Chitosan Nanocomplexes for the Delivery of ENaC Antisense Oligonucleotides to Airway Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanocomplexes
2.2. Determination of Size Distribution and Zeta Potential
2.3. Stability Measurements
2.4. Gel Retardation Assay
2.5. Cell Culture
2.6. MTT Assay
2.7. Transfection
2.8. Fluorescence Optical Experiments
2.9. Transepithelial Measurements
2.10. Statistical Analysis
3. Results
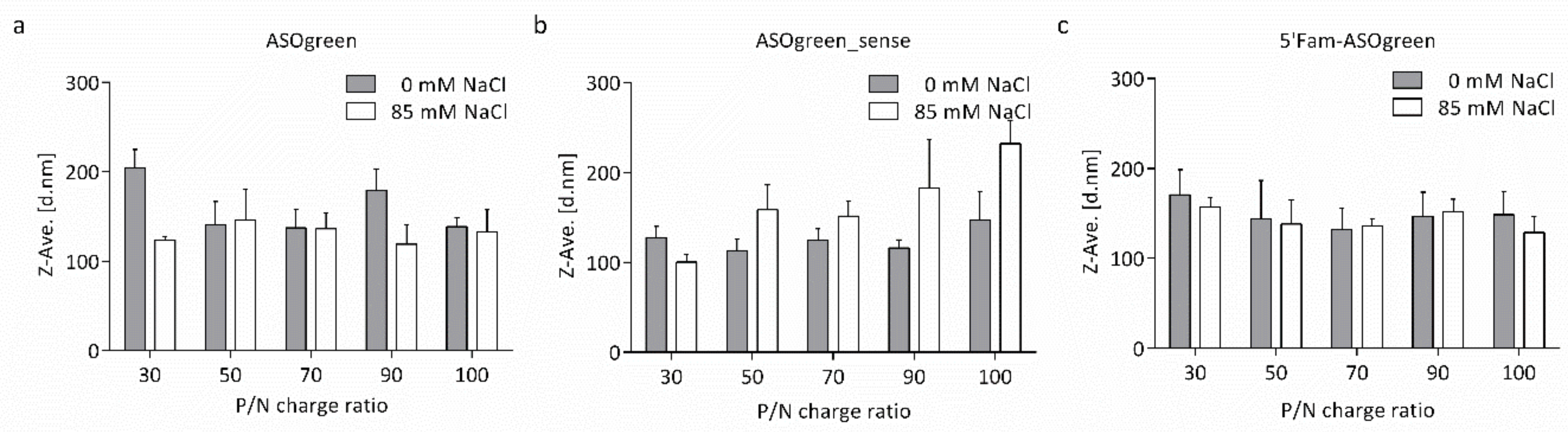
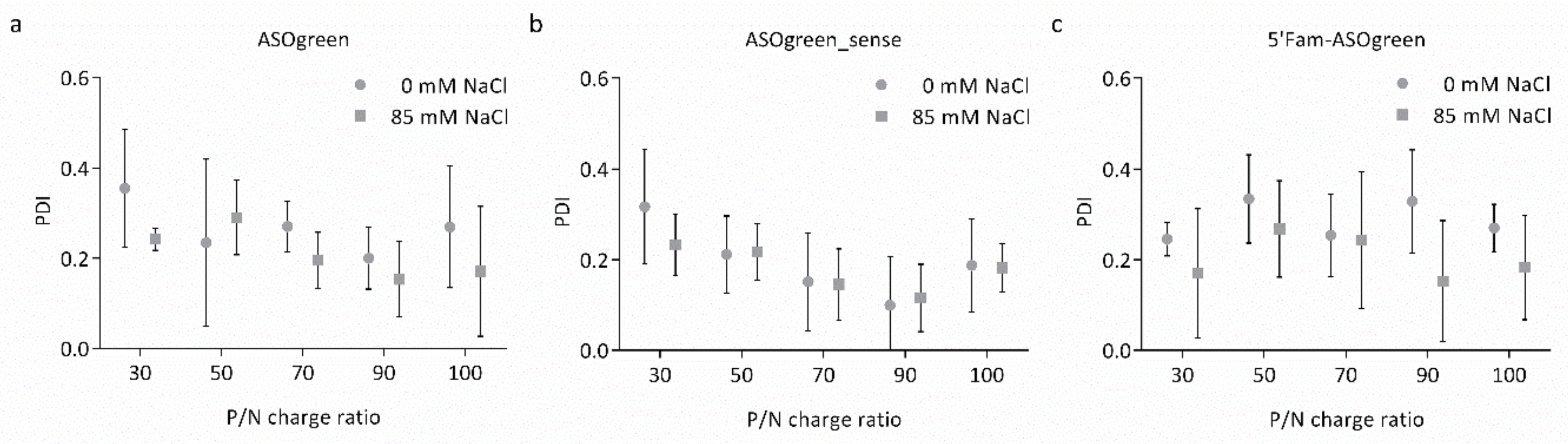
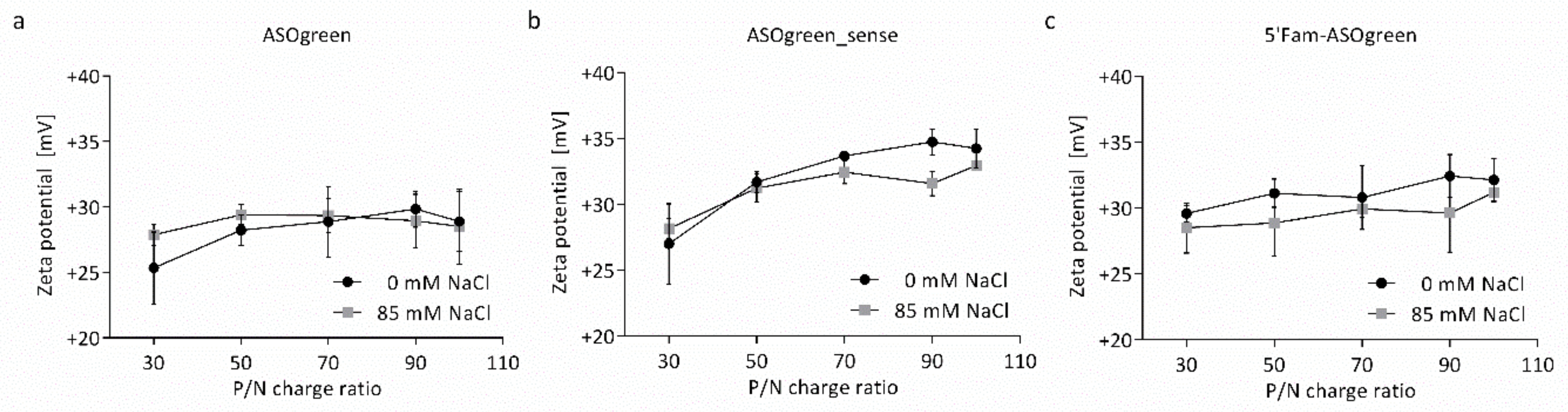
3.1. Physicochemical Characterization of Nanocomplexes
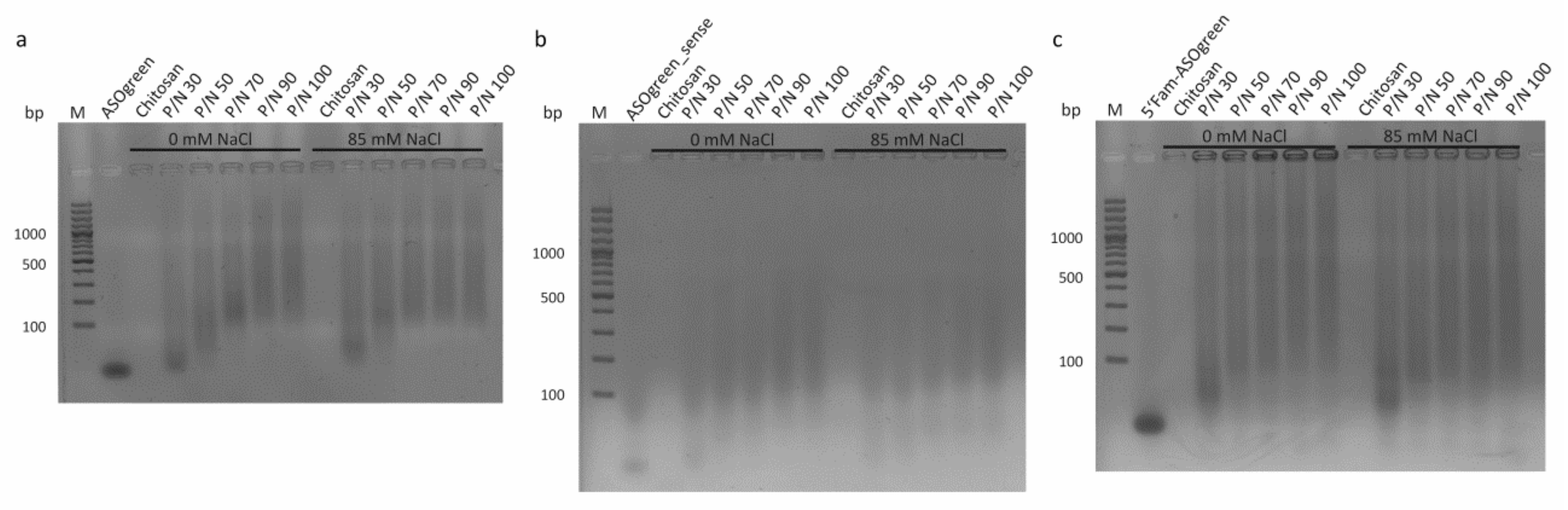
3.2. Stability of CS–ASO Nanocomplexes
3.3. Cell Culture Experiments with CS–ASO Nanocomplexes
4. Discussion
4.1. Salt Benefits the Formation of CS–ASO Nanocomplexes
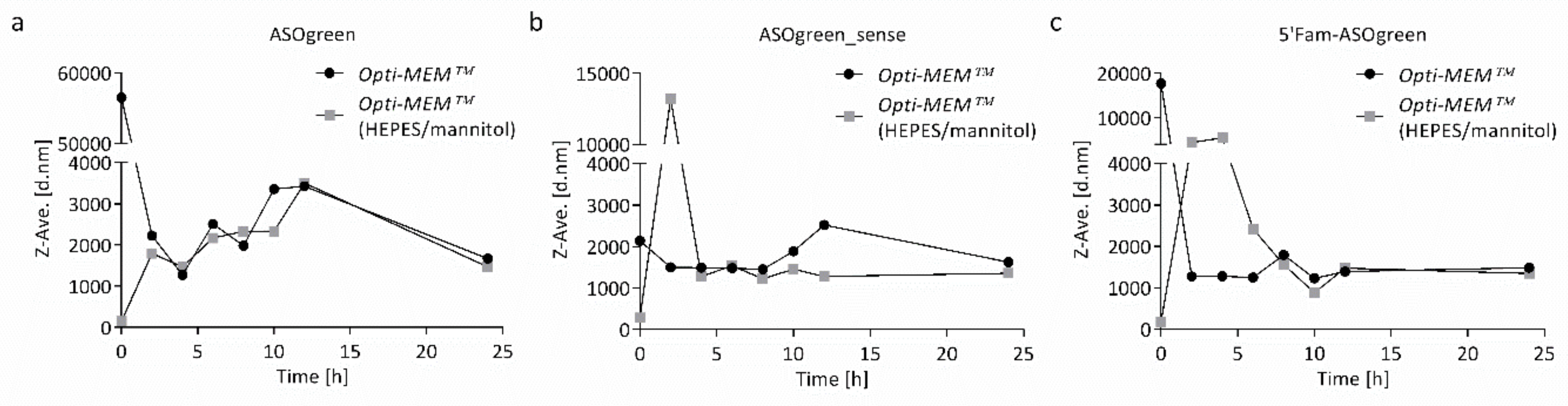
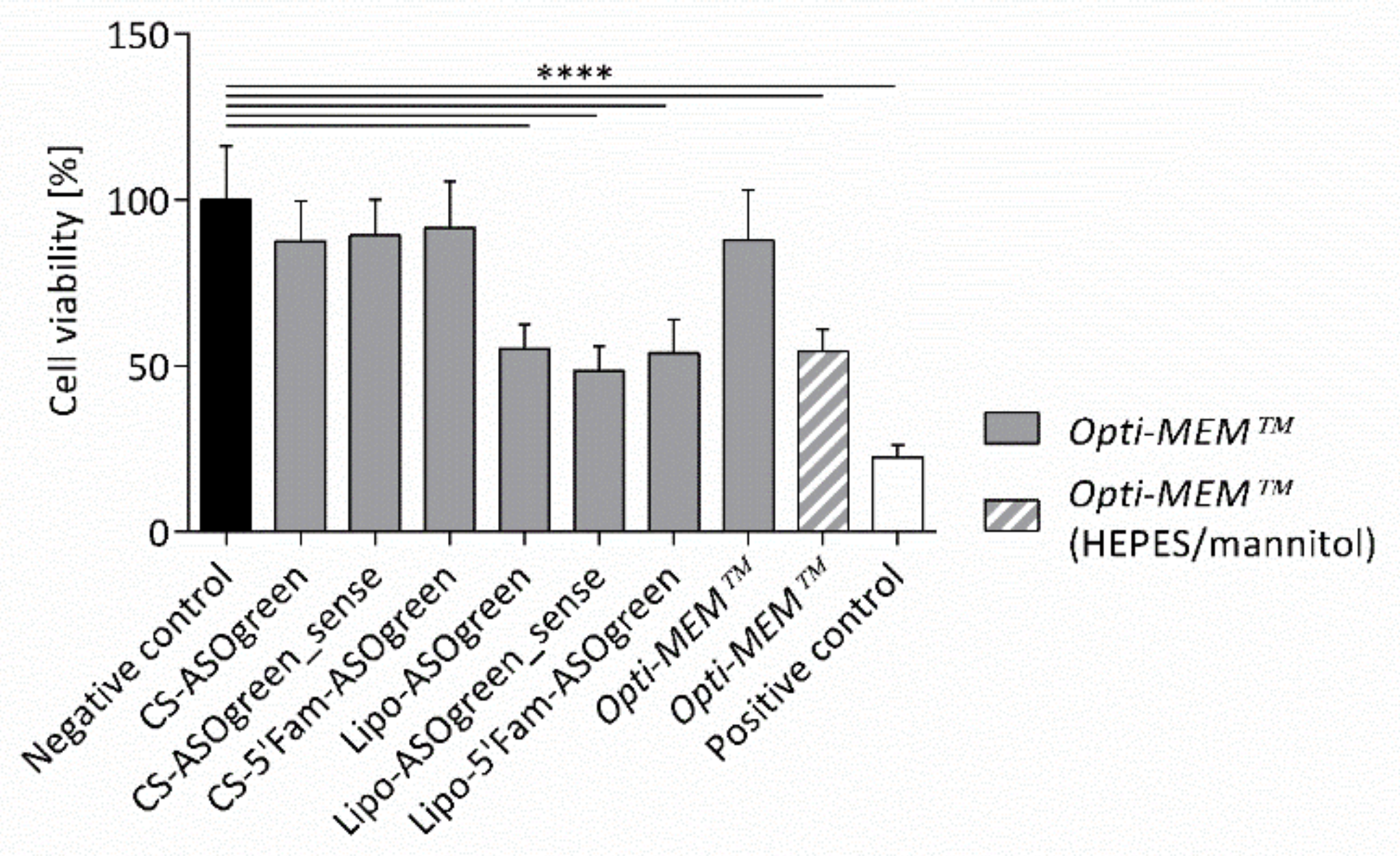
4.2. Supplemented Transfection Medium Stabilizes Nanocomplexes but Harms Human Respiratory Epithelial Cells
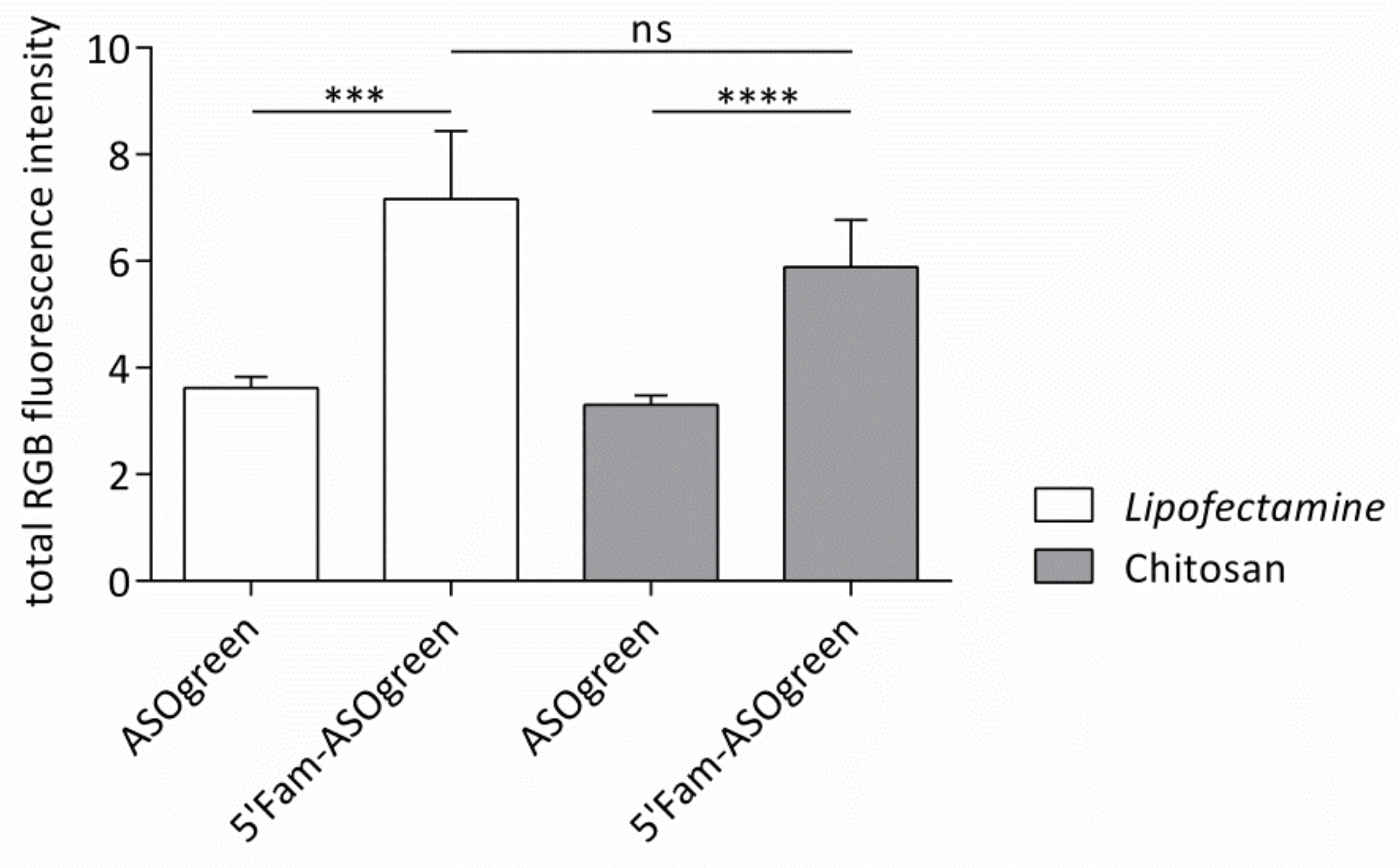
4.3. ASO Transfection Successfully Downregulates ENaC Activity in Human Respiratory Epithelial Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Quon, B.S.; Rowe, S.M. New and emerging targeted therapies for cystic fibrosis. BMJ 2016, 352, i859. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Naehrig, S.; Chao, C.-M.; Naehrlich, L. Cystic fibrosis. Dtsch. Arztebl. Int. 2017, 114, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Schwiebert, E.M.; Benos, D.J.; Egan, M.E.; Stutts, M.J.; Guggino, W.B. CFTR is a conductance regulator as well as a chloride channel. Physiol. Rev. 1999, 79, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Bangel, N.; Dahlhoff, C.; Sobczak, K.; Weber, W.-M.; Kusche-Vihrog, K. Upregulated expression of ENaC in human CF nasal epithelium. J. Cyst. Fibros. 2008, 7, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rückes-Nilges, C.; Weber, U.; Lindemann, H.; Münker, G.; Clauss, W.; Weber, W.-M. Minor Role of Cl− secretion in non-cystic fibrosis and cystic fibrosis human nasal epithelium. Cell. Physiol. Biochem. 1999, 9, 1–10. [Google Scholar] [CrossRef]
