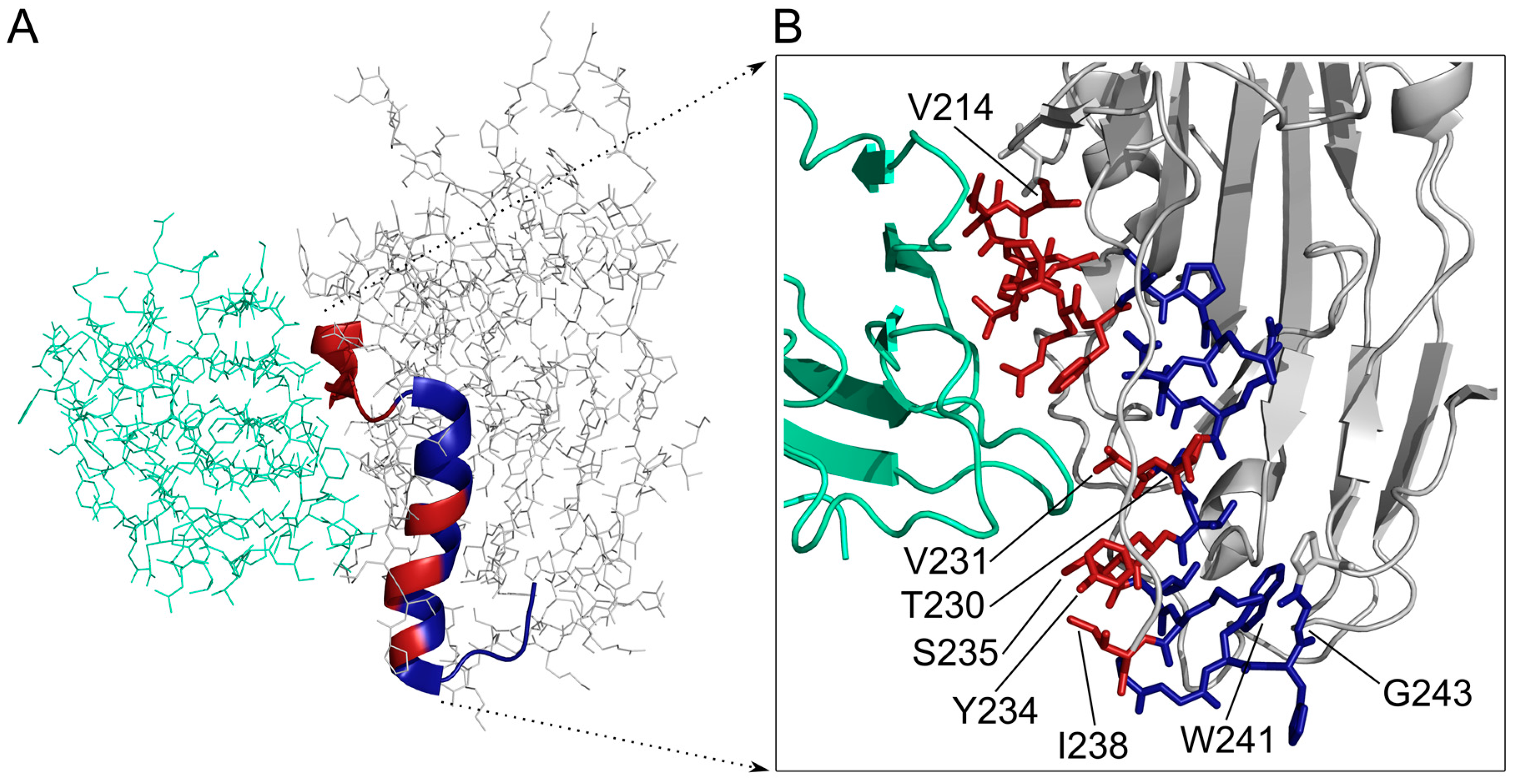
3.1. Synthesis and Characterisation of HSV-1 gD Peptides
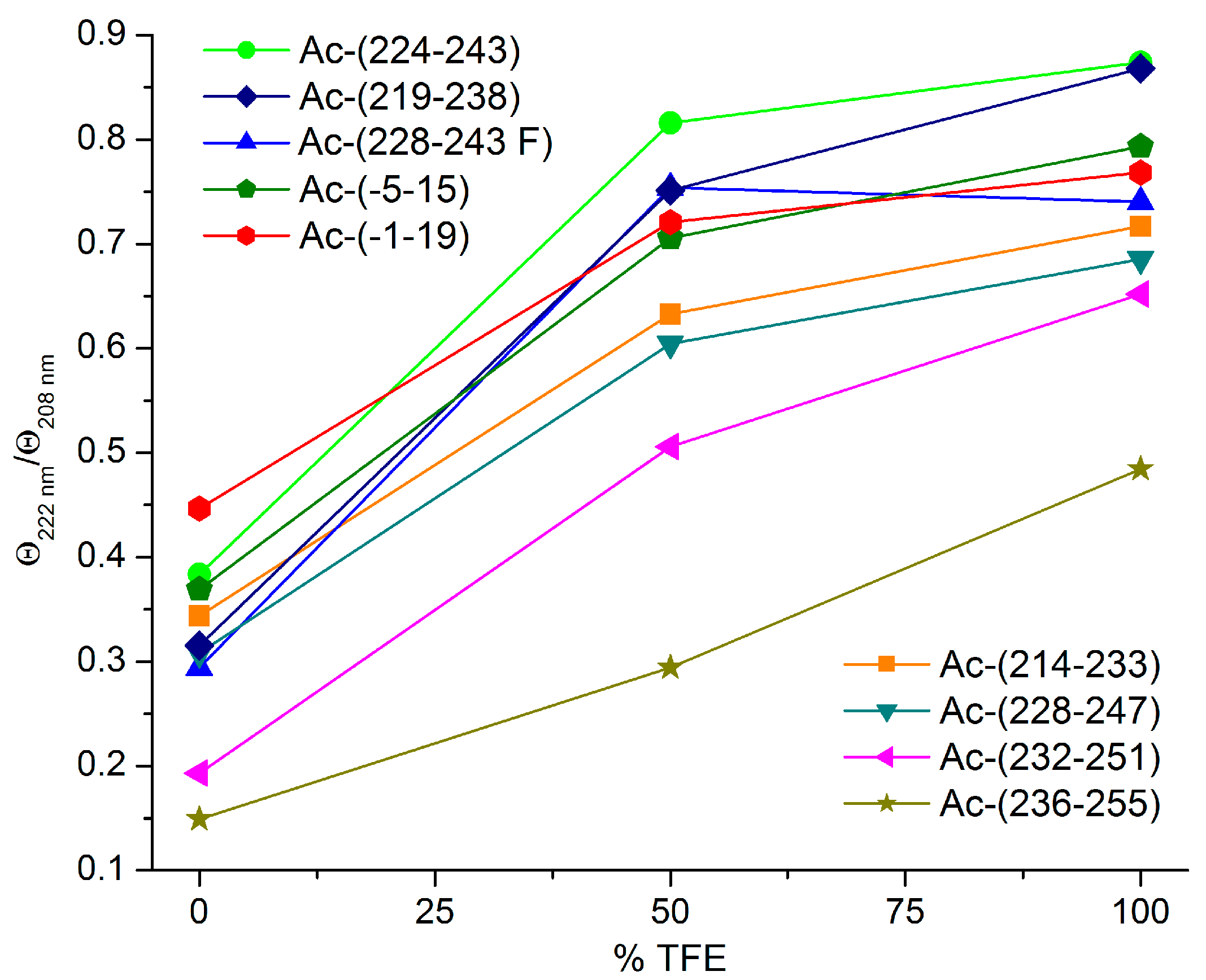
3.2. Secondary Structure Studies Using Electronic Circular Dichroism Spectroscopy
3.3. Cellular Uptake and Intracellular Localisation of HSV-1 gD Cf-Peptides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, M.; Sunderland, K.; Mao, C. Virus-Derived Peptides for Clinical Applications. Chem. Rev. 2017, 117, 10377–10402. [Google Scholar] [CrossRef] [PubMed]
- Vanova, J.; Hejtmankova, A.; Kalbacova, M.H.; Spanielova, H. The Utilization of Cell-Penetrating Peptides in the Intracellular Delivery of Viral Nanoparticles. Materials 2019, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.M.; Almeida Dias, S.; Flores, L.; Veiga, A.S.; Castanho, M.A. Mining viral proteins for antimicrobial and cell-penetrating drug delivery peptides. Bioinformatics 2015, 31, 2252–2256. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J. Herpes simplex viruses. In Filed Viruses, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; LWW: Philadelphia, PA, USA, 2001; pp. 2461–2509. [Google Scholar]
- Navaratnarajah, C.K.; Miest, T.S.; Carfi, A.; Cattaneo, R. Targeted entry of enveloped viruses: Measles and herpes simplex virus I. Curr. Opin. Virol. 2012, 2, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yan, J.; Lu, G.; Guo, Z.; Fan, Z.; Wang, J.; Shi, Y.; Qi, J.; Gao, G.F. Binding of herpes simplex virus glycoprotein D to nectin-1 exploits host cell adhesion. Nat. Commun. 2011, 2, 577. [Google Scholar] [CrossRef]
- Krummenacher, C.; Carfi, A.; Eisenberg, R.J.; Cohen, G.H. Entry of herpesviruses into cells: The enigma variations. Adv. Exp. Med. Biol. 2013, 790, 178–195. [Google Scholar] [CrossRef]
- Lazear, E.; Whitbeck, J.C.; Zuo, Y.; Carfi, A.; Cohen, G.H.; Eisenberg, R.J.; Krummenacher, C. Induction of conformational changes at the N-terminus of herpes simplex virus glycoprotein D upon binding to HVEM and nectin-1. Virology 2014, 448, 185–195. [Google Scholar] [CrossRef]
