Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment of Rb-ME
2.3. Cell Culture
2.4. Nitric Oxide (NO) Assay
2.5. MTT Assay
2.6. Animals
2.7. Isolation of Peritoneal Macrophages
2.8. High-Performance Liquid Chromatography (HPLC) Analysis
2.9. Semi-Quantitative RT-PCR
2.10. Enzyme Linked Immunosorbent Assay (ELISA)
2.11. Preparing Whole or Nuclear Lysates
2.12. Western Blotting
2.13. Luciferase Assay
2.14. Plasmid Transfection
2.15. In Vitro Kinase Assay
2.16. LPS-Induced Peritonitis In Vivo Model
2.17. HCl/EtOH-Gastritis In Vivo Model and Measurement of MPO Activity
2.18. Statistical Analysis
3. Results
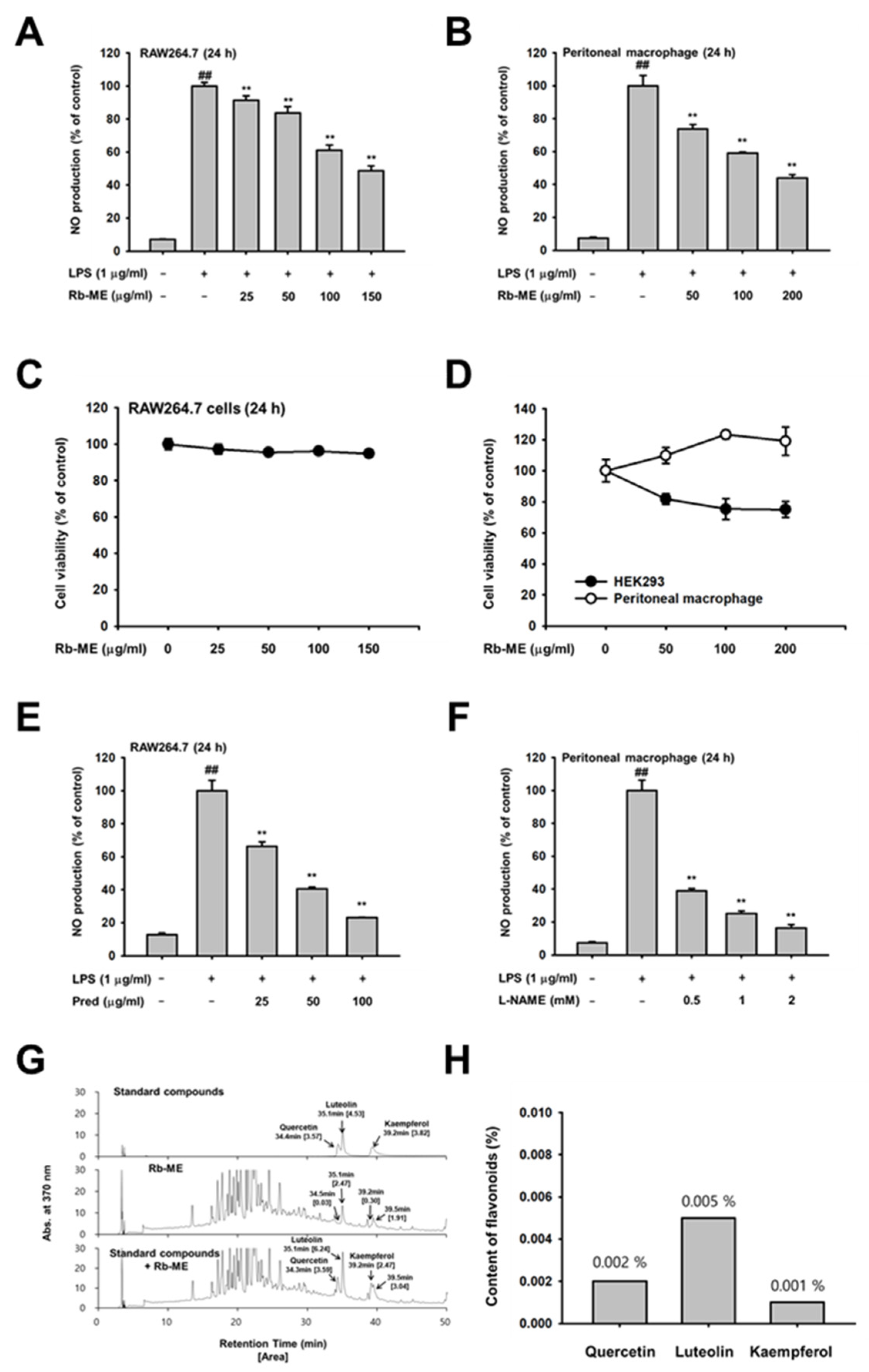
3.1. Inhibitory Effect of Rb-ME on NO Production in RAW264.7 Cells and Peritoneal Macrophages
3.2. Suppressive Effect of Rb-ME on Inflammatory Gene Expressions at a Transcriptional Level
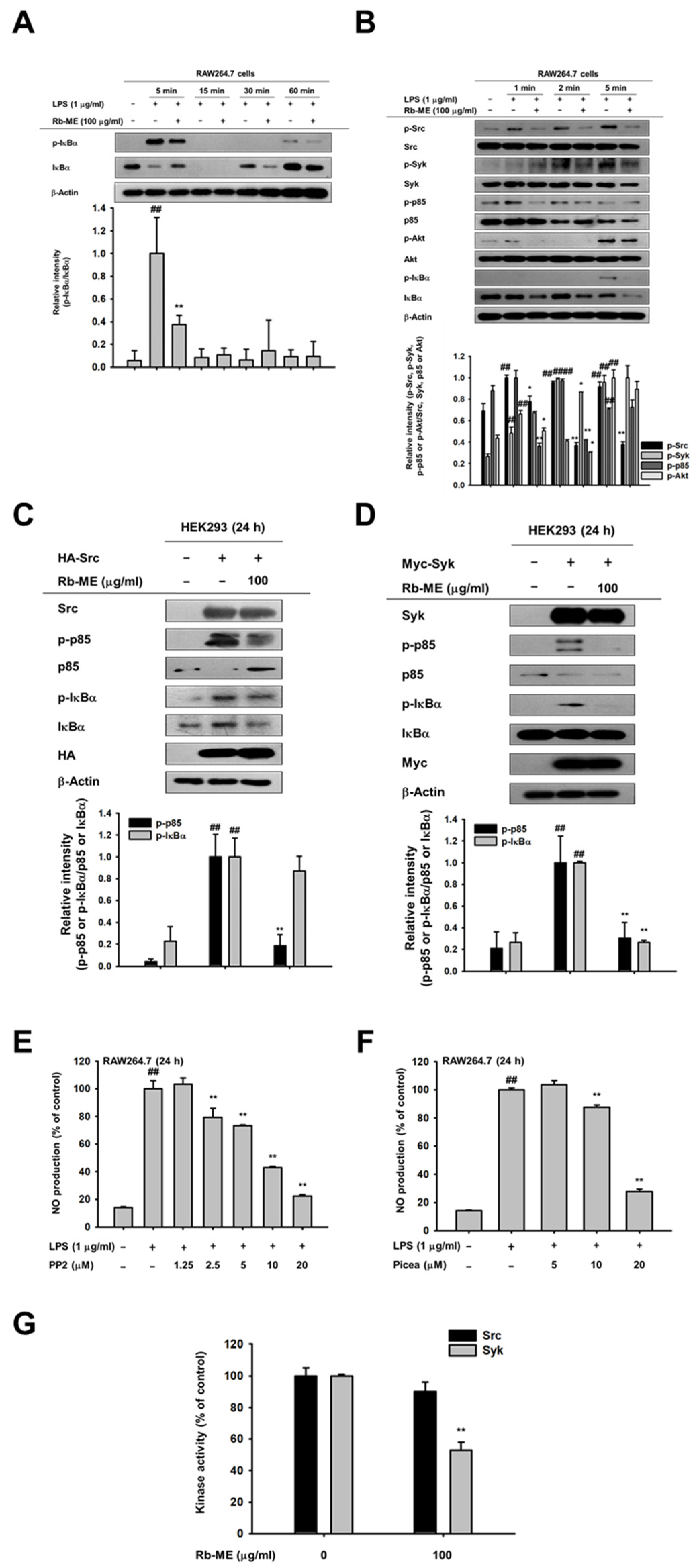
3.3. Effect of Rb-ME on the NF-κB Signaling Pathway
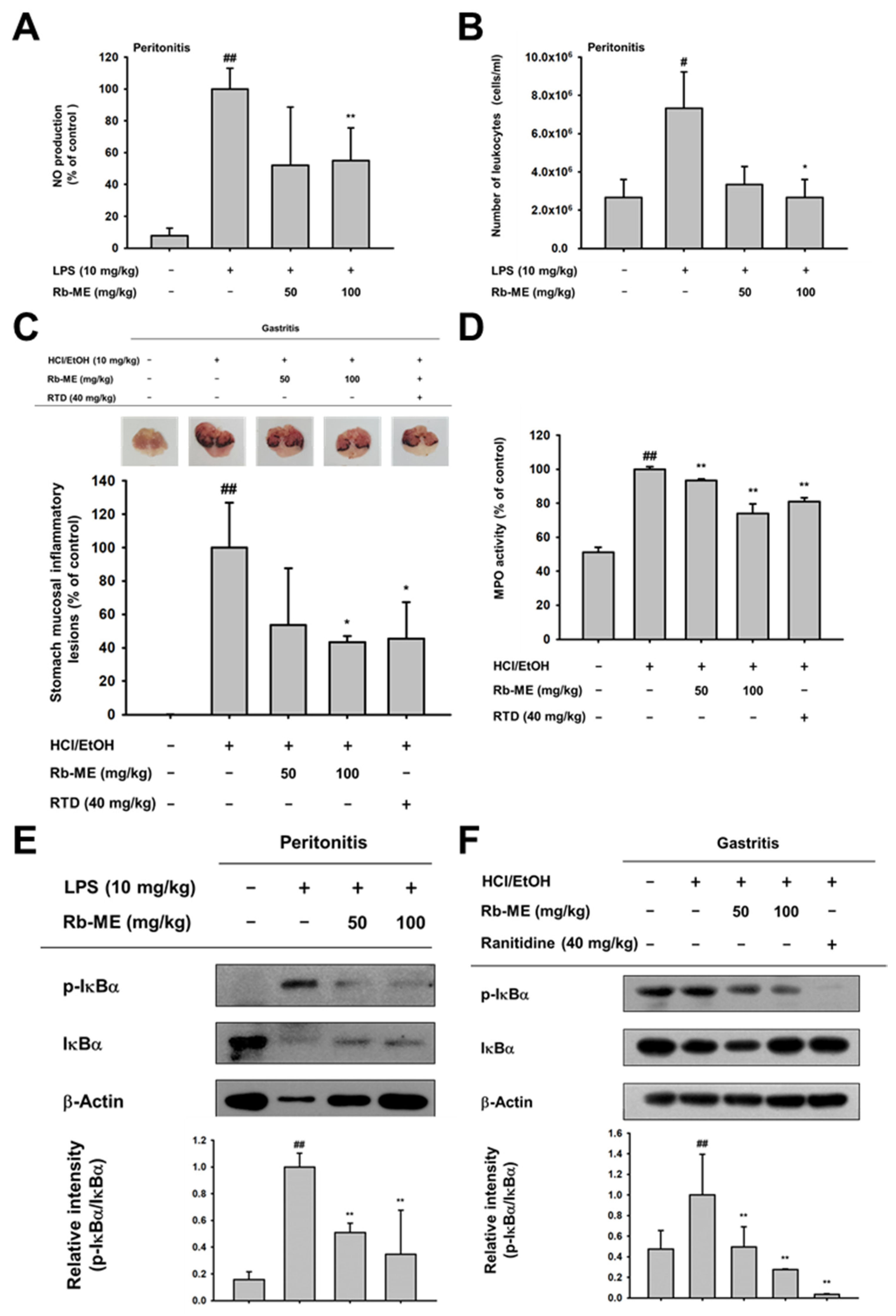
3.4. Anti-Inflammatory Effect of Rb-ME in LPS-Induced Peritonitis and HCl/EtOH-Induced Gastritis In Vivo Models
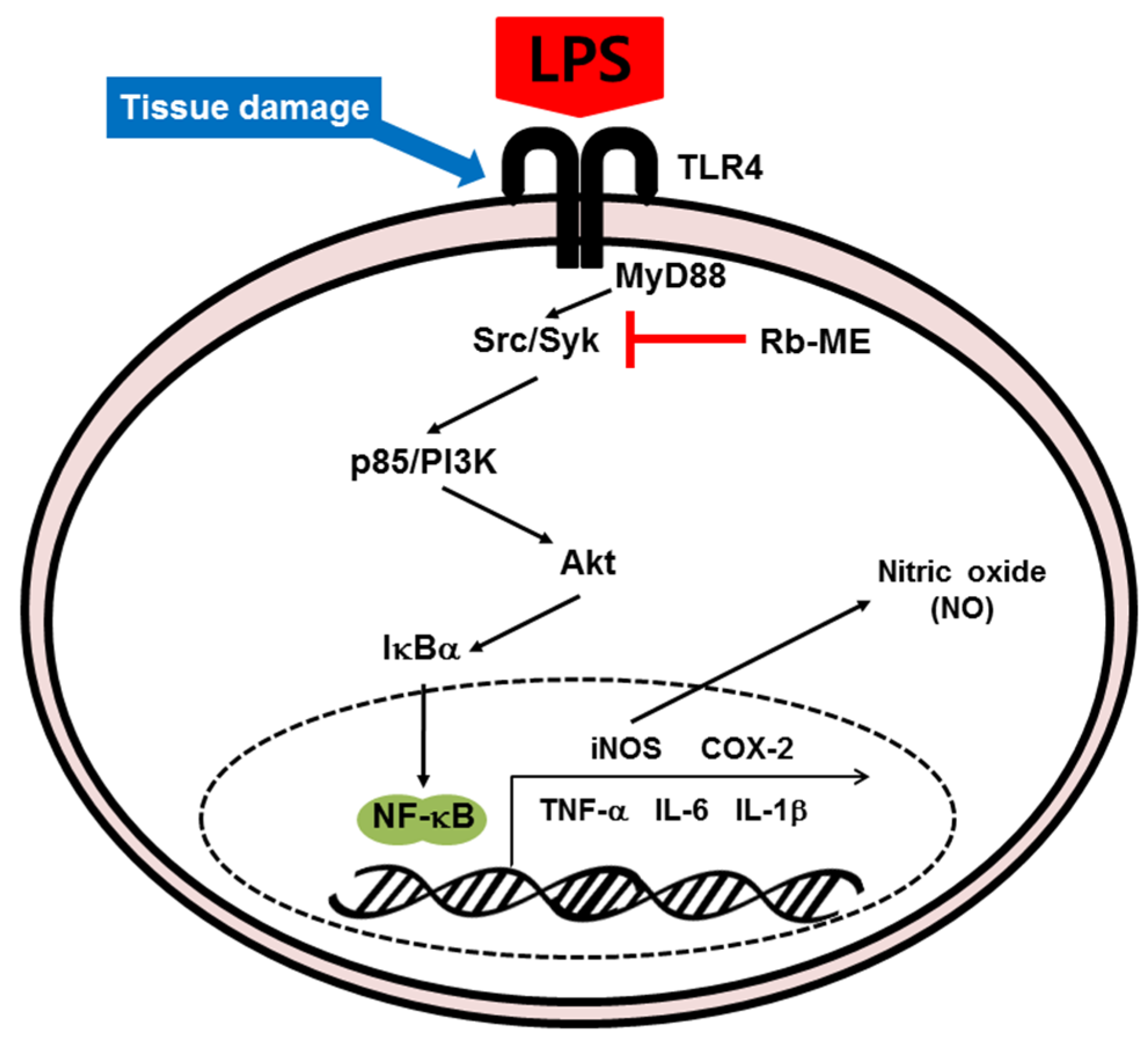
4. Discussions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PAMPs | Pathogen-associated molecular patterns |
| PRRs | Pattern recognition receptors |
| TLRs | Toll-like receptors |
| LPS | Lipopolysaccharide |
| Syk | Spleen tyrosine kinase |
| PI3K p85 | Phosphoinositide-3-kinase p85 |
| IκBα | Inhibitor of kappa B alpha |
| MAPK | Mitogen activated protein kinase |
| MYD88 | Myeloid differentiation primary response 88 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| COX-2 | Cyclooxygenase-2 |
| IL | Interleukin |
| RH | Right hand (Right side) |
| FRT | Front |
| ACC | Accessory |
| NO | Nitric oxide |
| Pred | Prednisolone |
| TNF-α | Tumor necrosis factor-alpha |