- Boucher, R. An overview of the pathogenesis of cystic fibrosis lung disease. Adv. Drug Deliv. Rev. 2002, 54, 1359–1371. [Google Scholar] [CrossRef]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Sobczak, K.; Segal, A.; Bangel-Ruland, N.; Semmler, J.; Van Driessche, W.; Lindemann, H.; Heermann, R.; Weber, W.-M. Specific inhibition of epithelial Na+ channels by antisense oligonucleotides for the treatment of Na+ hyperabsorption in cystic fibrosis. J. Gene Med. 2009, 11, 813–823. [Google Scholar] [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Drevinek, P.; Pressler, T.; Cipolli, M.; De Boeck, K.; Schwarz, C.; Bouisset, F.; Boff, M.; Henig, N.; Paquette-Lamontagne, N.; Montgomery, S.; et al. Antisense oligonucleotide eluforsen is safe and improves respiratory symptoms in F508DEL cystic fibrosis. J. Cyst. Fibros. 2020, 19, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Borchard, G. Chitosans for gene delivery. Adv. Drug Deliv. Rev. 2001, 52, 145–150. [Google Scholar] [CrossRef]
- Grenha, A.; Al-Qadi, S.; Seijo, B.; Remuñán-López, C. The potential of chitosan for pulmonary drug delivery. J. Drug Deliv. Sci. Technol. 2010, 20, 33–43. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Prego, C.; Torres, D.; Alonso, M.J. The potential of chitosan for the oral administration of peptides. Expert Opin. Drug Deliv. 2005, 2, 843–854. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef]
- Ma, L.; Shen, C.; Gao, L.; Li, D.; Shang, Y.; Yin, K.; Zhao, D.; Cheng, W.; Quan, D. Anti-inflammatory activity of chitosan nanoparticles carrying NF-κB/p65 antisense oligonucleotide in RAW264.7 macropghage stimulated by lipopolysaccharide. Colloids Surfaces B Biointerfaces 2016, 142, 297–306. [Google Scholar] [CrossRef]
