Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals
2.3. Estrus Detection and Artificial Insemination
2.4. Embryo Recovery and Embryo Transfer
2.5. Embryo and Corpora Lutea Evaluation
2.6. Tissue Collection and Preparation
2.7. Cytokine Analysis
2.8. Protein Measurement
2.9. Statistical Analysis
3. Results
3.1. Reproductive Performance at Day 18 or 24 of Pregnancy after AI or ET
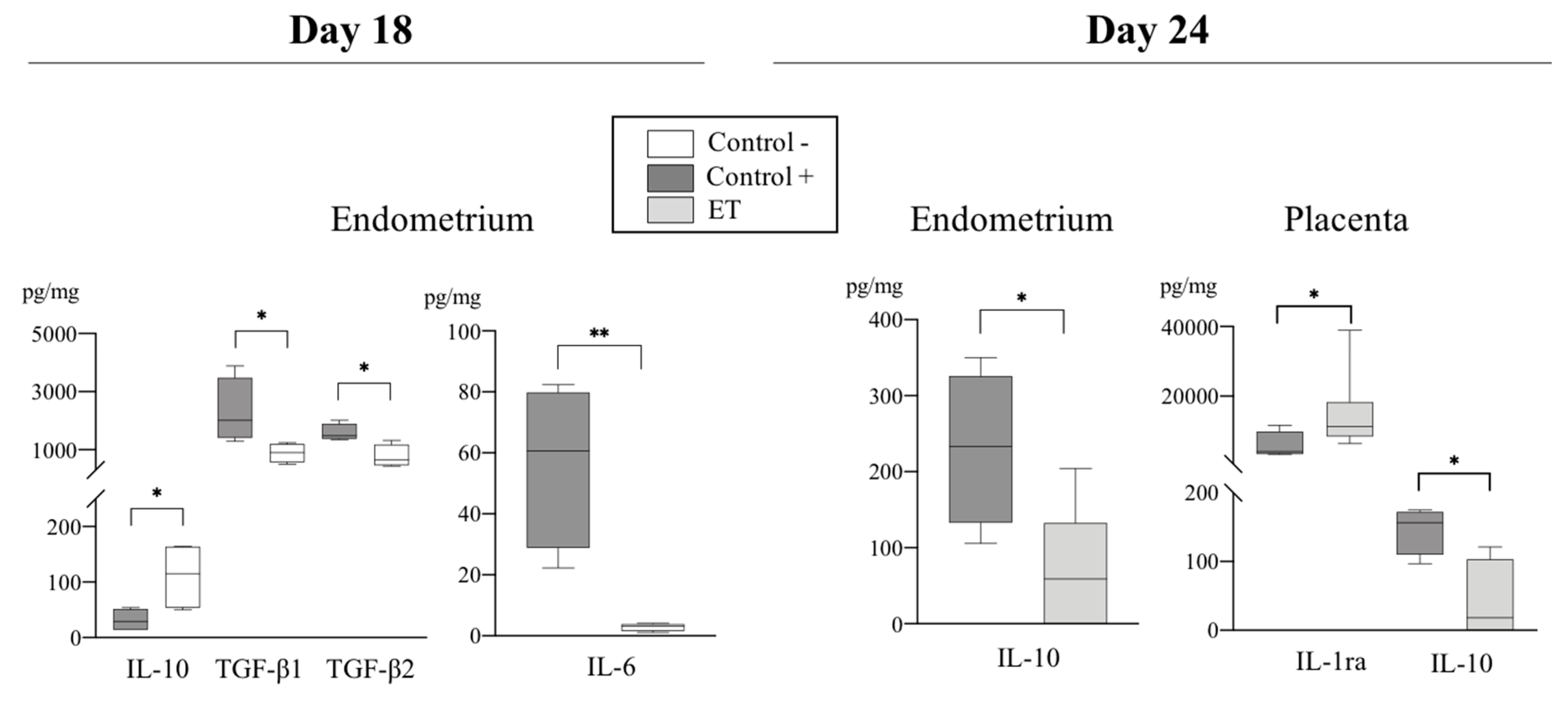
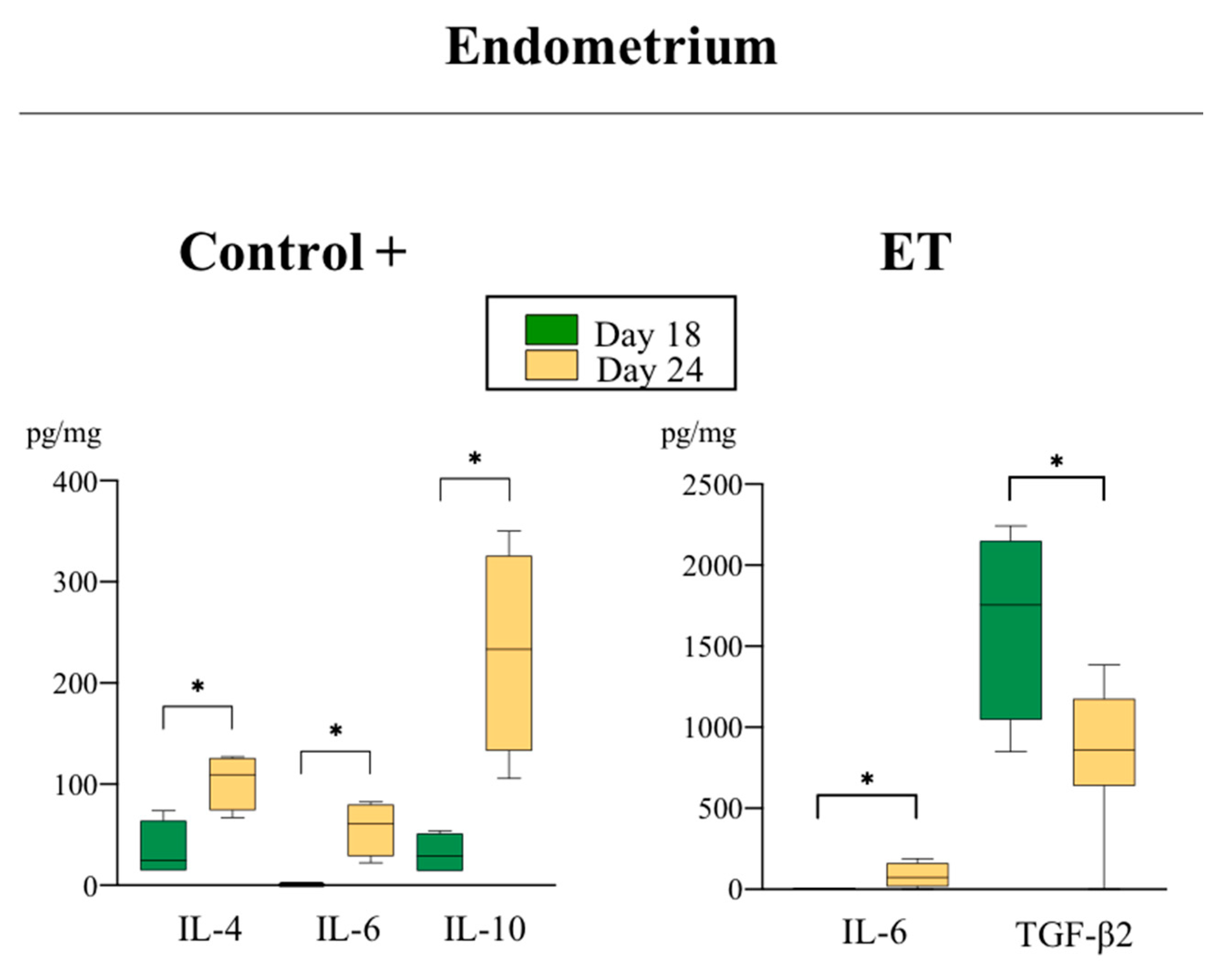
3.2. Variations on the Levels of Cytokines During the Peri-Implantation Period of Pregnancy
3.2.1. Pro-Inflammatory Cytokines
3.2.2. Anti-Inflammatory Cytokines
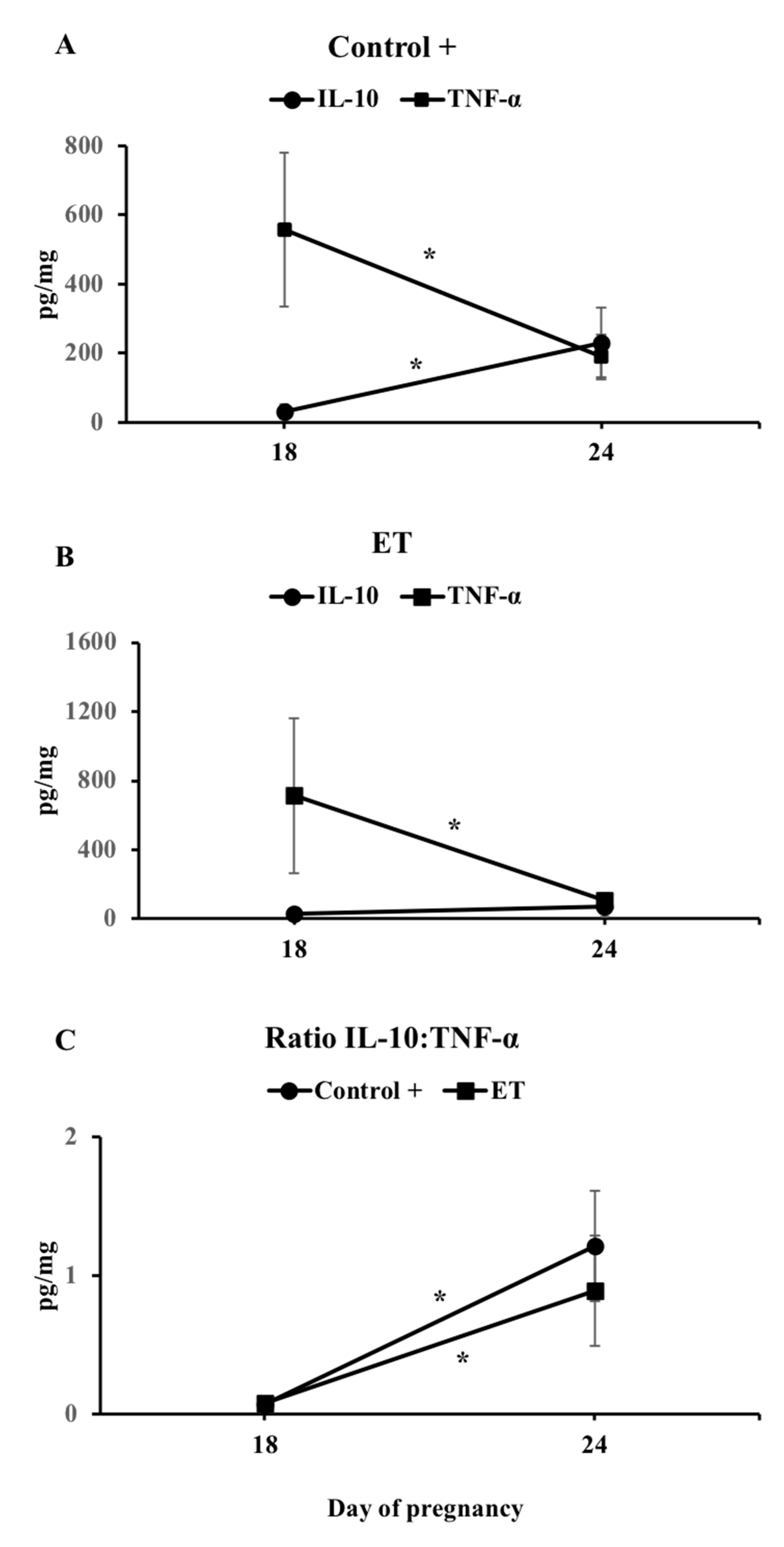
3.3. The IL-10:TNF-α Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bidarimath, M.; Tayade, C. Pregnancy and spontaneous fetal loss: A pig perspective. Mol. Reprod. Dev. 2017, 84, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Geisert, R.D.; Schmitt, R.A.M. Early embryonic survival in the pig: Can it be improved? J. Anim. Sci. 2002, 80, E54–E65. [Google Scholar]
- Ross, J.W.; Ashworth, M.D.; Stein, D.R.; Couture, O.P.; Tuggle, C.K.; Geisert, R.D. Identification of differential gene expression during porcine conceptus rapid trophoblastic elongation and attachment to uterine luminal epithelium. Physiol. Genomics 2009, 36, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.E.; McRae, M.A.; Plair, B.L.; Keller, G.L.; Dresser, B.L. Successful in vitro and in vivo development of in vitro fertilized two- to four-cell cat embryos following cryopreservation, culture and transfer. Theriogenology 1994, 42, 513–525. [Google Scholar] [CrossRef]
- Ducro-Steverink, D.W.B.; Peters, C.G.W.; Maters, C.C.; Hazeleger, W.; Merks, J.W.M. Reproduction results and offspring performance after non-surgical embryo transfer in pigs. Theriogenology 2004, 62, 522–531. [Google Scholar] [CrossRef]
- Martinez, E.A.; Angel, M.A.; Cuello, C.; Sanchez-Osorio, J.; Gomis, J.; Parrilla, I.; Vila, J.; Colina, I.; Diaz, M.; Reixach, J.; et al. Successful non-surgical deep uterine transfer of porcine morulae after 24 hour culture in a chemically defined medium. PLoS ONE 2014, 9, e104696. [Google Scholar] [CrossRef]
- Gundogan, F.; Bianchi, D.W.; Scherjon, S.A.; Roberts, D.J. Placental pathology in egg donor pregnancies. Fertil. Steril. 2010, 93, 397–404. [Google Scholar] [CrossRef]