- Di Giovine, P.; Settembre, E.C.; Bhargava, A.K.; Luftig, M.A.; Lou, H.; Cohen, G.H.; Eisenberg, R.J.; Krummenacher, C.; Carfi, A. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 2011, 7, e1002277. [Google Scholar] [CrossRef]
- Carfi, A.; Gong, H.; Lou, H.; Willis, S.H.; Cohen, G.H.; Eisenberg, R.J.; Wiley, D.C. Crystallization and preliminary diffraction studies of the ectodomain of the envelope glycoprotein D from herpes simplex virus 1 alone and in complex with the ectodomain of the human receptor HveA. Acta Crystallographica. Sect. Dbiological Crystallogr. 2002, 58, 836–838. [Google Scholar] [CrossRef]
- Cairns, T.M.; Ditto, N.T.; Atanasiu, D.; Lou, H.; Brooks, B.D.; Saw, W.T.; Eisenberg, R.J.; Cohen, G.H. Surface Plasmon Resonance Reveals Direct Binding of Herpes Simplex Virus Glycoproteins gH/gL to gD and Locates a gH/gL Binding Site on gD. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Connolly, S.A.; Jackson, J.O.; Jardetzky, T.S.; Longnecker, R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat. Reviews. Microbiol. 2011, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, F.; Fusco, D.; Menotti, L.; Gianni, T.; Eisenberg, R.J.; Cohen, G.H.; Campadelli-Fiume, G. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 2004, 101, 7445–7450. [Google Scholar] [CrossRef] [PubMed]
- Krummenacher, C.; Baribaud, I.; Eisenberg, R.J.; Cohen, G.H. Cellular localization of nectin-1 and glycoprotein D during herpes simplex virus infection. J. Virol. 2003, 77, 8985–8999. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Falanga, A.; Capasso, D.; Guarnieri, D.; Correale, S.; Galdiero, M.; Netti, P.A.; Zollo, M.; Galdiero, S.; Di Gaetano, S.; et al. gH625 is a viral derived peptide for effective delivery of intrinsically disordered proteins. Int. J. Nanomed. 2013, 8, 2555–2565. [Google Scholar] [CrossRef][Green Version]
- Falanga, A.; Tarallo, R.; Carberry, T.; Galdiero, M.; Weck, M.; Galdiero, S. Elucidation of the interaction mechanism with liposomes of gH625-peptide functionalized dendrimers. PLoS ONE 2014, 9, e112128. [Google Scholar] [CrossRef] [PubMed]