- Shilakari Asthana, G.; Asthana, A.; Kohli, D.V.; Vyas, S.P. Mannosylated chitosan nanoparticles for delivery of antisense oligonucleotides for macrophage targeting. Biomed Res. Int. 2014, 2014, 17. [Google Scholar] [CrossRef]
- Dung, T.H.; Lee, S.R.; Han, S.D.; Kim, S.J.; Ju, Y.M.; Kim, M.S.; Yoo, H. Chitosan-TPP nanoparticle as a release system of antisense oligonucleotide in the oral environment. J. Nanosci. Nanotechnol. 2007, 7, 3695–3699. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Taetz, S.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: Effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine 2007, 3, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Fernández Fernández, E.; Santos-Carballal, B.; Weber, W.-M.; Goycoolea, F.M. Chitosan as a non-viral co-transfection system in a cystic fibrosis cell line. Int. J. Pharm. 2016, 502, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kolonko, A.K.; Fernández Fernández, E.; Santos-Carballal, B.; Goycoolea, F.M.; Weber, W.-M. Functional restoring of defect CFTR by transfection of CFTR- mRNA using chitosan. JSM Genet. Genomics 2016, 3, 1016. [Google Scholar]
- Segal, A.; Van Driessche, W.; Weber, W.-M. Specific effects of antisense oligonucleotides on ENaC expressed in Xenopus laevis oocytes. Pflugers Arch. 2002, 443, 228. [Google Scholar]
- Gröhn, F. Electrostatic self-assembly as route to supramolecular structures. Macromol. Chem. Phys. 2008, 209, 2295–2301. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjøniksen, A.L.; Hiorth, M. Effects of ionic strength on the size and compactness of chitosan nanoparticles. Colloid Polym. Sci. 2012, 290, 919–929. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano-and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surfaces B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef]
- Santos-Carballal, B.; Aaldering, L.J.; Ritzefeld, M.; Pereira, S.; Sewald, N.; Moerschbacher, B.M.; Götte, M.; Goycoolea, F.M. Physicochemical and biological characterization of chitosan-microRNA nanocomplexes for gene delivery to MCF-7 breast cancer cells. Sci. Rep. 2015, 5, 13567. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir 2011, 27, 10392–10399. [Google Scholar] [CrossRef] [PubMed]