- Letur, H.; Peigne, M.; Ohl, J.; Cedrin-Durnerin, I.; Mathieu-D’Argent, E.; Scheffler, F.; Grzegorczyk-Martin, V.; de Mouzon, J. Hypertensive pathologies and egg donation pregnancies: Results of a large comparative cohort study. Fertil. Steril. 2016, 106, 284–290. [Google Scholar] [CrossRef][Green Version]
- Vallet, J.L.; McNeel, A.K.; Johnson, G.; Bazer, F.W. TRIENNIAL REPRODUCTION SYMPOSIUM: Limitations in uterine and conceptus physiology that lead to fetal losses1,2. J. Anim. Sci. 2013, 91, 3030–3040. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A. Pig blastocyst-uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A. Interferons and uterine receptivity. Semin. Reprod. Med. 2009, 27, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Blitek, A.; Kaczmarek, M.M.; Waclawik, A.; Ziecik, A.J.; Rodriguez-Martinez, H.; Soede, N.M.; Flowers, W.L. Embryo-Maternal Relationships during the Peri-Implantation Period-New and Old Players; Nottingham University Press: Nottingham, UK, 2013. [Google Scholar]
- Spencer, T.E.; Bazer, F.W. Uterine and placental factors regulating conceptus growth in domestic animals. J. Anim. Sci. 2004, 82 (Suppl. 13), E4–E13. [Google Scholar] [PubMed]
- Tilburgs, T.; Roelen, D.L.; van der Mast, B.J.; de Groot-Swings, G.M.; Kleijburg, C.; Scherjon, S.A.; Claas, F.H. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 2008, 180, 5737–5745. [Google Scholar] [CrossRef]
- Erlebacher, A. Immune surveillance of the maternal/fetal interface: Controversies and implications. Trends Endocrinol. Metab. 2010, 21, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Warning, J.C.; McCracken, S.A.; Morris, J.M. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011, 141, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Geisert, R.D.; Lucy, M.C.; Whyte, J.J.; Ross, J.W.; Mathew, D.J. Cytokines from the pig conceptus: Roles in conceptus development in pigs. J. Anim. Sci. Biotechnol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Hannan, N.J.; Salamonsen, L.A. Role of chemokines in the endometrium and in embryo implantation. Curr. Opin. Obstet. Gynecol. 2007, 19, 266–272. [Google Scholar] [CrossRef]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Wessels, J.M.; Koti, M.; Tayade, C. Altered expression of chemokines and their receptors at porcine maternal-fetal interface during early and mid-gestational fetal loss. Cell Tissue Res. 2016, 366, 747–761. [Google Scholar] [CrossRef]