| IL | Interleukin |
| AP-1 | Activator protein 1 |
| IP | Intraperitoneal |
| NOS | NO synthases |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
References
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Target Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.D.S.; Soldau, K.; Christen, U.; Tobias, P.S.; Ulevitch, R.J. Lipopolysaccharide Is in Close Proximity to Each of the Proteins in Its Membrane Receptor Complex. J. Boil. Chem. 2001, 276, 21129–21135. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Boil. 2016, 100, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Muroi, M.; Kinoshita, M.; Hamada, O.; Minai, Y.; Sugita-Konishi, Y.; Kamata, Y.; Tanamoto, K. NF-kappaB activation via MyD88-dependent Toll-like receptor signaling is inhibited by trichothecene mycotoxin deoxynivalenol. J. Toxicol. Sci. 2016, 41, 273–279. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, J.; Kim, E.; Kim, S.H.; Seo, D.B.; Kim, J.-H.; Shin, S.S.; Cho, J.Y. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J. Ginseng Res. 2017, 42, 496–503. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Lee, J.-O.; Choi, E.; Shin, K.K.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Hossain, M.A.; Kim, H.S.; Yi, Y.-S.; Kim, D.; et al. Compound K, a ginsenoside metabolite, plays an antiinflammatory role in macrophages by targeting the AKT1-mediated signaling pathway. J. Ginseng Res. 2018, 43, 154–160. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Sham, T.-T.; Chan, C.O.; Wang, Y.; Yang, J.-M.; Mok, D.K.-W.; Chan, S.-W. A Review on the Traditional Chinese Medicinal Herbs and Formulae with Hypolipidemic Effect. BioMed Res. Int. 2014, 2014, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dobriyal, R.M.; Singh, G.S.; Rao, K.S.; Saxena, K.G. Medicinal Plant Resources in Chhakinal Watershed in the Northwestern Himalaya. J. Herbs, Spices Med. Plants 1997, 5, 15–27. [Google Scholar] [CrossRef]
