Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases
Abstract
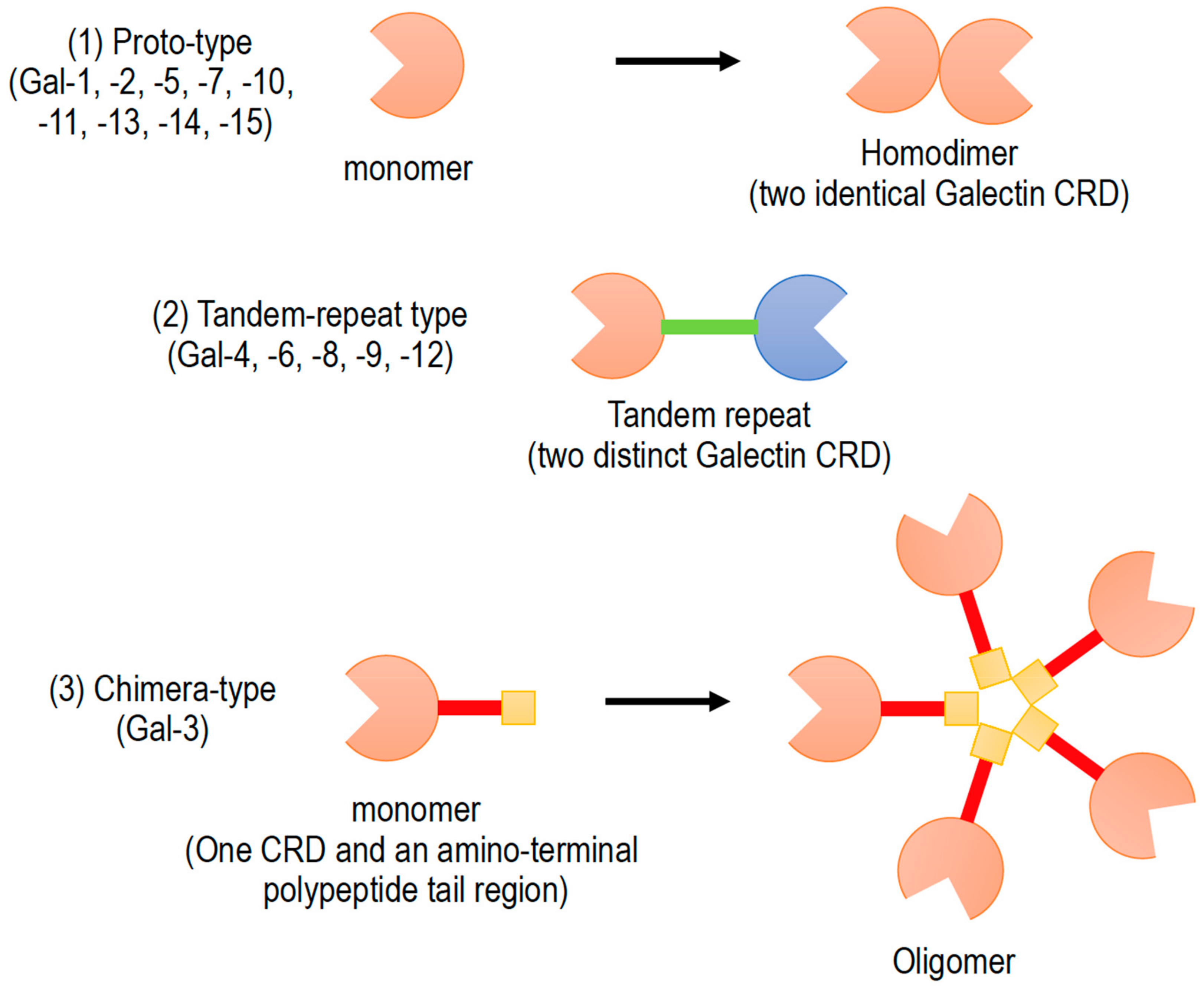
1. Introduction
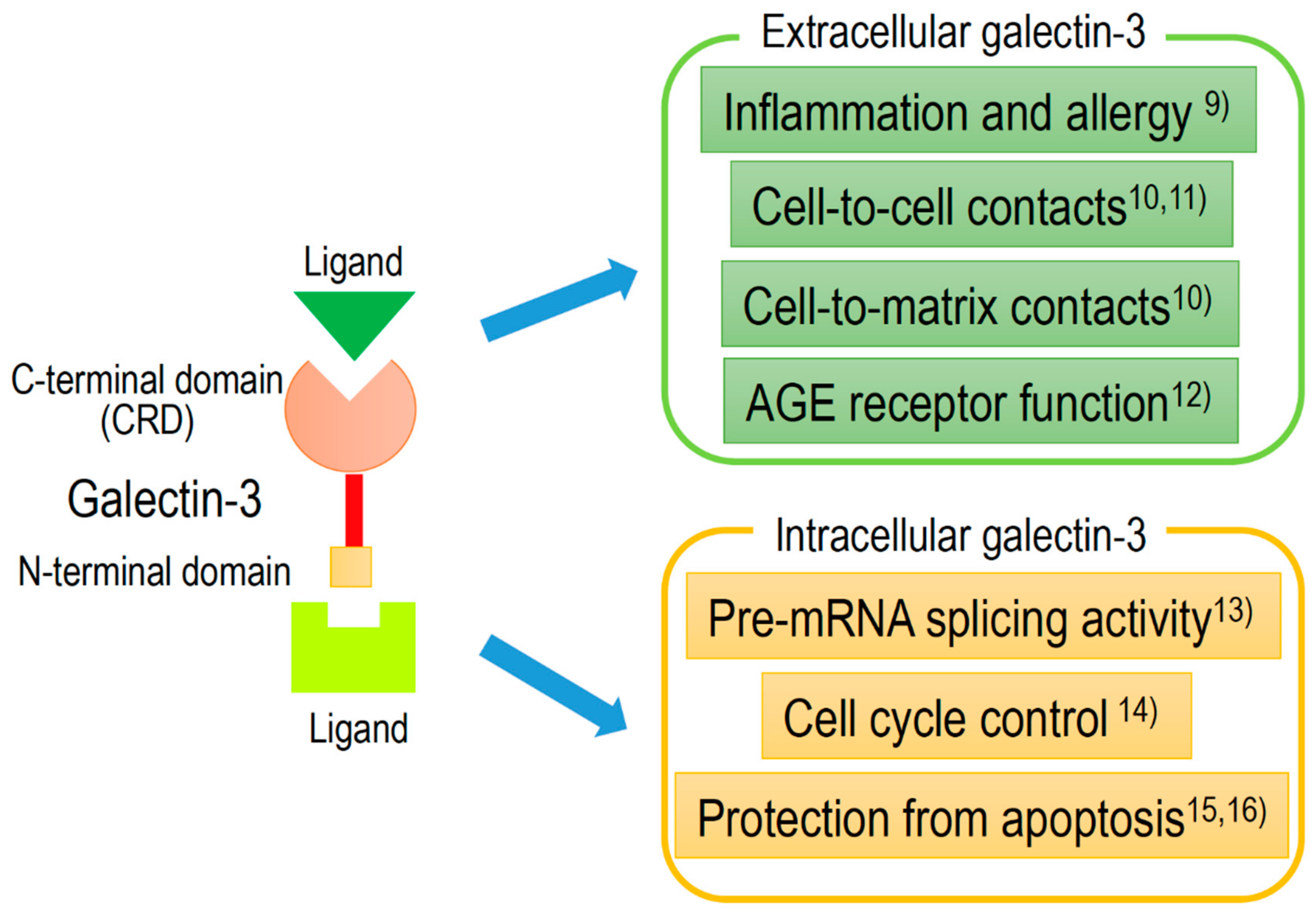
2. Clinical Significance and Applications of Galectine-3 (Gal-3)
3. Gal-3 and Heart Disease
3.1. Clinical Use of Gal-3 as a Possible Biomarker in Heart Disease
3.2. Established Biomarkers in Heart Disease
3.3. Gal-3 as a Biomarker of Fibrosis
3.4. Usefulness of Gal-3 in Animal Models
3.5. Clinical Use of Gal-3 as a Next-Generation Biomarker in the Future
4. Gal-3 in Various Nervous System Processes
4.1. Gal-3 in Microglial and Macrophages
4.2. Gal-3 as a Possible Biomarker in Neurodegenerative Disease
4.3. Gal-3 Is Associated with Cerebrovascular Disease
5. Gal-3 in Renal Disease
5.1. Cardiovascular Disease and Chronic Kidney Disease
5.2. Gal-3 as a Biomarker of CKD in Clinical Studies
5.3. Gal-3 in Animal Data for CKD
6. Gal-3 in Other Diseases
6.1. Assessing Liver Fibrosis
6.2. Rheumatoid Arthritis and Osteoarthritis
6.3. Connective Tissue Diseases
6.4. Skin Diseases and Its Repair
7. Gal-3 in Neoplasms
7.1. Gal-3 in Tumors and the Prognosis
7.2. Gal-3 in Tumor-Associated Macrophages
7.3. Gal-3 in Early Neoplastic Lesions
7.4. Complicated Mechanism of Gal-3 Expression in Neoplasms
8. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.T.; Patterson, R.J.; Wang, J.L. Intracellular functions of galectins. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Weng, I.C.; Hong, M.H.; Liu, F.T. Galectins as bacterial sensors in the host innate response. Curr. Opin. Microbiol. 2014, 17, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.W.; Cummings, R.D.; Drickamer, K.; Felzi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.I.; et al. Galectins: A family of animal β-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Paclik, D.; Werner, L.; Guckelberger, O.; Wiedenmann, B.; Sturm, A. Galectins distinctively regulate central monocyte and macrophage function. Cell. Immunol. 2011, 271, 97–103. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Stitt, A.W.; He, C.; Vlassara, H. Characterization of the advanced glycation end-product receptor complex in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 1999, 256, 549–556. [Google Scholar] [CrossRef]
- Inohara, H.; Raz, A. Functional Evidence that Cell Surface Galectin-3 Mediates Homotypic Cell Adhesion. Cancer Res. 1995, 55, 3267–3271. [Google Scholar]
- Ochieng, J.; Leite-Browning, M.L.; Warfield, P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem. Biophys. Res. Commun. 1998, 246, 788–791. [Google Scholar] [CrossRef]
- Vlassara, H.; Li, Y.M.; Imani, F.; Wojciechowicz, D.; Yang, Z.; Liu, F.T.; Cerami, A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): A new member of the AGE-receptor complex. Mol. Med. 1995, 1, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Dagher, S.F.; Wang, J.L.; Patterson, R.J. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc. Natl. Acad. Sci. USA 1995, 92, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.C.; Lin, H.M.; Biliran, H.; Raz, A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999, 59, 4148–4154. [Google Scholar] [PubMed]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.C.; Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar] [PubMed]
- Clementy, N.; Garcia, B.; André, C.; Bisson, A.; Benhenda, N.; Pierre, B.; Bernard, A.; Fauchier, L.; Piver, E.; Babuty, D. Galectin-3 level predicts response to ablation and outcomes in patients with persistent atrial fibrillation and systolic heart failure. PLoS ONE 2018, 13, e0201517. [Google Scholar] [CrossRef]
- Andre, C.; Piver, E.; Perault, R.; Bisson, A.; Pucheux, J.; Vermes, E.; Pierre, B.; Fauchier, L.; Babuty, D.; Clementy, N. Galectin-3 predicts response and outcomes after cardiac resynchronization therapy 11 Medical and Health Sciences 1102 Cardiorespiratory Medicine and Haematology. J. Transl. Med. 2018, 16, 299. [Google Scholar] [CrossRef]
- Zuern, C.S.; Floss, N.; Mueller, I.I.; Eick, C.; Duckheim, M.; Patzelt, J.; Gawaz, M.; May, A.E.; Mueller, K.A.L. Galectin-3 is associated with left ventricular reverse remodeling and outcome after percutaneous mitral valve repair. Int. J. Cardiol. 2018, 263, 104–110. [Google Scholar] [CrossRef]