- Wegmann, T.G. Foetal protection against abortion: is it immunosuppression or immunostimulation? Ann. Immunol. (Paris) 1984, 135D, 309–312. [Google Scholar]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef]
- Shiraishi, H.; Hayakawa, S.; Satoh, K. Murine experimental abortion by IL-2 administration is caused by activation of cytotoxic T lymphocytes and placental apoptosis. J. Clin. Lab. Immunol. 1996, 48, 93–108. [Google Scholar] [PubMed]
- Pursel, V.G.; Johnson, L.A. Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Martinez, C.A.; Nohalez, A.; Sanchez-Osorio, J.; Vazquez, J.M.; Roca, J.; Parrilla, I.; Gil, M.A.; Cuello, C. Nonsurgical deep uterine transfer of vitrified, in vivo-derived, porcine embryos is as effective as the default surgical approach. Sci. Rep. 2015, 5, 10587. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M. Photographic illustrations of embryo developmental stage and quality codes. In Manual of the International Embryo Transfer Society; IETS: Savoy, IL, USA, 1998; pp. 167–170. [Google Scholar]
- Nohalez, A.; Gil, M.A.; Sanchez-Osorio, J.; Cuello, C.; Martinez, C.A.; Parrilla, I.; Martinez, E.A.; Roca, J.; Vazquez, J.M. Nonsurgical deep uterine transfer of vitrified, in vivo-derived, porcine embryos is as effective as the default surgical approach. Sci. Rep. 2015, 5, 10587. [Google Scholar]
- Edwards, A.K.; Wessels, J.M.; Kerr, A.; Tayade, C. An overview of molecular and cellular mechanisms associated with porcine pregnancy success or failure. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 394–401. [Google Scholar] [CrossRef]
- Vigano, P.; Mangioni, S.; Pompei, F.; Chiodo, I. Maternal-conceptus cross talk—A review. Placenta 2003, 24 (Suppl. B), S56–S61. [Google Scholar] [CrossRef]
- Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Kaczynski, P.; Ziecik, A.J. Embryo-maternal dialogue during pregnancy establishment and implantation in the pig. Mol. Reprod. Dev. 2017, 84, 842–855. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wu, G.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Bayless, K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol. Hum. Reprod. 2009, 16, 135–152. [Google Scholar] [CrossRef]