- Pande, P.; Tiwari, L.; Pande, H. Ethnoveterinary plants of Uttaranchal—A Review; CSIR: Hobart, Australia, 2007. [Google Scholar]
- Mantle, D.; Eddeb, F.; Pickering, A.T. Comparison of relative antioxidant activities of British medicinal plant species in vitro. J. Ethnopharmacol. 2000, 72, 47–51. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ghedira, K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomed. 2009, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, W.S.; Yu, T.; Yi, Y.-S.; Park, J.G.; Jeong, D.; Kim, J.-H.; Oh, J.S.; Yoon, K.; Kim, J.-H.; et al. Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases. Biochem. Pharmacol. 2014, 88, 201–215. [Google Scholar] [CrossRef]
- Hossen, M.J.; Hong, Y.D.; Baek, K.-S.; Yoo, S.; Hong, Y.H.; Kim, J.H.; Lee, J.-O.; Kim, N.; Park, J.; Cho, J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2016, 41, 43–51. [Google Scholar] [CrossRef]
- Kim, E.; Yi, Y.-S.; Son, Y.-J.; Han, S.Y.; Kim, N.H.; Nam, G.; Hossain, M.A.; Kim, J.-H.; Park, J.; Cho, J.Y. BIOGF1K, a compound K-rich fraction of ginseng, plays an antiinflammatory role by targeting an activator protein-1 signaling pathway in RAW264.7 macrophage-like cells. J. Ginseng Res. 2018, 42, 233–237. [Google Scholar] [CrossRef]
- Hong, Y.H.; Kim, N.; Nam, G.; Yoo, S.; Han, S.Y.; Jeong, S.-G.; Kim, E.; Jeong, D.; Yoon, K.; Kim, S.; et al. Photoaging protective effects of BIOGF1K, a compound-K-rich fraction prepared from Panax ginseng. J. Ginseng Res. 2017, 42, 81–89. [Google Scholar] [CrossRef]
- Almela, L.; Sánchez-Muñoz, B.; Fernández-López, J.; Roca, M.J.; Rabe, V. Liquid chromatograpic–mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J. Chromatogr. A 2006, 1120, 221–229. [Google Scholar] [CrossRef]