- Singare, D.S.; Marella, S.; Gowthamrajan, K.; Kulkarni, G.T.; Vooturi, R.; Rao, P.S. Optimization of formulation and process variable of nanosuspension: An industrial perspective. Int. J. Pharm. 2010, 402, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Nah, J.-W.; Kwon, Y.; Koh, J.J.; Ko, K.S.; Kim, S.W. Water-soluble and low molecular weight chitosan-based plasmid DNA delivery. Pharm. Res. 2001, 18, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.-H.; Qiu, B.; Zhou, P.-H. Chitosan/hyaluronic acid/plasmid-DNA nanoparticles encoding interleukin-1 receptor antagonist attenuate inflammation in synoviocytes induced by interleukin-1 beta. J. Mater. Sci. Mater. Med. 2018, 29, 155. [Google Scholar] [CrossRef]
- Sanders, N.; Rudolph, C.; Braeckmans, K.; De Smedt, S.C.; Demeester, J. Extracellular barriers in respiratory gene therapy. Adv. Drug Deliv. Rev. 2009, 61, 115–127. [Google Scholar] [CrossRef]
- Broughton-Head, V.J.; Smith, J.R.; Shur, J.; Shute, J.K. Actin limits enhancement of nanoparticle diffusion through cystic fibrosis sputum by mucolytics. Pulm. Pharmacol. Ther. 2007, 20, 708–717. [Google Scholar] [CrossRef]
- Sanders, N.N.; De Smedt, S.C.; Van Rompaey, E.; Simoens, P.; De Baets, F.; Demeester, J. Cystic fibrosis sputum. Am. J. Respir. Crit. Care Med. 2000, 162, 1905–1911. [Google Scholar] [CrossRef]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Huang, M.; Fong, C.W.; Khor, E.; Lim, L.Y. Transfection efficiency of chitosan vectors: Effect of polymer molecular weight and degree of deacetylation. J. Control. Release 2005, 106, 391–406. [Google Scholar] [CrossRef]
- Freeman, E.C.; Weiland, L.M.; Meng, W.S. Modeling the proton sponge hypothesis: Examining proton sponge effectiveness for enhancing intracellular gene delivery through multiscale modeling. J. Biomater. Sci. Polym. Ed. 2013, 24, 398–416. [Google Scholar] [CrossRef]