- Moffett, A.; Loke, C. Implantation, embryo-maternal interactions, immunology and modulation of the uterine environment—A workshop report. Placenta 2006, 27 (Suppl. A), S54–S55. [Google Scholar] [CrossRef]
- Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002, 2, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, H.; Croy, B.A.; King, G.J. Conceptus influences the distribution of uterine leukocytes during early porcine pregnancy. Biol. Reprod. 2002, 66, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Moldenhauer, L.M. Immunological determinants of implantation success. Int. J. Dev. Biol. 2014, 58, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Taylor, H.S. The role of growth factors and cytokines during implantation: Endocrine and paracrine interactions. Semin. Reprod. Med. 2009, 27, 62–79. [Google Scholar] [CrossRef]
- White, F.J.; Kimball, E.M.; Wyman, G.; Stein, D.R.; Ross, J.W.; Ashworth, M.D.; Geisert, R.D. Estrogen and interleukin-1beta regulation of trophinin, osteopontin, cyclooxygenase-1, cyclooxygenase-2, and interleukin-1beta system in the porcine uterus. Soc. Reprod. Fertil. Suppl. 2009, 66, 203–204. [Google Scholar]
- Cencic, A.; Guillomot, M.; Koren, S.; La Bonnardiere, C. Trophoblastic interferons: do they modulate uterine cellular markers at the time of conceptus attachment in the pig? Placenta 2003, 24, 862–869. [Google Scholar] [CrossRef]
- Ross, J.W.; Malayer, J.R.; Ritchey, J.W.; Geisert, R.D. Characterization of the interleukin-1beta system during porcine trophoblastic elongation and early placental attachment. Biol. Reprod. 2003, 69, 1251–1259. [Google Scholar] [CrossRef]
- Geisert, R.D.; Whyte, J.J.; Meyer, A.E.; Mathew, D.J.; Juarez, M.R.; Lucy, M.C.; Prather, R.S.; Spencer, T.E. Rapid conceptus elongation in the pig: An interleukin 1 beta 2 and estrogen-regulated phenomenon. Mol. Reprod. Dev. 2017, 84, 760–774. [Google Scholar] [CrossRef]
- Seo, H.; Choi, Y.; Shim, J.; Choi, Y.; Ka, H. Regulatory mechanism for expression of IL1B receptors in the uterine endometrium and effects of IL1B on prostaglandin synthetic enzymes during the implantation period in pigs. Biol. Reprod. 2012, 87, 31. [Google Scholar] [CrossRef]