- Baek, K.-S.; Yi, Y.-S.; Son, Y.-J.; Yoo, S.; Sung, N.Y.; Kim, Y.; Hong, S.; Aravinthan, A.; Kim, J.-H.; Cho, J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J. Ginseng Res. 2016, 40, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, E.; Kim, J.H.; Yoon, K.; Kim, S.; Lee, J.; Cho, J.Y. AKT1-targeted proapoptotic activity of compound K in human breast cancer cells. J. Ginseng Res. 2019, 43, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Kim, M.-Y.; Cho, J.Y. MAPK/AP-1-Targeted Anti-Inflammatory Activities of Xanthium strumarium. Am. J. Chin. Med. 2016, 44, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.-S.; Jung, S.-H.; Kim, S.-Y.; Hyun, J.-W.; Ko, K.; Kim, W.-K.; Kim, H.-S. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005, 335, 1017–1025. [Google Scholar] [CrossRef]
- Yi, Y.-S.; Cho, J.Y.; Kim, D. Cerbera manghas methanol extract exerts anti-inflammatory activity by targeting c-Jun N-terminal kinase in the AP-1 pathway. J. Ethnopharmacol. 2016, 193, 387–396. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Jin, L.; Chen, G.; Li, C.; Qi, K.; Kong, D.; Wang, Y.; Song, M.; Ma, L. Effectiveness of and risk associated with aspirin therapy in hemodialysis patients with a background of antiplatelet factor 4/heparin complex antibody detection. Thromb. Res. 2015, 136, 61–68. [Google Scholar] [CrossRef]
- Park, J.G.; Kim, S.C.; Kim, Y.H.; Yang, W.S.; Kim, Y.; Hong, S.; Kim, K.-H.; Yoo, B.C.; Kim, S.H.; Kim, J.-H.; et al. Anti-Inflammatory and Antinociceptive Activities of Anthraquinone-2-Carboxylic Acid. Mediat. Inflamm. 2016, 2016, 1903849. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wagner, E.F. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann. Rheum. Dis. 2011, 70, 109–112. [Google Scholar] [CrossRef]