- Horvati, K.; Bacsa, B.; Mlinko, T.; Szabo, N.; Hudecz, F.; Zsila, F.; Bosze, S. Comparative analysis of internalisation, haemolytic, cytotoxic and antibacterial effect of membrane-active cationic peptides: Aspects of experimental setup. Amino Acids 2017, 49, 1053–1067. [Google Scholar] [CrossRef]
- Kiss, E.; Gyulai, G.; Pari, E.; Horvati, K.; Bosze, S. Membrane affinity and fluorescent labelling: Comparative study of monolayer interaction, cellular uptake and cytotoxicity profile of carboxyfluorescein-conjugated cationic peptides. Amino Acids 2018, 50, 1557–1571. [Google Scholar] [CrossRef]
- Geysen, H.M.; Meloen, R.H.; Barteling, S.J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 1984, 81, 3998–4002. [Google Scholar] [CrossRef]
- Baranyai, Z.; Kratky, M.; Vosatka, R.; Szabo, E.; Senoner, Z.; David, S.; Stolarikova, J.; Vinsova, J.; Bosze, S. In vitro biological evaluation of new antimycobacterial salicylanilide-tuftsin conjugates. Eur. J. Med. Chem. 2017, 133, 152–173. [Google Scholar] [CrossRef]
- Lamiable, A.; Thevenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tuffery, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Maupetit, J.; Derreumaux, P.; Tuffery, P. A fast method for large-scale de novo peptide and miniprotein structure prediction. J. Comput. Chem. 2010, 31, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Reed, T.A. A set of constructed type spectra for the practical estimation of peptide secondary structure from circular dichroism. Anal. Biochem. 1997, 254, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Amon, M.A.; Ali, M.; Bender, V.; Hall, K.; Aguilar, M.I.; Aldrich-Wright, J.; Manolios, N. Kinetic and conformational properties of a novel T-cell antigen receptor transmembrane peptide in model membranes. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2008, 14, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Datki, Z.; Juhasz, A.; Galfi, M.; Soos, K.; Papp, R.; Zadori, D.; Penke, B. Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma cells. Brain Res. Bull. 2003, 62, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Datki, Z.; Papp, R.; Zadori, D.; Soos, K.; Fulop, L.; Juhasz, A.; Laskay, G.; Hetenyi, C.; Mihalik, E.; Zarandi, M.; et al. In vitro model of neurotoxicity of Abeta 1-42 and neuroprotection by a pentapeptide: Irreversible events during the first hour. Neurobiol. Dis. 2004, 17, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Biedler, J.L.; Helson, L.; Spengler, B.A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar]
- Biedler, J.L.; Roffler-Tarlov, S.; Schachner, M.; Freedman, L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978, 38, 3751–3757. [Google Scholar]
- Toniolo, C.; Formaggio, F.; Woody, R. Comprehensive Chiroptical Spectroscopy, Volume 2: Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules; Berova, N., Polavarapu, P., Nakanishi, K., Woody, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 2, pp. 499–544. [Google Scholar]
- Buck, M. Trifluoroethanol and colleagues: Cosolvents come of age. Recent studies with peptides and proteins. Q. Rev. Biophys. 1998, 31, 297–355. [Google Scholar] [CrossRef]
- Sanavio, B.; Piccoli, A.; Gianni, T.; Bertucci, C. Helicity propensity and interaction of synthetic peptides from heptad-repeat domains of herpes simplex virus 1 glycoprotein H: A circular dichroism study. Biochim. Et Biophys. Acta 2007, 1774, 781–791. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yang, J.T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem. Biophys. Res. Commun. 1971, 44, 1285–1291. [Google Scholar] [CrossRef]
- Choy, N.; Raussens, V.; Narayanaswami, V. Inter-molecular coiled-coil formation in human apolipoprotein E C-terminal domain. J. Mol. Biol. 2003, 334, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Alland, C.; Moreews, F.; Boens, D.; Carpentier, M.; Chiusa, S.; Lonquety, M.; Renault, N.; Wong, Y.; Cantalloube, H.; Chomilier, J.; et al. RPBS: A web resource for structural bioinformatics. Nucleic Acids Res. 2005, 33, W44–W49. [Google Scholar] [CrossRef] [PubMed]