- Mathew, D.J.; Newsom, E.M.; Guyton, J.M.; Tuggle, C.K.; Geisert, R.D.; Lucy, M.C. Activation of the transcription factor nuclear factor-kappa B in uterine luminal epithelial cells by interleukin 1 Beta 2: A novel interleukin 1 expressed by the elongating pig conceptus. Biol. Reprod. 2015, 92, 107. [Google Scholar] [CrossRef]
- Mathew, D.J.; Lucy, M.C.; D Geisert, R. Interleukins, interferons, and establishment of pregnancy in pigs. Reproduction 2016, 151, R111–R122. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.E.; Pfeiffer, C.A.; Brooks, K.E.; Spate, L.D.; Benne, J.A.; Cecil, R.; Samuel, M.S.; Murphy, C.N.; Behura, S.; McLean, M.K.; et al. New perspective on conceptus estrogens in maternal recognition and pregnancy establishment in the pigdagger. Biol. Reprod. 2019, 101, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Qiu, B.; Mei, F.; Liu, F.; Feng, Z.; Fan, J.; Nie, J.; Huang, L.; Liao, X.; Wang, Z.; et al. Interleukin-1alpha leads to growth hormone deficiency in adamantinomatous craniopharyngioma by targeting pericytes: Implication in pituitary fibrosis. Metabolism. 2019, 101, 153998. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef]
- Massuto, D.A.; Kneese, E.C.; Johnson, G.A.; Burghardt, R.C.; Hooper, R.N.; Ing, N.H.; Jaeger, L.A. Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: Proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction 2010, 139, 465–478. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Pillars Article: Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Simmen, R.; Simmen, F. Regulation of uterine and conceptus secretory activity in the pig. J. Reprod. Fertil. Suppl. 1990, 40, 279–292. [Google Scholar]
- Mobini, M.; Mortazavi, M.; Nadi, S.; Zare-Bidaki, M.; Pourtalebi, S.; Arababadi, M.K. Significant roles played by interleukin-10 in outcome of pregnancy. Iran. J. Basic Med. Sci. 2016, 19, 119–124. [Google Scholar]
- Busse, M.; Campe, K.-N.J.; Nowak, D.; Schumacher, A.; Plenagl, S.; Langwisch, S.; Tiegs, G.; Reinhold, A.; Zenclussen, A.C. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci. Rep. 2019, 9, 9335. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, J.E.; Sharma, S. Interleukin-10: A multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 2010, 63, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.Y.; Raghupathy, R. IL-10 and pregnancy complications. Clin. Exp. Obstet. Gynecol. 2017, 44, 252–258. [Google Scholar] [PubMed]