- Lee, Y.G.; Chain, B.M.; Cho, J.Y. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of kappaB alpha via activation of the phosphoinositide-3-kinase and Akt pathways. Int. J. Biochem. Cell Biol. 2009, 41, 811–821. [Google Scholar] [CrossRef]
- Aslam, M.S.; Choudhary, B.A.; Uzair, M.; Ijaz, A.S. The genus Ranunculus: A phytochemical and ethnopharmacological review. Int. J. Pharm. Pharm. Sci. 2012, 4, 15–22. [Google Scholar]
- Hill, R.; Van Heyningen, R. Ranunculin: The precursor of the vesicant substance of the buttercup. Biochem. J. 1951, 49, 332–335. [Google Scholar] [CrossRef] [PubMed]
- An, I.; Ucmak, D.; Esen, M.; Gevher, O.D. Phytocontact dermatitis due to Ranunculus arvensis: Report of three cases. North. Clin. Istanb. 2018, 6, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.-W.; Nathan, C. Nitric Oxide and Macrophage Function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Röszer, T. The Biology of Subcellular Nitric Oxide; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Hear. J. 2011, 33, 829–837. [Google Scholar] [CrossRef]
- Xue, Q.-J.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar] [CrossRef]
- Iuvone, T.; Carnuccio, R.; Di Rosa, M. Modulation of granuloma formation by endogenous nitric oxide. Eur. J. Pharmacol. 1994, 265, 89–92. [Google Scholar] [CrossRef]
- McCartney-Francis, N.; Allen, J.B.; Mizel, D.E.; Albina, J.E.; Xie, Q.W.; Nathan, C.F.; Wahl, S.M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 1993, 178, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Stratman, N.C.; Carter, D.B.; Sethy, V.H. Ibuprofen: Effect on inducible nitric oxide synthase. Mol. Brain Res. 1997, 50, 107–112. [Google Scholar] [CrossRef]