- Asleh, R.; Enriquez-Sarano, M.; Jaffe, A.S.; Manemann, S.M.; Weston, S.A.; Jiang, R.; Roger, V.L. Galectin-3 Levels and Outcomes After Myocardial Infarction: A Population-Based Study. J. Am. Coll. Cardiol. 2019, 73, 2286–2295. [Google Scholar] [CrossRef]
- Cui, Y.; Qi, X.; Huang, A.; Li, J.; Hou, W.; Liu, K. Differential and Predictive Value of Galectin-3 and Soluble Suppression of Tumorigenicity-2 (sST2) in Heart Failure with Preserved Ejection Fraction. Med. Sci. Monit. 2018, 24, 5139–5146. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.M.; Kuster, N.; Curinier, C.; Huet, F.; Plawecki, M.; Solecki, K.; Roubille, F.; Cristol, J.P. Exploring collagen remodeling and regulation as prognosis biomarkers in stable heart failure. Clin. Chim. Acta 2019, 490, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Bhambhani, V.; Christenson, R.H.; Meijers, W.C.; de Boer, R.A.; Levy, D.; Larson, M.G.; Ho, J.E. Longitudinal Change in Galectin-3 and Incident Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2018, 72, 3246–3254. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.B.; Cheung, C.-L.; Lee, A.C.H.; Lam, J.K.Y.; Wong, Y.; Shiu, S.W.M. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia 2018, 61, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.L.; Katz, R.; Bellovich, K.A.; Bhat, Z.Y.; Brosius, F.C.; de Boer, I.H.; Gadegbeku, C.A.; Gipson, D.S.; Hawkins, J.J.; Himmelfarb, J.; et al. Soluble ST2 and Galectin-3 and Progression of CKD. Kidney Int. Rep. 2019, 4, 103–111. [Google Scholar] [CrossRef]
- Savoj, J.; Becerra, B.; Kim, J.K.; Fusaro, M.; Gallieni, M.; Lombardo, D.; Lau, W.L. Utility of Cardiac Biomarkers in the Setting of Kidney Disease. Nephron 2019, 141, 227–235. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, P.L. The role of galectin-3 in the kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef]
- Gopal, D.M.; Ayalon, N.; Wang, Y.C.; Siwik, D.; Sverdlov, A.; Donohue, C.; Perez, A.; Downing, J.; Apovian, C.; Silva, V.; et al. Galectin-3 is associated with stage B metabolic heart disease and pulmonary hypertension in young obese patients. J. Am. Heart Assoc. 2019, 8, e011100. [Google Scholar] [CrossRef]
- Sato, S.; Ouellet, M.; St-Pierre, C.; Tremblay, M.J. Glycans, galectins, and HIV-1 infection. Ann. N. Y. Acad. Sci. 2012, 1253, 133–148. [Google Scholar] [CrossRef]
- Nielsen, C.T.; Østergaard, O.; Rasmussen, N.S.; Jacobsen, S.; Heegaard, N.H.H. A review of studies of the proteomes of circulating microparticles: Key roles for galectin-3-binding protein-expressing microparticles in vascular diseases and systemic lupus erythematosus. Clin. Proteom. 2017, 14, 11. [Google Scholar] [CrossRef]
- Noguchi, K.; Tomita, H.; Kanayama, T.; Niwa, A.; Hatano, Y.; Hoshi, M.; Sugie, S.; Okada, H.; Niwa, M.; Hara, A. Time-course analysis of cardiac and serum galectin-3 in viral myocarditis after an encephalomyocarditis virus inoculation. PLoS ONE 2019, 14, e0210971. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Niwa, M.; Hoshi, M.; Saito, K.; Hisamatsu, K.; Hatano, Y.; Tomita, H.; Miyazaki, T.; Hara, A. Early microlesion of viral encephalitis confirmed by galectin-3 expression after a virus inoculation. Neurosci. Lett. 2015, 592, 107–112. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and autoimmune diseases. Exp. Biol. Med. 2015, 240, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Dhirapong, A.; Lleo, A.; Leung, P.; Gershwin, M.E.; Liu, F.T. The immunological potential of galectin-1 and -3. Autoimmun. Rev. 2009, 8, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Shin, T. The pleiotropic effects of galectin-3 in neuroinflammation: A review. Acta Histochem. 2013, 115, 407–411. [Google Scholar] [CrossRef]
- Saccon, F.; Gatto, M.; Ghirardello, A.; Iaccarino, L.; Punzi, L.; Doria, A. Role of galectin-3 in autoimmune and non-autoimmune nephropathies. Autoimmun. Rev. 2017, 16, 34–47. [Google Scholar] [CrossRef]