- Funkhouser, J.D.; Aronson, N.N. Chitinase family GH18: Evolutionary insights from the genomic history of a diverse protein family. BMC Evol. Biol. 2007, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Boot, R.G.; Blommaart, E.F.C.; Swart, E.; Vlugt, K.G.d.; Bijl, N.; Moe, C.; Place, A.; Aerts, J.M.F.G. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 2001, 276, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Potter, J.; Kumar, S.; Zou, Y.; Quintanilla, R.; Sridharan, M.; Carte, J.; Chen, W.; Roark, N.; Ranganathan, S.; et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 2015, 208, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, D.; Giupponi, E.; Mantovani, D.; Candiani, G. Size matters for in vitro gene delivery: Investigating the relationships among complexation protocol, transfection medium, size and sedimentation. Sci. Rep. 2017, 7, 44134. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Chao, A.; Tsai, C.-L.; Sue, S.-C.; Lin, C.-Y.; Lee, Y.-Z.; Hung, Y.-L.; Chao, A.-S.; Cheng, A.-J.; Wang, H.-S.; et al. Utilization of HEPES for enhancing protein transfection into mammalian cells. Mol. Ther. - Methods Clin. Dev. 2019, 13, 99–111. [Google Scholar] [CrossRef]
- Riedl, S.; Kaiser, P.; Raup, A.; Synatschke, C.; Jérôme, V.; Freitag, R. Non-viral transfection of human T lymphocytes. Processes 2018, 6, 188. [Google Scholar] [CrossRef]
- Fernández Fernández, E.; Santos-Carballal, B.; de Santi, C.; Ramsey, J.; MacLoughlin, R.; Cryan, S.-A.; Greene, C. Biopolymer-based nanoparticles for cystic fibrosis lung gene therapy studies. Materials 2018, 11, 122. [Google Scholar] [CrossRef]
- Waymouth, C. Osmolality of mammalian blood and of media for culture of mammalian cells. In Vitro 1970, 6, 109–127. [Google Scholar] [CrossRef]
- Kastl, L.; Isbach, M.; Dirksen, D.; Schnekenburger, J.; Kemper, B. Quantitative phase imaging for cell culture quality control. Cytom. Part A 2017, 91, 470–481. [Google Scholar] [CrossRef]