- DiGirolamo, G.; Farina, M.; Riberio, M.L.; Ogando, D.; Aisemberg, J.; Santos, A.R.D.L.; Marti, M.L.; Franchi, A.M. Effects of cyclooxygenase inhibitor pretreatment on nitric oxide production, nNOS and iNOS expression in rat cerebellum. Br. J. Pharmacol. 2003, 139, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Franchi, A.; Di Girolamo, G.; Farina, M.; Santos, A.D.L.; Martí, M.; Gimeno, M. In Vivo and in Vitro Effects of Lysine Clonixinate on Nitric Oxide Synthase in LPS-Treated and Untreated Rat Lung Preparations. Nitric Oxide 2001, 5, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Triadafilopoulos, G. Epidemiology of NSAID induced gastrointestinal complications. J. Rheumatol. Suppl. 1999, 56, 18–24. [Google Scholar]
- Ng, S.C.; Chan, F.K.L. NSAID-induced gastrointestinal and cardiovascular injury. Curr. Opin. Gastroenterol. 2010, 26, 611–617. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Christman, J.W. The Role of Nuclear Factor- κ B in Cytokine Gene Regulation. Am. J. Respir. Cell Mol. Boil. 1997, 17, 3–9. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPSinduced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NFkappaB, STAT3 or AP1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar]
- Cui, S.; Bilitewski, U. Anti-inflammatory effect of Syk inhibitor in LPS stimulated macrophages. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2013, 29, 1024–1027. [Google Scholar]
- Lee, H.S.; Moon, C.; Lee, H.W.; Park, E.-M.; Cho, M.-S.; Kang, J.L. Src tyrosine kinases mediate activations of NF-kappaB and integrin signal during lipopolysaccharide-induced acute lung injury. J. Immunol. 2007, 179, 7001–7011. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Boil. 2009, 1, a001651. [Google Scholar]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Invest. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.; Albanese, C.; Steer, J.; Fu, M.; Bouzahzah, B.; Pestell, R.G. NF-kappaB and cell-cycle regulation: The cyclin connection. Cytokine Growth Factor Rev. 2001, 12, 73–90. [Google Scholar] [CrossRef]