- Satoh, K.; Niwa, M.; Binh, N.H.; Nakashima, M.; Kobayashi, K.; Takamatsu, M.; Hara, A. Increase of galectin-3 expression in microglia by hyperthermia in delayed neuronal death of hippocampal CA1 following transient forebrain ischemia. Neurosci. Lett. 2011, 504, 199–203. [Google Scholar] [CrossRef]
- Hisamatsu, K.; Niwa, M.; Kobayashi, K.; Miyazaki, T.; Hirata, A.; Hatano, Y.; Tomita, H.; Hara, A. Galectin-3 expression in hippocampal CA2 following transient forebrain ischemia and its inhibition by hypothermia or antiapoptotic agents. Neuroreport 2016, 27, 311–317. [Google Scholar] [CrossRef][Green Version]
- Satoh, K.; Niwa, M.; Goda, W.; Binh, N.H.; Nakashima, M.; Takamatsu, M.; Hara, A. Galectin-3 expression in delayed neuronal death of hippocampal CA1 following transient forebrain ischemia, and its inhibition by hypothermia. Brain Res. 2011, 1382, 266–274. [Google Scholar] [CrossRef]
- Siew, J.J.; Chen, H.M.; Chen, H.Y.; Chen, H.L.; Chen, C.M.; Soong, B.W.; Wu, Y.R.; Chang, C.P.; Chan, Y.C.; Lin, C.H.; et al. Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington’s disease. Nat. Commun. 2019, 10, 3473. [Google Scholar] [CrossRef]
- Ashraf, G.M.; Baeesa, S.S. Investigation of Gal-3 Expression Pattern in Serum and Cerebrospinal Fluid of Patients Suffering From Neurodegenerative Disorders. Front. Neurosci. 2018, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Rotshenker, S. The role of Galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J. Mol. Neurosci. 2009, 39, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Binh, N.H.; Satoh, K.; Kobayashi, K.; Takamatsu, M.; Hatano, Y.; Hirata, A.; Tomita, H.; Kuno, T.; Hara, A. Galectin-3 in preneoplastic lesions of glioma. J. Neurooncol. 2013, 111, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, J.W.; Owusu, L.; Sun, M.Z.; Wu, J.; Zhang, J. Galectin-3 in cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef]
- Xin, M.; Dong, X.W.; Guo, X.L. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Ruvolo, P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 427–437. [Google Scholar] [CrossRef]
- Zeinali, M.; Adelinik, A.; Papian, S.; Khorramdelazad, H.; Abedinzadeh, M. Role of galectin-3 in the pathogenesis of bladder transitional cell carcinoma. Hum. Immunol. 2015, 76, 770–774. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.L. Molecular regulation of galectin-expression and therapeutic implication in cancer progression. Biomed. Pharmacother. 2016, 78, 165–171. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and cancer stemness. Glycobiology 2018, 28, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, X.; Ma, L.; Zhuang, Y.; Wei, Y.; Zhang, L.; Jin, S.; Liang, W.; Shen, X.; Li, C.; et al. Galectin-3 may serve as a marker for poor prognosis in colorectal cancer: A meta-analysis. Pathol. Res. Pract. 2019, 215, 152612. [Google Scholar] [PubMed]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Dimitroff, C.J. Galectin-binding O-glycosylations as regulators of malignancy. Cancer Res. 2015, 75, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- De Couto, G.; Ouzounian, M.; Liu, P.P. Early detection of myocardial dysfunction and heart failure. Nat. Rev. Cardiol. 2010, 7, 334–344. [Google Scholar] [CrossRef]
- Hashmi, S.; Al-Salam, S. Galectin-3 is expressed in the myocardium very early post-myocardial infarction. Cardiovasc. Pathol. 2015, 24, 213–223. [Google Scholar] [CrossRef]
- Van Der Velde, A.R.; Meijers, W.C.; Ho, J.E.; Brouwers, F.P.; Rienstra, M.; Bakker, S.J.L.; Muller Kobold, A.C.; Van Veldhuisen, D.J.; Van Gilst, W.H.; Van Der Harst, P.; et al. Serial galectin-3 and future cardiovascular disease in the general population. Heart 2016, 102, 1134–1141. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Shrestha, K.; Shao, Z.; Borowski, A.G.; Troughton, R.W.; Thomas, J.D.; Klein, A.L. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am. J. Cardiol. 2011, 108, 385–390. [Google Scholar] [CrossRef]
- Lõpez, B.; González, A.; Querejeta, R.; Zubillaga, E.; Larman, M.; Díez, J. Galectin-3 and histological, molecular and biochemical aspects of myocardial fibrosis in heart failure of hypertensive origin. Eur. J. Heart Fail. 2015, 17, 385–392. [Google Scholar] [CrossRef]