- Kaislasuo, J.; Simpson, S.; Petersen, J.F.; Peng, G.; Aldo, P.; Lokkegaard, E.; Paidas, M.; Pal, L.; Guller, S.; Mor, G. IL-10 to TNFalpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am. J. Reprod. Immunol. 2019. [Google Scholar] [CrossRef]
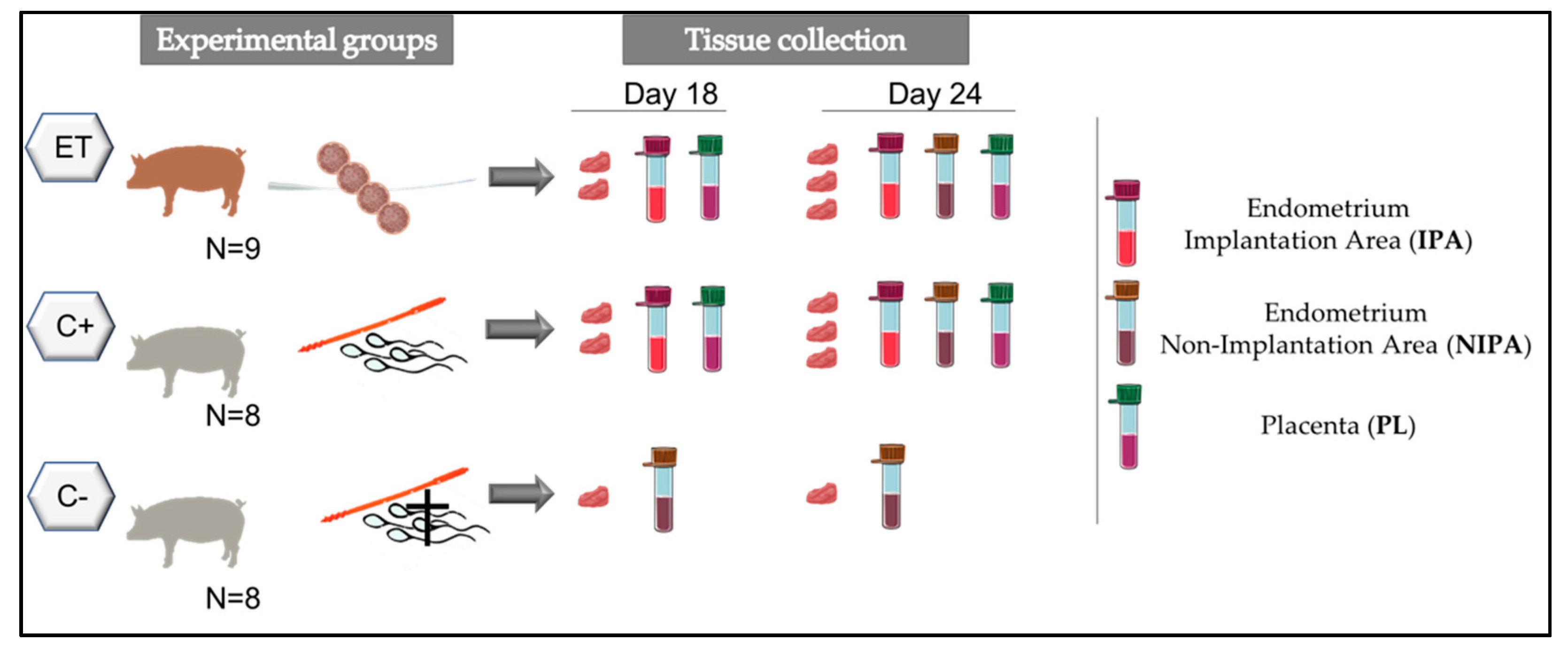
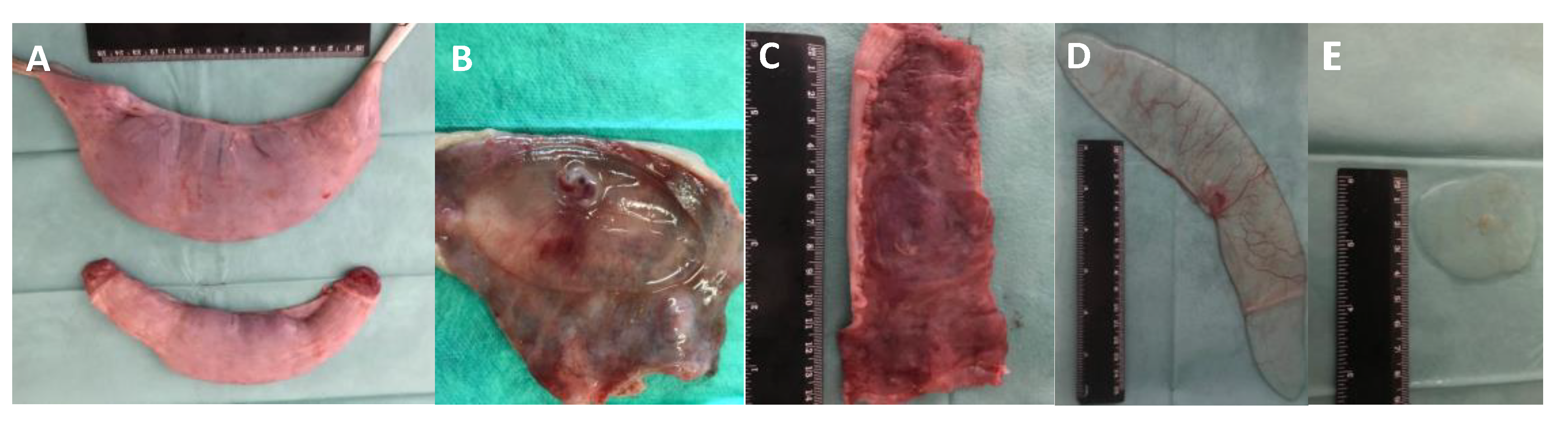
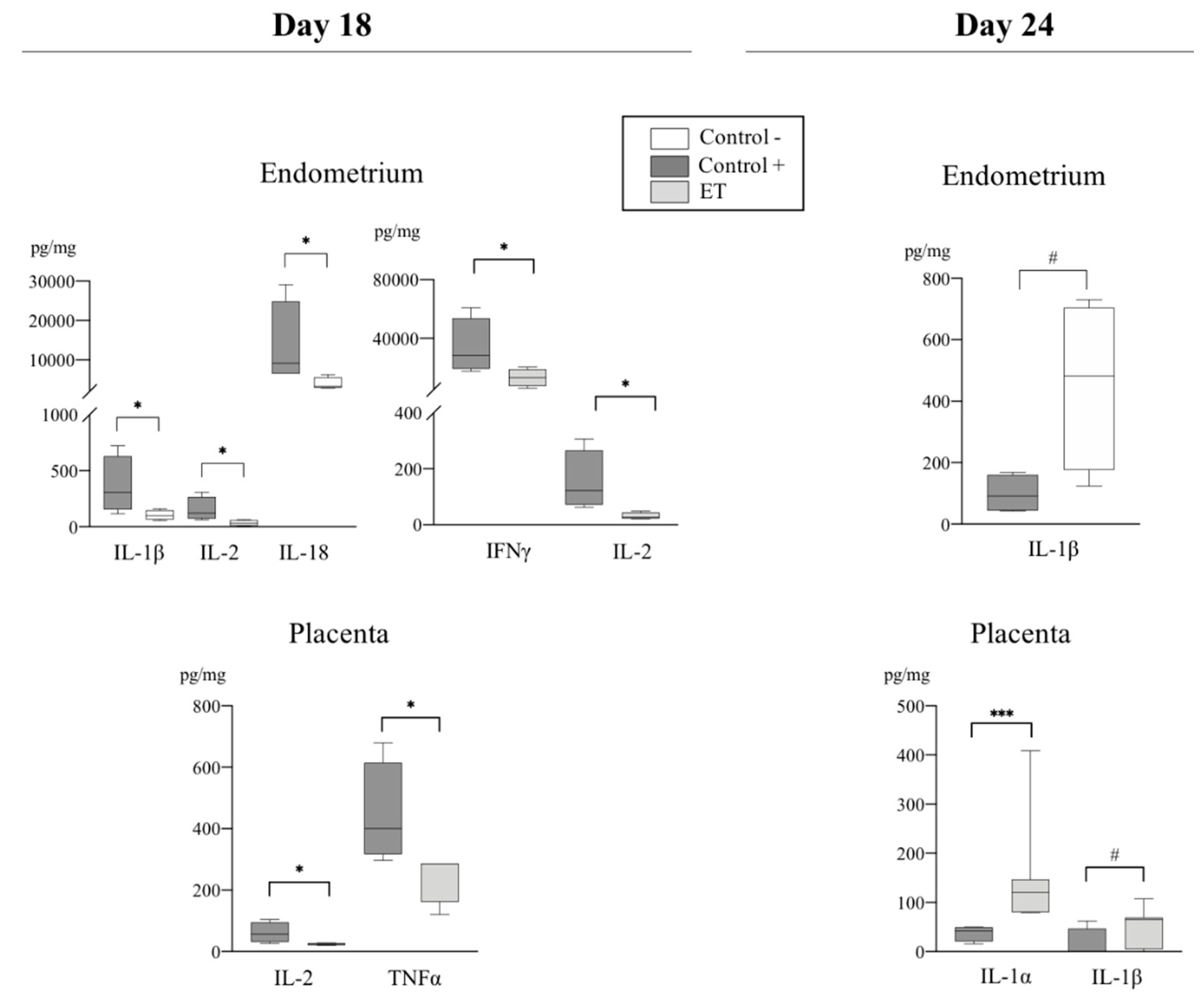
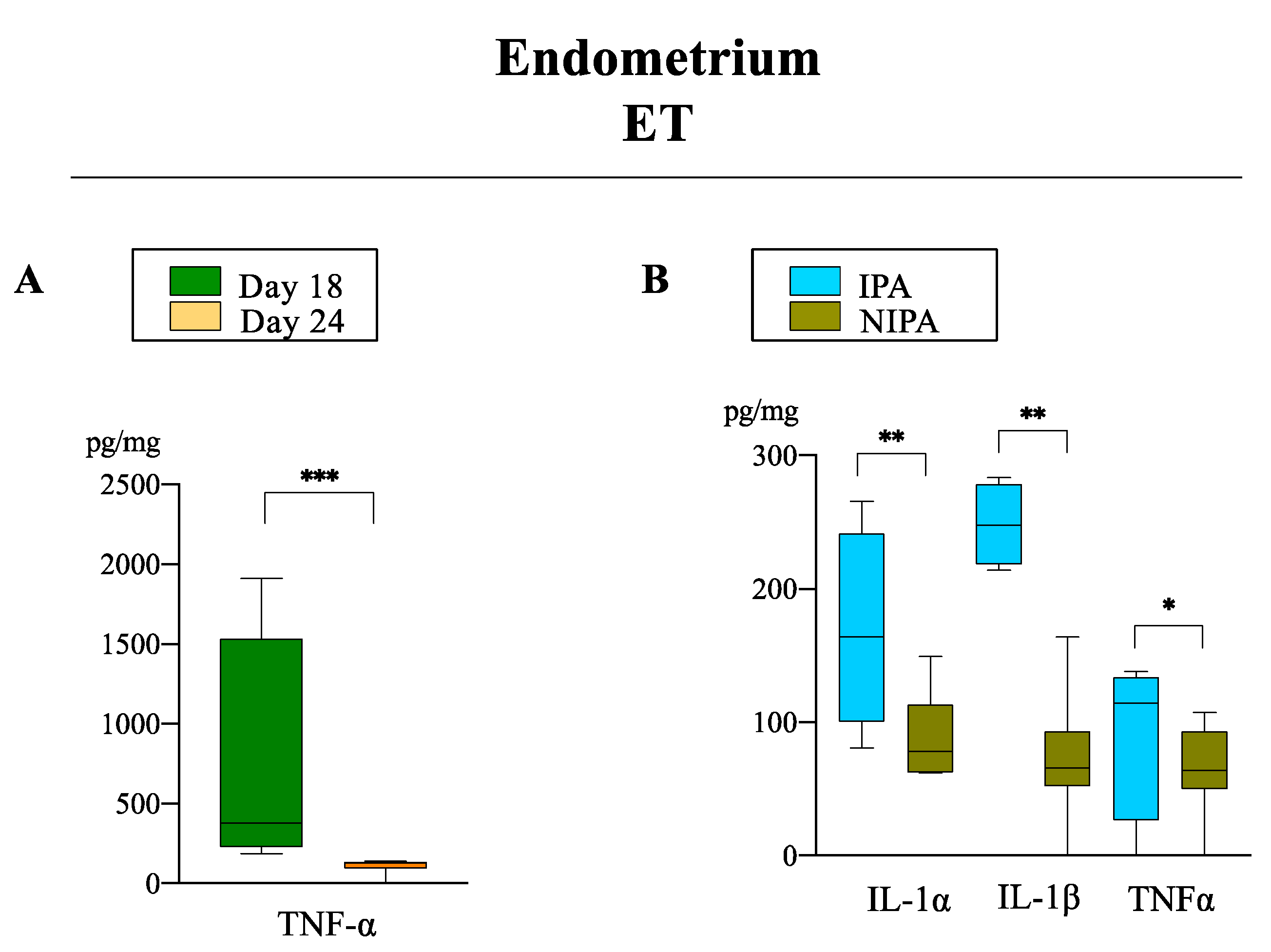
| Group | Corpora Lutea (n, mean ± sem) | Transferred Morulae/ET (n) | Total Recovered Embryos n (%) * | Normal Recovered Embryos n (%) * | Delayed Recovered Embryos n (%) ** |
|---|---|---|---|---|---|
| C+ | 94 (23.5 ± 1.0) | - | 76 (80.9) a | 72 (76.6) a | 4 (5.3) c |
| ET | 90 (22.5 ± 1.6) | 92 | 54 (58.7) b | 42 (45.6) b | 12 (22.2) d |
| Day 18 | Day 24 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | C− | C+ | C+ PL | ET | ET PL | C− | C+ IPA | C+ PL | C+ NIPA | ET IPA | ET PL | ET NIPA |
| GM-CSF | 40 ± 9.1 | 75.7 ± 19.2 | 52.3 ± 11.6 | 41.48 ± 13.5 | 42.1 ± 9.5 | 128.6 ± 74.5 | 47.2 ± 21.3 | 108.3 ± 13.1 | 113.0 ± 51 | 25.8 ± 6.2 | 84.1 ± 13.1 | 17.7± 5.7 |
| IFN-γ | 20,899.9 ± 5552.5 | 33,831.3 ± 9468.4 | 22,930.4 ± 5044.1 | 13,246.1 ± 2992.2 | 12,487.8 ± 2810 | 11,582.9 ± 2623.8 | 14,606.5 ± 634.5 | 21,030 ± 3019.4 | 30,452.2 ± 14,028.2 | 16,247.2 ± 5057.7 | 27,062.1 ± 7529.8 | 7820.2 ± 1636.2 |
| IL-12 | 37.5 ± 30.7 | 23.3 ± 6.5 | 23.7 ± 4.5 | 29.9 ± 2.5 | 19.7 ± 2.5 | 187.4 ± 99.5 | 41.3 ± 15.7 | 0.0 ± 0.0 | 150.1 ± 101.5 | 28.3 ± 7.6 | 0.0 ± 0.0 | 30.3 ± 5.6 |
| IL-18 | 3961.9 ± 778.3 | 13,493.9 ± 5363.5 | 6785.1 ± 482.5 | 11,497.2 ± 1106.5 | 8027.5 ± 1299.7 | 8914.1 ± 2895.4 | 7225.7 ± 2657.5 | 10,320.4 ± 2786.1 | 9367.8 ± 1691.2 | 8990.7 ± 2252 | 13,509.3 ± 3322.1 | 3403.2 ± 738.3 |
| IL-1α | 91.6 ± 20.2 | 113.2 ± 24.4 | 112.3 ± 26.5 | 147.2 ± 15.7 | 97 ± 31.2 | 98.05 ± 30.7 | 96.7 ± 17.9 | 37.4 ± 7.9 | 118.6 ± 38.8 | 168.6 ± 24.5 | 147.5 ± 38.5 | 88.7 ± 13.4 |
| IL-1β | 103.6 ± 21.7 | 363.3 ± 129.1 | 6170.5 ± 2716.6 | 253.5 ± 38.2 | 7674.7 ± 1759.4 | 454.3 ± 138.1 | 98.2 ± 31.7 | 15.6 ± 15.6 | 242.3 ± 156.7 | 202.4 ± 37.8 | 49.3 ± 13.6 | 73.9 ± 18.6 |
| IL-1RA | 719.5 ± 272.1 | 2042 ± 801.2 | 1749.2 ± 299.6 | 1575.6 ± 179.7 | 1682.7 ± 367.2 | 914.5 ± 95.3 | 2344.1 ± 647.4 | 5775.5 ± 1973.9 | 1714.2 ± 320.3 | 1279.8 ± 289.9 | 14,924.7 ± 3739.3 | 462.8 ± 135.5 |