- Myerburg, M.M.; King, J.D.; Oyster, N.M.; Fitch, A.C.; Magill, A.; Baty, C.J.; Watkins, S.C.; Kolls, J.K.; Pilewski, J.M.; Hallows, K.R. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2010, 42, 676–684. [Google Scholar] [CrossRef]
- Rubenstein, R.C.; Lockwood, S.R.; Lide, E.; Bauer, R.; Suaud, L.; Grumbach, Y. Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2011, 300, L88–L101. [Google Scholar] [CrossRef] [PubMed]
- Murgia, X.; Yasar, H.; Carvalho-Wodarz, C.; Gordon, S.; Schwarzkopf, K. Modelling the bronchial barrier in pulmonary drug delivery: A human bronchial epithelial cell line supplemented with human tracheal mucus. Eur. J. Pharm. Biopharm. 2017, 118, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, R.; Auerbach, S.D.; Li, X.; Loftus, R.W.; Husted, R.F.; Stokes, J.B.; Thomas, C.P. Glucocorticoid induction of epithelial sodium channel expression in lung and renal epithelia occurs via trans-activation of a hormone response element in the 5′-flanking region of the human epithelial sodium channel alpha subunit gene. J. Biol. Chem. 1999, 274, 12431–12437. [Google Scholar] [CrossRef]
- Zabner, J.; Karp, P.; Seiler, M.; Phillips, S.L.; Mitchell, C.J.; Saavedra, M.; Welsh, M.; Klingelhutz, A.J. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L844–L854. [Google Scholar] [CrossRef]
- Uhlmann, E.; Peyman, A. Antisense oligonucleotides: A new therapeutic principle. Chem. Rev. 1990, 90, 543–584. [Google Scholar] [CrossRef]
- Wang, T.; Larcher, L.; Ma, L.; Veedu, R. Systematic screening of commonly used commercial transfection reagents towards efficient transfection of single-stranded oligonucleotides. Molecules 2018, 23, 2564. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Live-cell imaging to compare the transfection and gene silencing efficiency of calcium phosphate nanoparticles and a liposomal transfection agent. Gene Ther. 2017, 24, 282–289. [Google Scholar] [CrossRef]