- Erkilet, G.; Özpeker, C.; Böthig, D.; Kramer, F.; Röfe, D.; Bohms, B.; Morshuis, M.; Gummert, J.; Milting, H. The biomarker plasma galectin-3 in advanced heart failure and survival with mechanical circulatory support devices. J. Heart Lung Transplant. 2013, 32, 221–230. [Google Scholar] [CrossRef]
- Beiras-Fernandez, A.; Weis, F.; Rothkopf, J.; Kaczmarek, I.; Ledderose, C.; Dick, A.; Keller, T.; Beiras, A.; Kreth, S. Local expression of myocardial galectin-3 does not correlate with its serum levels in patients undergoing heart transplantation. Ann. Transplant. 2013, 18, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Van Kimmenade, R.R.; Januzzi, J.L.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of Amino-Terminal Pro-Brain Natriuretic Peptide, Galectin-3, and Apelin for the Evaluation of Patients With Acute Heart Failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Bivona, G.; Lo Sasso, B.; Scazzone, C.; Bazan, V.; Bellia, C.; Ciaccio, M. Galectin-3 in acute coronary syndrome. Clin. Biochem. 2017, 50, 797–803. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Lok, D.J.A.; Jaarsma, T.; Van Der Meer, P.; Voors, A.A.; Hillege, H.L.; Van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef]
- Lopez-Andrés, N.; Rossignol, P.; Iraqi, W.; Fay, R.; Nuée, J.; Ghio, S.; Cleland, J.G.F.; Zannad, F.; Lacolley, P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail. 2012, 14, 74–81. [Google Scholar] [CrossRef]
- Weir, R.A.P.; Petrie, C.J.; Murphy, C.A.; Clements, S.; Steedman, T.; Miller, A.M.; McInnes, I.B.; Squire, I.B.; Ng, L.L.; Dargie, H.J.; et al. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circ. Heart Fail. 2013, 6, 492–498. [Google Scholar] [CrossRef]
- Yan, X.J.; Yu, G.F.; Jie, Y.Q.; Fan, X.F.; Huang, Q.; Dai, W.M. Role of galectin-3 in plasma as a predictive biomarker of outcome after acute intracerebral hemorrhage. J. Neurol. Sci. 2016, 368, 121–127. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhao, J.; Liu, H.; He, S. Prognostic value of plasma galectin-3 levels after aneurysmal subarachnoid hemorrhage. Brain Behav. 2016, 6, e00543. [Google Scholar] [CrossRef]
- Sävman, K.; Heyes, M.P.; Svedin, P.; Karlsson, A. Microglia/Macrophage-Derived Inflammatory Mediators Galectin-3 and Quinolinic Acid are Elevated in Cerebrospinal Fluid from Newborn Infants after Birth Asphyxia. Transl. Stroke Res. 2013, 4, 228–235. [Google Scholar] [CrossRef]
- Kang, E.H.; Moon, K.C.; Lee, E.Y.; Lee, Y.J.; Lee, E.B.; Ahn, C.; Song, Y.W. Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus 2009, 18, 22–28. [Google Scholar] [CrossRef]
- Shirabe, K.; Bekki, Y.; Gantumur, D.; Araki, K.; Ishii, N.; Kuno, A.; Narimatsu, H.; Mizokami, M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: More than a biomarker of liver fibrosis. J. Gastroenterol. 2018, 53, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.F.; Christensen, A.F.; Lindegaard, H.M.; Hetland, M.L.; Hørslev-Petersen, K.; Stengaard-Pedersen, K.; Ejbjerg, B.J.; Lottenburger, T.; Ellingsen, T.; Pedersen, J.K.; et al. Galectin-3 is Persistently Increased in Early Rheumatoid Arthritis (RA) and Associates with Anti-CCP Seropositivity and MRI Bone Lesions, While Early Fibrosis Markers Correlate with Disease Activity. Scand. J. Immunol. 2017, 86, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.R.; Tan, G.Z.; Meng, Z.; Yu, M.; Li, K.W.; Yin, J.; Wei, K.H.; Luo, Y.J.; Jia, S.Q.; Zhang, S.J.; et al. Association of anti-acidic ribosomal protein P0 and anti-galectin 3 antibodies with the development of skin lesions in systemic lupus erythematosus. Arthritis Rheumatol. 2015, 67, 193–203. [Google Scholar] [CrossRef]
- Faludi, R.; Nagy, G.; Tőkés-Füzesi, M.; Kovács, K.; Czirják, L.; Komócsi, A. Galectin-3 is an independent predictor of survival in systemic sclerosis. Int. J. Cardiol. 2017, 233, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Boutas, I.; Potiris, A.; Brenner, W.; Lebrecht, A.; Hasenburg, A.; Kalantaridou, S.; Schmidt, M. The expression of galectin-3 in breast cancer and its association with chemoresistance: A systematic review of the literature. Arch. Gynecol. Obstet. 2019, 300, 1113–1120. [Google Scholar] [CrossRef]