| IL-2 | 33.9 ± 13.5 | 153.2 ± 53.7 | 61.2 ± 16.6 | 30 ± 6.2 | 24.1 ± 1.6 | 519.7 ± 174.3 | 70.9 ± 15.1 | 268.3 ± 59.3 | 250.4 ± 169.5 | 30.2 ± 9.8 | 300.2 ± 83.1 | 29.1 ± 8.3 |
| IL-4 | 49.7 ± 16.2 | 34.3 ± 13.9 | 20.9 ± 4.6 | 14 ± 3.8 | 20.4 ± 7.2 | 81.1 ± 49.3 | 103.1 ± 13.9 | 28.7 ± 1.7 | 333.2 ± 228.2 | 41.3 ± 18.3 | 19.5 ± 5.1 | 33.8 ± 12.2 |
| IL-6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 56.4 ± 13.3 | 40.9 ± 18.4 | 35.7 ± 20 | 85.8 ± 25.4 | 136.2 ± 34.2 | 11.7 ± 5.4 |
| IL-8 | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.04 | 0.6 ± 0.0 | 0.8 ± 0.8 | 1.1 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.06 | 0.6 ± 0.1 | 1.1 ± 0.1 |
| TGF- β 1 | 888.3 ± 162.6 | 2300.4 ± 563.9 | 1739.5 ± 192.5 | 2092.4 ± 300.1 | 1995.1 ± 153.5 | 2062.3 ± 417.9 | 1358.2 ± 211.1 | 2936.9± | 2848.5 ± 987.7 | 1387.5 ± 230.3 | 1856.1 ± 327.8 | 1208.1 ± 82.3 |
| TGF- β 2 | 764 ± 195.6 | 1584.9 ± 147.6 | 2887.6 ± 688.1 | 1650.5 ± 294.1 | 3652.3 ± 552.1 | 1158.7 ± 228.5 | 1012.8 ± 237.3 | 1549.9 ± 397.4 | 1642.4 ± 631.9 | 842.8 ± 150.7 | 1343.9 ± 145.7 | 1217 ± 274.4 |
| TGF- β 3 | 14.5 ± 5.1 | 21.4 ± 3.1 | 15.7 ± 3.3 | 22.8 ± 2.6 | 25.3 ± 7.4 | 16.9 ± 3.8 | 16.3 ± 2.5 | 15.9 ± 4.5 | 31.9 ± 10.8 | 18.1 ± 3.5 | 17.6 ± 3.4 | 22.6 ± 3.8 |
| IL-10 | 110.9 ± 30.7 | 31.5 ± 10.1 | 62.0 ± 14.3 | 30.4 ± 5.4 | 76.3 ± 11.7 | 769.2 ± 359.6 | 230.6 ± 50.5 | 146.2 ± 17.3 | 608.6 ± 396.4 | 71.7 ± 16.1 | 41.2 ± 18.7 | 63.5 ± 24 |
| TNF-α | 375 ± 113.3 | 557.9 ± 178.4 | 444.1 ± 82.5 | 713.9 ± 402.2 | 244 ± 41.1 | 168.7 ± 27.2 | 189.8 ± 32.2 | 459.8 ± 85.7 | 305.3 ± 123.8 | 105.4 ± 26.6 | 548.9 ± 124.7 | 63.8 ± 13.3 |
| IL-10:TNF-α (ratio) | 0.4 ± 0.1 | 0.07 ± 0.02 | 0.15 ± 0.04 | 0.08 ± 0.02 | 0.36 ± 0.1 | 4.8 ± 2.6 | 1.21 ± 0.2 | 0.36 ± 0.09 | 1.58 ± 0.4 | 0.89 ± 0.2 | 0.2 ± 0.05 | 1.59 ± 0.2 |
| Treatment-Day 18 | Treatment-Day 24 | Endometrial Area-Day 24 | Day of Pregnancy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CK | C+ vs. C− EN | C+ vs. ET EN | C+ vs. ET PL | C+ vs. C− EN | C+ vs. ET EN | C+ vs. ET PL | IPA vs. NIPA C+ | IPA vs. NIPA ET | D18 vs. D24 C+ | D18 vs. D24 ET |
| GM-CSF | 0.149 | 0.248 | 0.564 | 0.248 | 0.234 | 0.126 | 0.248 | 0.561 | 0.149 | 0.308 |