- Lim, S.; Forbes, B.; Martin, G.; Brown, M. In vivo and in vitro characterization of novel microparticulates based on hyaluronan and chitosan hydroglutamate. AAPS PharmSciTech 2001, 2, 1–12. [Google Scholar] [CrossRef]
- Florea, B.I.; Thanou, M.; Junginger, H.E.; Borchard, G. Enhancement of bronchial octreotide absorption by chitosan and N-trimethyl chitosan shows linear in vitro/in vivo correlation. J. Control. Release 2006, 110, 353–361. [Google Scholar] [CrossRef]
- Jain, L.; Chen, X.-J.; Malik, B.; Al-Khalili, O.; Eaton, D.C. Antisense oligonucleotides against the α-subunit of ENaC decrease lung epithelial cation-channel activity. Am. J. Physiol. Cell. Mol. Physiol. 1999, 276, L1046–L1051. [Google Scholar] [CrossRef]
- Griesenbach, U.; Kitson, C.; Garcia, E.S.; Farley, R.; Singh, C.; Somerton, L.; Painter, H.; Smith, R.L.; Gill, D.R.; Hyde, S.C.; et al. Inefficient cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir. Res. 2006, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Crosby, J.R.; Zhao, C.; Jiang, C.; Bai, D.; Katz, M.; Greenlee, S.; Kawabe, H.; McCaleb, M.; Rotin, D.; Guo, S.; et al. Inhaled ENaC antisense oligonucleotide ameliorates cystic fibrosis-like lung disease in mice. J. Cyst. Fibros. 2017, 16, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Semaniakou, A.; Croll, R.P.; Chappe, V. Animal models in the pathophysiology of cystic fibrosis. Front. Pharmacol. 2018, 9, 1475. [Google Scholar] [CrossRef] [PubMed]


| Oligo Name | Sequence 5′-3′ | Orientation |
|---|---|---|
| ASOgreen | TGG ATG GTG GTG TTG T | antisense |
| ASOgreen_sense | ACA ACA CCA CCA TCC A | sense (negative control) |
| 5′Fam-ASOgreen | 6-Fam-TGG ATG GTG GTG TTG T | antisense (fluorescent) |
| Oligo Name | Charge Ratio | ASO | Chitosan | ||
|---|---|---|---|---|---|
| P/N 1 | (nmol) 2 | (µg/µL) | (nmol) 3 | (µg/µL) | |
| 30 | 4.6 | 0.3 | 137.0 | 2.8 | |
| 50 | 4.6 | 0.3 | 228.4 | 4.6 | |
| ASOgreen | 70 | 4.6 | 0.3 | 319.7 | 6.5 |