- Neron, B.; Menager, H.; Maufrais, C.; Joly, N.; Maupetit, J.; Letort, S.; Carrere, S.; Tuffery, P.; Letondal, C. Mobyle: A new full web bioinformatics framework. Bioinformatics 2009, 25, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Thevenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tuffery, P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. [Google Scholar] [CrossRef]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tuffery, P. Improved PEP-FOLD Approach for Peptide and Miniprotein Structure Prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. [Google Scholar] [CrossRef]
- Smith, G. Herpesvirus transport to the nervous system and back again. Annu Rev. Microbiol 2012, 66, 153–176. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Kuny, C.V.; Szpara, M.L. Differentiated Human SH-SY5Y Cells Provide a Reductionist Model of Herpes Simplex Virus 1 Neurotropism. J. Virol 2017, 91. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- Yin, K.; Baillie, G.J.; Vetter, I. Neuronal cell lines as model dorsal root ganglion neurons: A transcriptomic comparison. Mol. Pain 2016, 12. [Google Scholar] [CrossRef]
- Mezo, G.; Kalaszi, A.; Remenyi, J.; Majer, Z.; Hilbert, A.; Lang, O.; Kohidai, L.; Barna, K.; Gaal, D.; Hudecz, F. Synthesis, conformation, and immunoreactivity of new carrier molecules based on repeated tuftsin-like sequence. Biopolymers 2004, 73, 645–656. [Google Scholar] [CrossRef]
- Bai, K.B.; Lang, O.; Orban, E.; Szabo, R.; Kohidai, L.; Hudecz, F.; Mezo, G. Design, synthesis, and in vitro activity of novel drug delivery systems containing tuftsin derivatives and methotrexate. Bioconjugate Chem. 2008, 19, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Horvati, K.; Bacsa, B.; Szabo, N.; David, S.; Mezo, G.; Grolmusz, V.; Vertessy, B.; Hudecz, F.; Bosze, S. Enhanced cellular uptake of a new, in silico identified antitubercular candidate by peptide conjugation. Bioconjugate Chem. 2012, 23, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Cichorek, M.; Ronowska, A.; Gensicka-Kowalewska, M.; Deptula, M.; Pelikant-Malecka, I.; Dzierzbicka, K. Novel therapeutic compound acridine-retrotuftsin action on biological forms of melanoma and neuroblastoma. J. Cancer Res. Clin. Oncol. 2019, 145, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.L.; Dowdy, S.F. Cell penetrating peptides in drug delivery. Pharm. Res. 2004, 21, 389–393. [Google Scholar] [CrossRef]