- Jiang, X.N.; Dang, Y.F.; Gong, F.L.; Guo, X.L. Role and regulation mechanism of Gal-3 in non-small cell lung cancer and its potential clinical therapeutic significance. Chem. Biol. Interact. 2019, 309, 108724. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Wang, Y.; Wu, X. The role of galectins in cervical cancer biology and progression. Biomed. Res. Int. 2018, 2018, 2175927. [Google Scholar] [CrossRef]
- Chiu, C.G.; Strugnell, S.S.; Griffith, O.L.; Jones, S.J.M.; Gown, A.M.; Walker, B.; Nabi, I.R.; Wiseman, S.M. Diagnostic utility of galectin-3 in thyroid cancer. Am. J. Pathol. 2010, 176, 2067–2081. [Google Scholar] [CrossRef]
- Liu, Y.H.; D’Ambrosio, M.; Liao, T.D.; Peng, H.; Rhaleb, N.E.; Sharma, U.; Andre, S.; Gabius, H.J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 404–412. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; Van Brakel, T.J.; Van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qian, X.; Shen, M.; Jiang, R.; Wagner, M.B.; Ding, G.; Chen, G.; Shen, B. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 513–521. [Google Scholar] [CrossRef]
- Jiang, H.-R.; Al Rasebi, Z.; Mensah-Brown, E.; Shahin, A.; Xu, D.; Goodyear, C.S.; Fukada, S.Y.; Liu, F.-T.; Liew, F.Y.; Lukic, M.L. Galectin-3 Deficiency Reduces the Severity of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2009, 182, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Hara, A.; Isagawa, T.; Manabe, I.; Takeda, K.; MaruYama, T. The nuclear IκB family protein IκBNS influences the susceptibility to experimental autoimmune encephalomyelitis in a murine model. PLoS ONE 2014, 9, e110838. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.P.; Lang, B.T.; Vemuganti, R.; Dempsey, R.J. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res. 2009, 1288, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Bertocchi, A.P.; Campanhole, G.; Wang, P.H.M.; Gonçalves, G.M.; Damião, M.J.; Cenedeze, M.A.; Beraldo, F.C.; De Paula Antunes Teixeira, V.; Dos Reis, M.A.; Mazzali, M.; et al. A Role for galectin-3 in renal tissue damage triggered by ischemia and reperfusion injury. Transpl. Int. 2008, 21, 999–1007. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef]
- Okamura, D.M.; Pasichnyk, K.; Lopez-Guisa, J.M.; Collins, S.; Hsu, D.K.; Liu, F.T.; Eddy, A.A. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am. J. Physiol. Ren. Physiol. 2011, 300, 245–253. [Google Scholar] [CrossRef]
- Iacobini, C.; Menini, S.; Ricci, C.; Scipioni, A.; Sansoni, V. Advanced lipoxidation end-products mediate lipid-induced glomerular injury: Role of receptor-mediated mechanisms. J. Pathol. 2009, 218, 360–369. [Google Scholar] [CrossRef]
- Pejnovic, N.; Jeftic, I.; Jovicic, N.; Arsenijevic, N.; Lukic, M.L. Galectin-3 and IL-33/ST2 axis roles and interplay in dietinduced steatohepatitis. World J. Gastroenterol. 2016, 22, 9706–9717. [Google Scholar] [CrossRef]
- Kang, H.G.; Kim, D.H.; Kim, S.J.; Cho, Y.; Jung, J.; Jang, W.; Chun, K.H. Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget 2016, 7, 68229–68241. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.V.R.; Servato, J.P.S.; Loyola, A.M.; Cardoso, S.V.; Chammas, R.; Liu, F.T.; Silva, M.J.B.; de Faria, P.R. Expression of APC protein during tongue malignant transformation in galectin-3-deficient mice challenged by the carcinogen 4-nitroquniline-n-oxide. Int. J. Clin. Exp. Pathol. 2014, 7, 3255–3263. [Google Scholar] [PubMed]
- Besler, C.; Lang, D.; Urban, D.; Rommel, K.P.; Von Roeder, M.; Fengler, K.; Blazek, S.; Kandolf, R.; Klingel, K.; Thiele, H.; et al. Plasma and cardiac galectin-3 in patients with heart failure reflects both inflammation and fibrosis: Implications for its use as a biomarker. Circ. Heart Fail. 2017, 10, e003804. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef]
- De Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Paul, S.; Lindenfeld, J.; Mann, D.L.; Albert, N.M.; Boehmer, J.P.; Collins, S.P.; Ezekowitz, J.A.; Givertz, M.M.; Katz, S.D.; Klapholz, M.; et al. Heart Failure Practice Guideline HFSA 2010 Comprehensive Heart Failure Practice Guideline. J. Card. Fail. 2010, 16, e1–e2. [Google Scholar]