| 90 | 4.6 | 0.3 | 411.0 | 8.3 | |
| 100 | 4.6 | 0.3 | 456.7 | 9.3 | |
| 30 | 4.8 | 0.3 | 144.3 | 2.9 | |
| 50 | 4.8 | 0.3 | 240.5 | 4.9 | |
| ASOgreen_sense | 70 | 4.8 | 0.3 | 336.7 | 6.8 |
| 90 | 4.8 | 0.3 | 433.0 | 8.8 | |
| 100 | 4.8 | 0.3 | 481.1 | 9.8 | |
| 30 | 4.7 | 0.3 | 140.0 | 2.8 | |
| 50 | 4.7 | 0.3 | 233.1 | 4.7 | |
| 5′Fam-ASOgreen | 70 | 4.7 | 0.3 | 326.4 | 6.6 |
| 90 | 4.7 | 0.3 | 419.6 | 8.5 | |
| 100 | 4.7 | 0.3 | 466.2 | 9.5 | |
| Attribute | ASOgreen | ASOgreen_sense | 5‘Fam-ASOgreen |
|---|---|---|---|
| Hydrodynamic diameter [d.nm] | 119.0 ± 17.9 | 182.9 ± 44.3 | 151.7 ± 11.6 |
| PDI | 0.15 ± 0.07 | 0.12 ± 0.06 | 0.15 ± 0.11 |
| Zeta potential [mV] | +28.9 ± 1.7 | +31.6 ± 0.7 | +29.6 ± 2.4 |
| Medium | Osmolality (mOsmol/kg) |
|---|---|
| NCI-H441 cell culture medium | 270.3 ± 1.7 |
| Opti-MEM™ | 272.7 ± 2.6 |
| Opti-MEM™ + HEPES (20 mM) + mannitol (270 mM) | 582.7 ± 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolonko, A.K.; Bangel-Ruland, N.; Goycoolea, F.M.; Weber, W.-M. Chitosan Nanocomplexes for the Delivery of ENaC Antisense Oligonucleotides to Airway Epithelial Cells. Biomolecules 2020, 10, 553. https://doi.org/10.3390/biom10040553
Kolonko AK, Bangel-Ruland N, Goycoolea FM, Weber W-M. Chitosan Nanocomplexes for the Delivery of ENaC Antisense Oligonucleotides to Airway Epithelial Cells. Biomolecules. 2020; 10(4):553. https://doi.org/10.3390/biom10040553
Chicago/Turabian StyleKolonko, A. Katharina, Nadine Bangel-Ruland, Francisco M. Goycoolea, and Wolf-Michael Weber. 2020. "Chitosan Nanocomplexes for the Delivery of ENaC Antisense Oligonucleotides to Airway Epithelial Cells" Biomolecules 10, no. 4: 553. https://doi.org/10.3390/biom10040553
APA StyleKolonko, A. K., Bangel-Ruland, N., Goycoolea, F. M., & Weber, W.-M. (2020). Chitosan Nanocomplexes for the Delivery of ENaC Antisense Oligonucleotides to Airway Epithelial Cells. Biomolecules, 10(4), 553. https://doi.org/10.3390/biom10040553