- Vives, E.; Schmidt, J.; Pelegrin, A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Et Biophys. Acta 2008, 1786, 126–138. [Google Scholar] [CrossRef]
- Reissmann, S. Cell penetration: Scope and limitations by the application of cell-penetrating peptides. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2014, 20, 760–784. [Google Scholar] [CrossRef]
- Guo, Z.; Peng, H.; Kang, J.; Sun, D. Cell-penetrating peptides: Possible transduction mechanisms and therapeutic applications. Biomed. Rep. 2016, 4, 528–534. [Google Scholar] [CrossRef]
- Henriques, S.T.; Melo, M.N.; Castanho, M.A. Cell-penetrating peptides and antimicrobial peptides: How different are they? Biochem. J. 2006, 399, 1–7. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar]
- Cartier, R.; Reszka, R. Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems. Gene Ther. 2002, 9, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D. Homology, similarity, and identity in peptide epitope immunodefinition. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2012, 18, 487–494. [Google Scholar] [CrossRef] [PubMed]
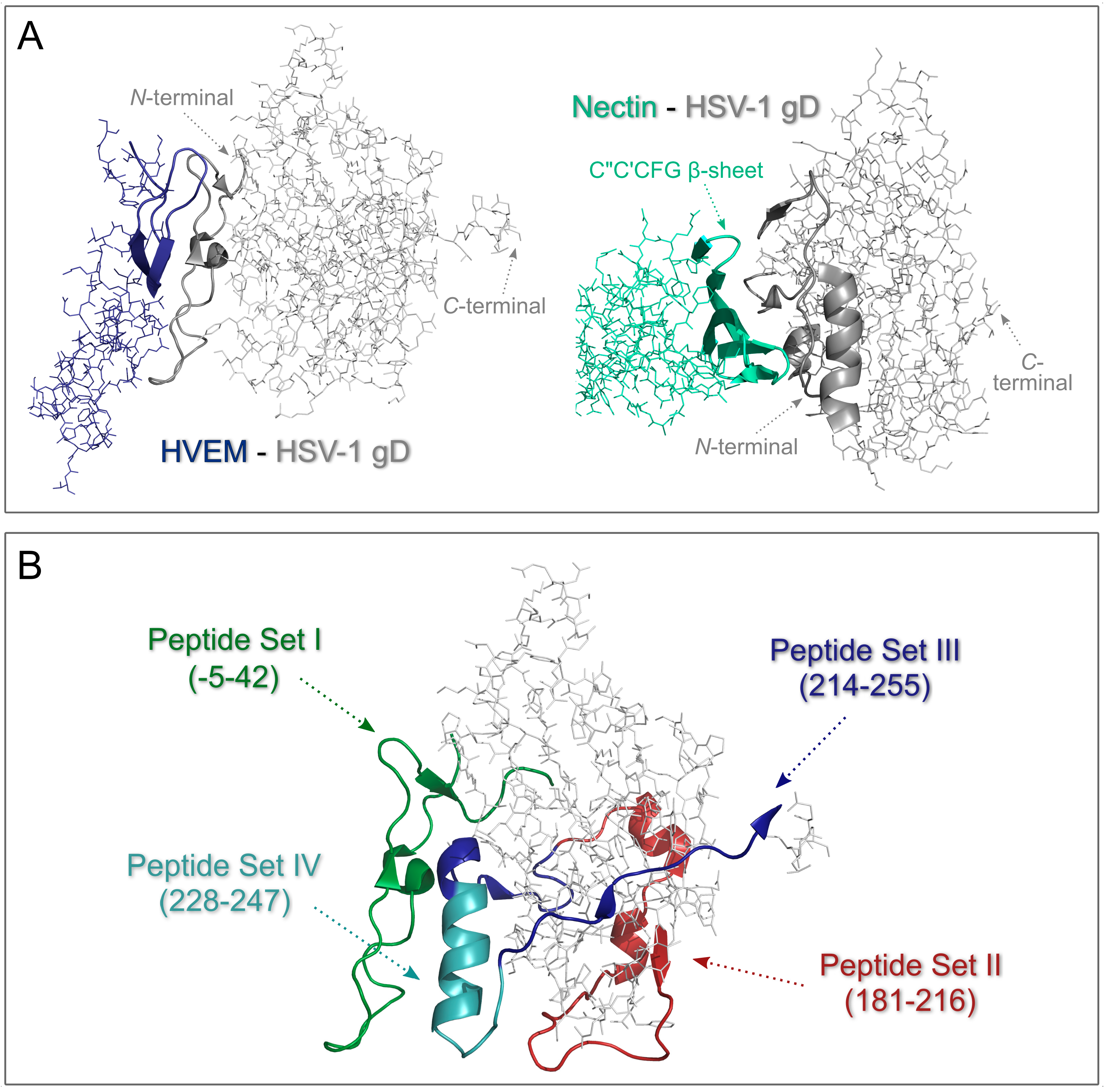
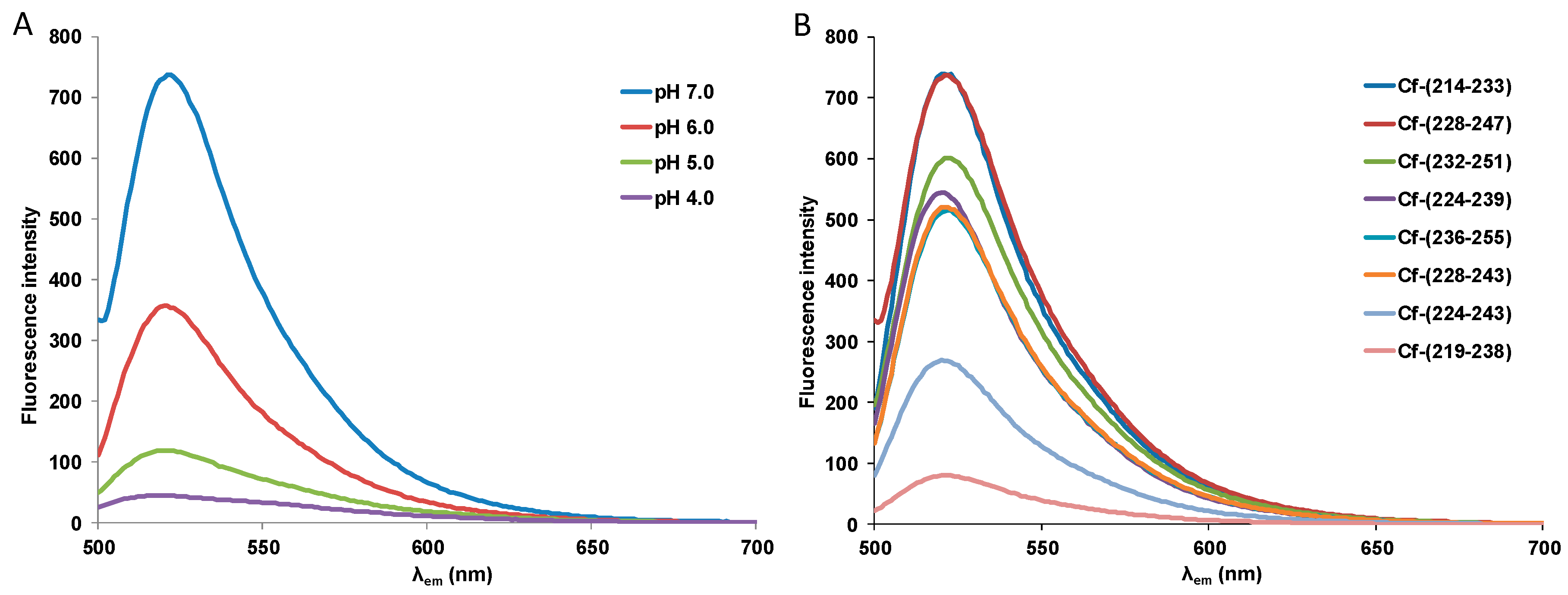
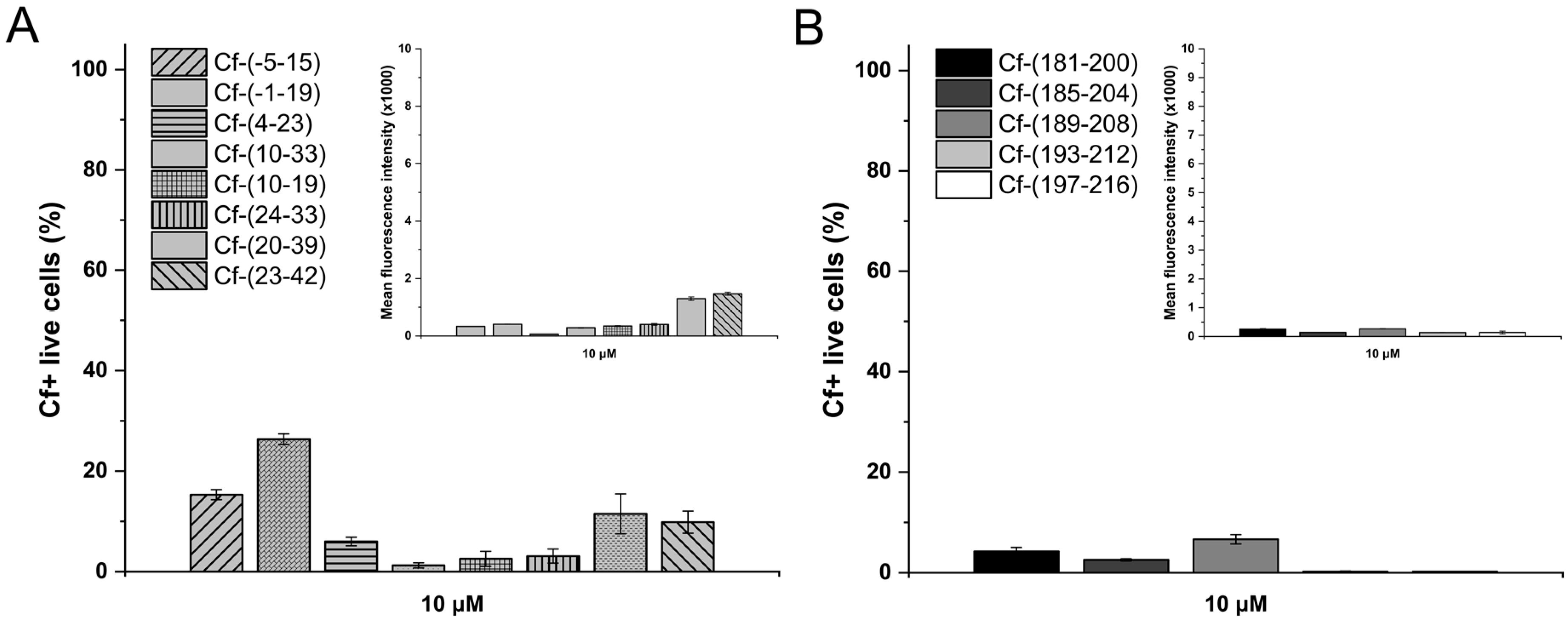
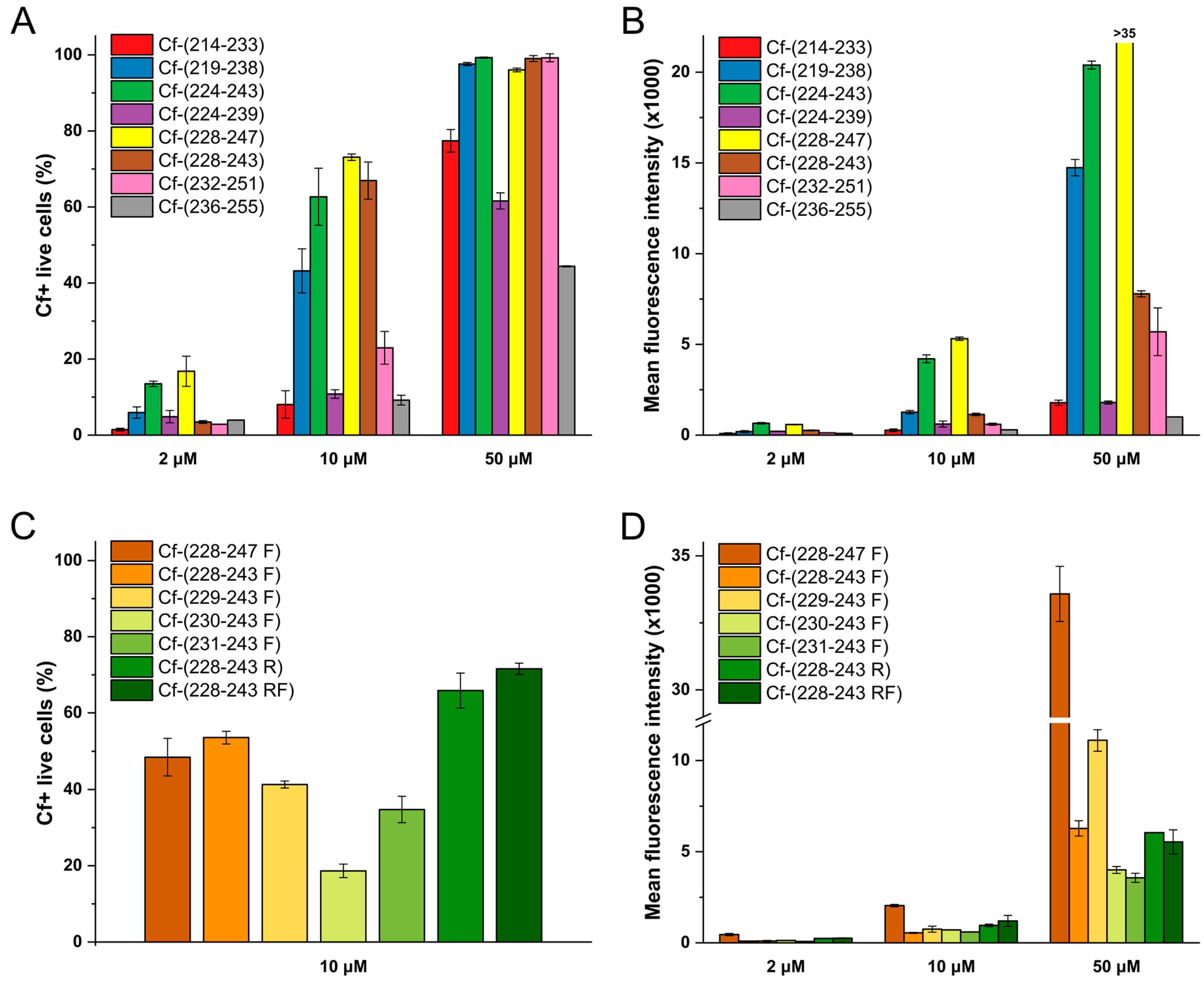
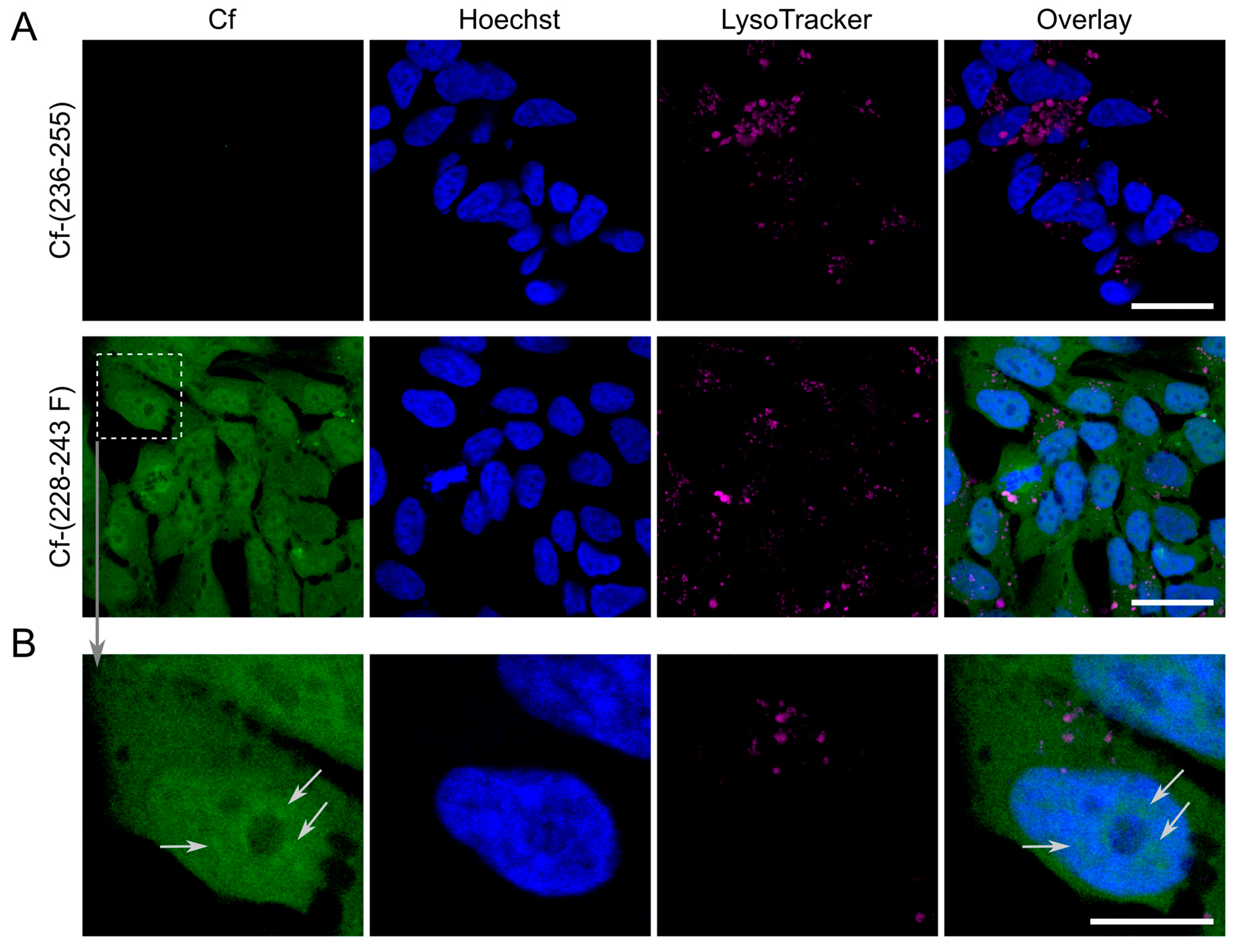
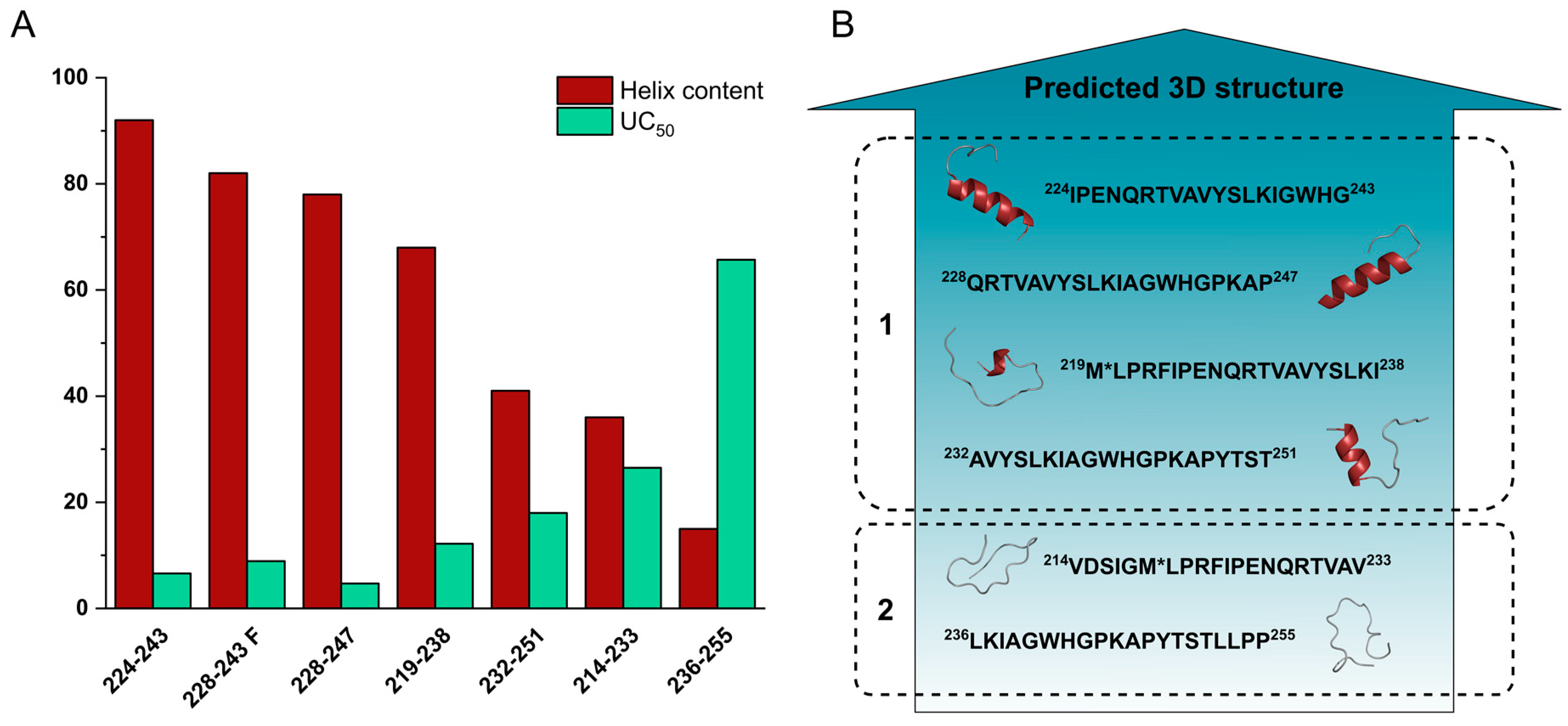
| Code | Set | Sequence | Mw calc / measa | UC50b |
|---|---|---|---|---|
| Cf-(-5–15) | I | Cf--5HGVRGKYALADASLKMADPN15 | 2471.7/2471.7 | 21.1 |
| Cf-(-1–19) | Cf--1GKYALADASLKMADPNRFRG19 | 2538.8/2539.0 | 17.5 | |
| Cf-(4–23) | Cf-4LADASLKMADPNRFRGKDLP23 | 2572.8/2572.8 | 91.9 | |
| Cf-(10–33) | Cf-10KMADPNRFRGKDLPVLDQLTDPPG33 | 3038.4/3038.5 | 40.3 | |
| Cf-(10–19) | Cf-10KMADPNRFRG19 | 1548.7/1548.6 | 35.1 | |
| Cf-(24–33) | Cf-24VLDQLTDPPG33 | 1411.5/1410.8 | 31.6 | |
| Cf-(20–39) | Cf-20KDLPVLDQLTDPPGVRRVYH39 | 2676.0/2675.1 | 23.0 | |