- Yancy, C.W.; Mariell Jessup, C.; Chair Biykem Bozkurt, V.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation 2017, 136, 137–161. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Francis, G.S.; Morrow, D.A.; Newby, L.K.; Cannon, C.P.; Jesse, R.L.; Storrow, A.B.; Christenson, R.H.; Christenson, R.H.; Apple, F.S.; et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical Utilization of Cardiac Biomarker Testing in Heart Failure. Clin. Biochem. 2008, 41, 210–221. [Google Scholar] [CrossRef]
- Sarhene, M.; Wang, Y.; Wei, J.; Huang, Y.; Li, M.; Li, L.; Acheampong, E.; Zhengcan, Z.; Xiaoyan, Q.; Yunsheng, X.; et al. Biomarkers in heart failure: The past, current and future. Heart Fail. Rev. 2019, 24, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, V.; George, M.; Shanmugam, E. Utility of galectin-3 as a prognostic biomarker in heart failure: Where do we stand? Eur. J. Prev. Cardiol. 2015, 22, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi-Ueda, H.; Matsuyama, T.A.; Ohta-Ogo, K.; Ikeda, Y. Significance and value of endomyocardial biopsy based on our own experience. Circ. J. 2017, 81, 417–426. [Google Scholar] [CrossRef]
- Basso, C.; Calabrese, F.; Angelini, A.; Carturan, E.; Thiene, G. Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Fail. Rev. 2013, 18, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Basso, C.; Baandrup, U.T.; Bruneval, P.; Butany, J.; Gallagher, P.J.; Halushka, M.K.; Miller, D.V.; Padera, R.F.; Radio, S.J.; et al. Recommendations for processing cardiovascular surgical pathology specimens: A consensus statement from the Standards and Definitions Committee of the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Andréoletti, L.; Lévêque, N.; Boulagnon, C.; Brasselet, C.; Fornes, P. Viral causes of human myocarditis. Arch. Cardiovasc. Dis. 2009, 102, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, C.; Chrzaszcz, M.; Choi, N.; Wainwright, M.S. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J. Neuroinflamm. 2010, 7, 32. [Google Scholar] [CrossRef]
- Shen, Y.F.; Yu, W.H.; Dong, X.Q.; Du, Q.; Yang, D.B.; Wu, G.Q.; Zhang, Z.Y.; Wang, H.; Jiang, L. The change of plasma galectin-3 concentrations after traumatic brain injury. Clin. Chim. Acta 2016, 456, 75–80. [Google Scholar] [CrossRef]
- McKerracher, L.; David, S. Easing the brakes on spinal cord repair. Nat. Med. 2004, 10, 1052–1053. [Google Scholar] [CrossRef]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef]
- Cengiz, T.; Türkboyları, S.; Gençler, O.S.; Anlar, Ö. The roles of galectin-3 and galectin-4 in the idiopatic Parkinson disease and its progression. Clin. Neurol. Neurosurg. 2019, 184, 105373. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, A.; Hardas, S.; Patel, N.; Singh Bajaj, N.; Arora, G.; Arora, P. Galectin-3: An emerging biomarker in stroke and cerebrovascular diseases. Eur. J. Neurol. 2018, 25, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nakatsuka, Y.; Shiba, M.; Kawakita, F.; Fujimoto, M.; Suzuki, H. Increased Plasma Galectin-3 Preceding the Development of Delayed Cerebral Infarction and Eventual Poor Outcome in Non-Severe Aneurysmal Subarachnoid Hemorrhage. Transl. Stroke Res. 2018, 9, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Niwa, M.; Iwai, T.; Nakashima, M.; Yano, H.; Uematsu, T.; Yoshimi, N.; Mori, H. Transport of fragmented DNA in apical dendrites of gerbil CA1 pyramidal neurons following transient fore ischemia. Brain Res. 1998, 806, 274–277. [Google Scholar] [CrossRef]
- Chowdhury, P.; Kehl, D.; Choudhary, R.; Maisel, A. The use of biomarkers in the patient with heart failure. Curr. Cardiol. Rep. 2013, 15, 372. [Google Scholar] [CrossRef]
- Mahmood, U.; Johnson, D.W.; Fahim, M.A. Cardiac biomarkers in dialysis. AIMS Genet. 2016, 4, 1–20. [Google Scholar] [CrossRef]