| IFN-γ | 0.248 | 0.043 | 0.149 | 0.021 | 0.174 | 0.865 | 0.773 | 0.105 | 0.021 | 1 |
| IL-12 | 0.386 | 0.564 | 0.386 | 0.149 | 0.496 | 1 | 0.248 | 0.908 | 0.386 | 0.865 |
| IL-18 | 0.021 | 0.773.5 | 0.248 | 0.773 | 0.61 | 0.496 | 0.386 | 0.081 | 0.386 | 0.496 |
| IL-1α | 0.564 | 0.248 | 0.564 | 1 | 0.062 | 0.007 | 0.773 | 0.01 | 0.773 | 0.734 |
| IL-1β | 0.043 | 0.564.1 | 0.564 | 0.083 | 0.126 | 0.078 | 0.564 | 0.024 | 0.083 | 0.396 |
| IL-1RA | 0.083 | 0.773 | 0.564 | 0.149 | 0.174 | 0.042 | 0.386 | 0.015 | 0.773 | 0.308 |
| IL-2 | 0.043 | 0.021 | 0.043 | 0.021 | 0.06 | 0.734 | 0.386 | 0.814 | 0.149 | 0.864 |
| IL-4 | 0.386 | 0.248 | 0.773 | 0.248 | 0.04 | 0.307 | 0.564 | 0.765 | 0.043 | 0.494 |
| IL-6 | 1 | 1 | 1 | 0.014 | 0.61 | 0.173 | 0.248 | 0.045 | 0.014 | 0.03 |
| IL-8 | 0.386 | 0.248 | 0.248 | 0.248 | 0.126 | 0.497 | 0.083 | 0.045 | 0.083 | 0.007 |
| TGF- β 1 | 0.021 | 0.773 | 0.564 | 0.083 | 0.497 | 0.174 | 0.083 | 0.2 | 0.083 | 0.126 |
| TGF- β 2 | 0.021 | 0.564 | 0.248 | 0.564 | 0.61 | 0.497 | 0.564 | 0.183 | 0.149 | 0.042 |
| TGF- β 3 | 0.248 | 0.773 | 0.248 | 1 | 0.497 | 0.734 | 0.149 | 0.563 | 0.149 | 0.234 |
| IL-10 | 0.043 | 0.773 | 0.564 | 0.386 | 0.026 | 0.016 | 0.773 | 0.952 | 0.021 | 0.494 |
| TNF-α | 0.564 | 0.773 | 0.021 | 0.773 | 0.062 | 0.734 | 0.773 | 0.024 | 0.021 | 0.007 |
| IL-10:TNF-α (ratio) | 0.234 | 0.631 | 0.3 | 0.112 | 0.1 | 0.564 | 0.126 | 0.105 | 0.002 | 0.009 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, C.A.; Rubér, M.; Rodriguez-Martinez, H.; Alvarez-Rodriguez, M. Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death? Biomolecules 2020, 10, 554. https://doi.org/10.3390/biom10040554
Martinez CA, Rubér M, Rodriguez-Martinez H, Alvarez-Rodriguez M. Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death? Biomolecules. 2020; 10(4):554. https://doi.org/10.3390/biom10040554
Chicago/Turabian StyleMartinez, Cristina A., Marie Rubér, Heriberto Rodriguez-Martinez, and Manuel Alvarez-Rodriguez. 2020. "Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death?" Biomolecules 10, no. 4: 554. https://doi.org/10.3390/biom10040554
APA StyleMartinez, C. A., Rubér, M., Rodriguez-Martinez, H., & Alvarez-Rodriguez, M. (2020). Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death? Biomolecules, 10(4), 554. https://doi.org/10.3390/biom10040554