| Cf-(23–42) | Cf-23PVLDQLTDPPGVRRVYHIQA42 | 2631.9/2631.3 | 23.1 | |
| Cf-(181–200) | II | Cf-181LEHRAKGSCKYALPLRIPPS220 | 2665.1/2665.2 | 36.8 |
| Cf-(185–204) | Cf-185AKGSCKYALPLRIPPSACLS204 | 2575.0/2575.5 | 92.9 | |
| Cf-(189–208) | Cf-189CKYALPLRIPPSACLSPQAY208 | 2691.1/2691.0 | 96.6 | |
| Cf-(193–212) | Cf-193LPLRIPPSACLSPQAYQQGV212 | 2566.9/2566.9 | 115.4 | |
| Cf-(197–216) | Cf-197IPPSACLSPQAYQQGVTVDS216 | 2489.7/2489.5 | 108.3 | |
| Cf-(214–233) c | III | Cf-214VDSIGM*LPRFIPENQRTVAV233 | 2581.9/2581.2 | 26.5 |
| Cf-(219–238) | Cf-219M*LPRFIPENQRTVAVYSLKI238 | 2715.1/2713.8 | 12.2 | |
| Cf-(224–243) | Cf-224IPENQRTVAVYSLKIAGWHG243 | 2596.8/2596.1 | 6.6 | |
| Cf-(224–239) | Cf-224IPENQRTVAVYSLKIA239 | 2159.4/2159.0 | 23.7 | |
| Cf-(228–247) | Cf-228QRTVAVYSLKIAGWHGPKAP247 | 2536.9/2537.2 | 4.7 | |
| Cf-(228–243) | Cf-228QRTVAVYSLKIAGWHG243 | 2143.4/2143.3 | 6.5 | |
| Cf-(232–251) | Cf-232AVYSLKIAGWHGPKAPYTST251 | 2504.8/2505.0 | 18.0 | |
| Cf-(236–255) | Cf-236LKIAGWHGPKAPYTSTLLPP255 | 2504.9/2505.0 | 65.7 | |
| Cf-(228–247 F) c | IV | Cf-228QRTVAVYSLKIAGFHGPKAP247 | 2497.8/2498.0 | 10.5 |
| Cf-(228–243 F) | Cf-228QRTVAVYSLKIAGFHG243 | 2104.3/2104.4 | 8.9 | |
| Cf-(229–243 F) | Cf-229RTVAVYSLKIAGFHG243 | 1976.2/1975.6 | 12.7 | |
| Cf-(230–243 F) | Cf-230TVAVYSLKIAGFHG243 | 1820.0/1819.1 | 19.1 | |
| Cf-(231–243 F) | Cf-231VAVYSLKIAGFHG243 | 1718.9/1718.5 | 14.7 | |
| Cf-(228–243 R) | Cf-228QRTVAVYSLRIAGWHG243 | 2171.4/2171.3 | 6.7 | |
| Cf-(228–243 RF) | Cf-228QRTVAVYSLRIAGFHG243 | 2132.3/2132.0 | 6.0 |
| H2O | H2O:TFE | TFE | H2O | H2O:TFE | TFE | |
|---|---|---|---|---|---|---|
| -5HGVRGKYALADASLKMADPN15 | -1GKYALADASLKMADPNRF19 | |||||
| α-helix | 0 (3) | 39 (33) | 66 (60) | 8 (7) | 50 (42) | 70 (56) |
| β-sheet | 15 | 0 | 0 | 4 | 0 | 0 |
| disordered | 70 | 30 | 2 | 64 | 10 | 0 |
| turn | 15 | 31 | 32 | 24 | 40 | 30 |
| R2 | 0.9833 | 0.9961 | 0.9952 | 0.9931 | 0.9909 | 0.9804 |
| 214VDSIGM*LPRFIPENQRTVAV233 | 219M*LPRFIPENQRTVAVYSLKI238 | |||||
| α-helix | 6 (7) | 18 (22) | 36 (28) | 8 (5) | 48 (51) | 68 (76) |
| β-sheet | 0 | 8 | 0 | 0 | 20 | 19 |