- Desmedt, V.; Desmedt, S.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Galectin-3 in Renal Pathology: More than Just an Innocent Bystander? Am. J. Nephrol. 2016, 43, 305–317. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- De Oliveira, F.L.; Carneiro, K.; Brito, J.M.; Cabanel, M.; Pereira, J.X.; de Paiva, L.A.; Syn, W.; Henderson, N.C.; El-Cheikh, M.C. Galectin-3, histone deacetylases, and Hedgehog signaling: Possible convergent targets in schistosomiasis-induced liver fibrosis. PLoS Negl. Trop. Dis. 2017, 11, e0005137. [Google Scholar] [CrossRef]
- Issa, S.F.; Duer, A.; Østergaard, M.; Hørslev-Petersen, K.; Hetland, M.L.; Hansen, M.S.; Junker, K.; Lindegaard, H.M.; Møller, J.M.; Junker, P. Increased galectin-3 may serve as a serologic signature of pre-rheumatoid arthritis while markers of synovitis and cartilage do not differ between early undifferentiated arthritis subsets. Arthritis Res. Ther. 2017, 19, 80. [Google Scholar] [CrossRef]
- Koca, S.S.; Akbas, F.; Ozgen, M.; Yolbas, S.; Ilhan, N.; Gundogdu, B.; Isik, A. Serum galectin-3 level in systemic sclerosis. Clin. Rheumatol. 2014, 33, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Chen, H.Y.; Saegusa, J.; Liu, F.T. Galectin-3 and the skin. J. Dermatol. Sci. 2011, 64, 85–91. [Google Scholar] [CrossRef] [PubMed]
- McLeod, K.; Walker, J.T.; Hamilton, D.W. Galectin-3 regulation of wound healing and fibrotic processes: Insights for chronic skin wound therapeutics. J. Cell Commun. Signal. 2018, 12, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.R.; Tan, G.Z.; Cao, C.X.; Han, Y.F.; Meng, Z.; Man, X.Y.; Jiang, Z.X.; Zhang, Y.P.; Dang, N.N.; Wei, K.H.; et al. Decrease of galectin-3 in keratinocytes: A potential diagnostic marker and a critical contributor to the pathogenesis of psoriasis. J. Autoimmun. 2018, 89, 30–40. [Google Scholar] [CrossRef]
- Pepe, D.; Elliott, C.G.; Forbes, T.L.; Hamilton, D.W. Detection of galectin-3 and localization of advanced glycation end products (AGE) in human chronic skin wounds. Histol. Histopathol. 2014, 29, 251–258. [Google Scholar] [PubMed]
- Griffioen, A.W.; Thijssen, V.L. Galectins in tumor angiogenesis. Ann. Transl. Med. 2014, 2, 90. [Google Scholar] [PubMed]
- Kaltner, H.; Toegel, S.; Caballero, G.G.; Manning, J.C.; Ledeen, R.W.; Gabius, H.J. Galectins: Their network and roles in immunity/tumor growth control. Histochem. Cell Biol. 2017, 147, 239–256. [Google Scholar] [CrossRef]
- Dubé-Delarosbil, C.; St-Pierre, Y. The emerging role of galectins in high-fatality cancers. Cell. Mol. Life Sci. 2018, 75, 1215–1226. [Google Scholar] [CrossRef]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin targeted therapy in oncology: Current knowledge and perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef]
- Chou, F.C.; Chen, H.Y.; Kuo, C.C.; Sytwu, H.K. Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar] [CrossRef]
- Dings, R.P.M.; Miller, M.C.; Griffin, R.J.; Mayo, K.H. Galectins as molecular targets for therapeutic intervention. Int. J. Mol. Sci. 2018, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta Rev. Cancer 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Sawa-Wejksza, K.; Kandefer-Szerszeń, M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. 2018, 66, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jeong, H.; Bae, Y.; Shin, K.; Kang, S.; Kim, H.; Oh, J.; Bae, H. Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J. Immunother. Cancer 2019, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.M.L.; Andrade, L.N.S.; Teixeira, V.R.; Costa, F.F.; Melo, C.M.; dos Santos, S.N.; Nonogaki, S.; Liu, F.T.; Bernardes, E.S.; Camargo, A.A.; et al. Galectin-3 disruption impaired tumoral angiogenesis by reducing VEGF secretion from TGFβ1-induced macrophages. Cancer Med. 2014, 3, 201–214. [Google Scholar] [CrossRef]
- Wijesundera, K.K.; Wijesundera, K.K.; Izawa, T.; Izawa, T.; Tennakoon, A.H.; Tennakoon, A.H.; Murakami, H.; Murakami, H.; Golbar, H.M.; Golbar, H.M.; et al. M1- and M2-macrophage polarization in rat liver cirrhosis induced by thioacetamide (TAA), focusing on Iba1 and galectin-3. Exp. Mol. Pathol. 2014, 96, 382–392. [Google Scholar] [CrossRef]
- Quenum Zangbede, F.O.; Chauhan, A.; Sharma, J.; Mishra, B.B. Galectin-3 in M2 macrophages plays a protective role in resolution of neuropathology in brain parasitic infection by regulating neutrophil turnover. J. Neurosci. 2018, 38, 6737–6750. [Google Scholar] [CrossRef]