- Chen, F.; Castranova, V.; Shi, X. New Insights into the Role of Nuclear Factor-κB in Cell Growth Regulation. Am. J. Pathol. 2001, 159, 387–397. [Google Scholar] [CrossRef]
- Fraser, C.C. Exploring the Positive and Negative Consequences of NF-κB Inhibition for the Treatment of Human Disease. Cell Cycle 2006, 5, 1160–1163. [Google Scholar] [CrossRef]

| Targets | Direction | Sequences (5′ to 3′) |
|---|---|---|
| iNOS | Forward | GGAGCCTTTAGACCTCAACAGA |
| Reverse | TGAACGAGGAGGGTGGTG | |
| COX-2 | Forward | CACTACATCCTGACCCACTT |
| Reverse | ATGCTCCTGCTTGAGTATGT | |
| TNF-α | Forward | GCCTCTTCTCATTCCTGCTTG |
| Reverse | CTGATGAGAGGGAGGCCATT | |
| IL-1β | Forward | CAACCAACAAGTGATATTCTCCATG |
| Reverse | GATCCACACACTCCAGCTGCA | |
| IL-6 | Forward | CTAGGTTTGCCGAGTAGATCTC |
| Reverse | GACAAAGCCAGAGTCCTTCAGAGA | |
| GAPDH | Forward | CAATGAATACGGCTACAGCAAC |
| Reverse | AGGGAGATGCTCAGTGTTGG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.H.; Kim, J.H.; Cho, J.Y. Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling. Biomolecules 2020, 10, 546. https://doi.org/10.3390/biom10040546
Hong YH, Kim JH, Cho JY. Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling. Biomolecules. 2020; 10(4):546. https://doi.org/10.3390/biom10040546
Chicago/Turabian StyleHong, Yo Han, Ji Hye Kim, and Jae Youl Cho. 2020. "Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling" Biomolecules 10, no. 4: 546. https://doi.org/10.3390/biom10040546
APA StyleHong, Y. H., Kim, J. H., & Cho, J. Y. (2020). Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling. Biomolecules, 10(4), 546. https://doi.org/10.3390/biom10040546