| disordered | 74 | 40 | 36 | 78 | 26 | 2 |
| turn | 20 | 34 | 28 | 14 | 6 | 11 |
| R2 | 0.9819 | 0.9957 | 0.9916 | 0.9810 | 0.9954 | 0.9921 |
| 224IPENQRTVAVYSLKIAGWHG243 | 228QRTVAVYSLKIAGWHGPKAP247 | |||||
| α-helix | 8 (3) | 73 (72) | 92 (89) | 0 (3) | 48 (41) | 78 (57) |
| β-sheet | 22 | 20 | 8 | 0 | 0 | 0 |
| disordered | 70 | 7 | 0 | 73 | 38 | 0 |
| turn | 0 | 0 | 0 | 27 | 14 | 22 |
| R2 | 0.9747 | 0.9950 | 0.9957 | 0.9863 | 0.9945 | 0.9939 |
| 232AVYSLKIAGWHGPKAPYTST251 | 236LKIAGWHGPKAPYTSTLLPP255 | |||||
| α-helix | 0 (0) | 32 (18) | 41 (25) | 0 (0) | 6 (5) | 15 (10) |
| β-sheet | 0 | 8 | 0 | 0 | 4 | 0 |
| disordered | 48 | 52 | 42 | 60 | 60 | 64 |
| turn | 52 | 8 | 17 | 40 | 30 | 21 |
| R2 | 0.9950 | 0.9800 | 0.9776 | 0.9767 | 0.9920 | 0.9942 |
| 228QRTVAVYSLKIAGFHG243 | ||||||
| α-helix | 0 (0) | 56 (52) | 82 (67) | |||
| β-sheet | 20 | 20 | 2 | |||
| disordered | 68 | 10 | 4 | |||
| turn | 12 | 14 | 12 | |||
| R2 | 0.9841 | 0.9889 | 0.9872 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bősze, S.; Zsila, F.; Biri-Kovács, B.; Szeder, B.; Majer, Z.; Hudecz, F.; Uray, K. Tailoring Uptake Efficacy of HSV-1 gD Tailoring Uptake Efficacy of Hsv-1 GD Derived Carrier Peptides. Biomolecules 2020, 10, 721. https://doi.org/10.3390/biom10050721
Bősze S, Zsila F, Biri-Kovács B, Szeder B, Majer Z, Hudecz F, Uray K. Tailoring Uptake Efficacy of HSV-1 gD Tailoring Uptake Efficacy of Hsv-1 GD Derived Carrier Peptides. Biomolecules. 2020; 10(5):721. https://doi.org/10.3390/biom10050721
Chicago/Turabian StyleBősze, Szilvia, Ferenc Zsila, Beáta Biri-Kovács, Bálint Szeder, Zsuzsa Majer, Ferenc Hudecz, and Katalin Uray. 2020. "Tailoring Uptake Efficacy of HSV-1 gD Tailoring Uptake Efficacy of Hsv-1 GD Derived Carrier Peptides" Biomolecules 10, no. 5: 721. https://doi.org/10.3390/biom10050721
APA StyleBősze, S., Zsila, F., Biri-Kovács, B., Szeder, B., Majer, Z., Hudecz, F., & Uray, K. (2020). Tailoring Uptake Efficacy of HSV-1 gD Tailoring Uptake Efficacy of Hsv-1 GD Derived Carrier Peptides. Biomolecules, 10(5), 721. https://doi.org/10.3390/biom10050721