- Strik, H.M.; Deininger, M.H.; Frank, B.; Schluesener, H.J.; Meyermann, R. Galectin-3: Cellular distribution and correlation with WHO-grade in human gliomas. J. Neurooncol. 2001, 53, 13–20. [Google Scholar] [CrossRef]
| Diseases | Usage of Biomarker | Potential Use as Biomarkers | Refs. | |
|---|---|---|---|---|
| Heart disease | acute heart failure | plasma level | combination with natriuretic peptide | [62] |
| acute heart failure | plasma level | promising prognostic marker | [63] | |
| chronic heart failure | plasma level | useful in HF patients with preserved LVEF | [64] | |
| chronic heart failure | myocardial and plasma level | not associated with histology | [65] | |
| acute myocardial infarction | serum level | no definite relationship with ventricular remodeling | [66] | |
| Nervous system diseases | myelin degeneration | tissues | activation of the phagocytosis of degenerated myelin | [42] |
| intracerebral hemorrhage | plasma level | prognostic predictor | [67] | |
| subarachnoid hemorrhage | plasma level | prognostic predictor | [68] | |
| global brain ischemia | cerebrospinal fluid | prognostic marker and inflammatory mediator | [69] | |
| Renal disease | CKD | plasma level | associated with progression of CKD | [25] |
| SLE nephritis | serum and biopsy specimens | associated with SLE patients, particularly in SLE nephritis | [70] | |
| Liver disease | liver fibrosis | serum Gal-3 related binding protein | assessing liver fibrosis | [71] |
| Connective tissue diseases | rheumatoid arthritis | serum level | increased in early rheumatoid arthritis | [72] |
| SLE | serum anti-Gal-3 antibody | a key role in SLE skin lesions | [73] | |
| systemic sclerosis | serum level | predictor of mortality | [74] | |
| Neoplasms | colorectal cancer | serum and tissues | related to tumor progression | [52] |
| breast cancer | human cell lines | important factor for treatment | [75] | |
| non-small cell lung cancer | tumor expression | promotion of invasion and metastasis | [76] | |
| lung and prostate cancers | tumor expression | therapeutic target of tumor immunity | [77] | |
| cervical cancer | tumor expression | targets of multifunctional cancer treatment | [78] | |
| thyroid cancer | tumor expression | diagnostic marker | [79] |
| Animal Models | Experimental Methods | Experimental Findings | Refs. | |
|---|---|---|---|---|
| Heart disease | chronic heart failure | intrapericardial injection of recombinant Gal-3 | myocardial fibrosis and its pharmacological inhibition | [80] |
| chronic heart failure | intrapericardial infusion of low-dose Gal-3 | increased Gal-3 in hypertrophied hearts | [81] | |
| chronic heart failure | banding of the pulmonary artery | increase of Gal-3 in ventricles | [82] | |
| acute heart failure | viral myocarditis | time-course analysis of cardiac and serum Gal-3 | [31] | |
| Nervous system diseases | multiple sclerosis | EAE in Gal-3-deficient mice | reduction in severity of EAE | [83] |
| multiple sclerosis | spinal cord in EAE | Gal-3 observed at early stage before symptoms | [84] | |
| Huntington’s disease | mouse model of Huntington’s disease | plasma and brain Gal3 levels correlated with disease severity | [40] | |
| brain ischemia | time course analysis of Gal-3 in hippocampus | usefulness of early stage of Gal-3 for ischemic brain | [39] | |
| brain ischemia | temperature-dependent enhancement of Gal-3 | hypothermic prevention of Gal-3 | [30] | |
| cerebral infarction | cerebral artery occlusion | up-regulated Gal-3 in late stage of permanent ischemia | [85] | |
| Renal disease | renal ischemia | Gal-3 deficient mice | less acute renal tubular necrosis | [86] |
| renal fibrosis | unilateral ureteral obstruction | renal fibroblast activation by macrophage-secreted Gal-3 | [87] | |
| renal ischemia | unilateral ureteral obstruction | Gal-3 protect renal tubules | [88] | |
| lipid-induced renal injury | Gal-3 deficient mice | more marked in Gal-3 deficient mice | [89] | |
| Liver disease | NAFLD/NASH | Gal-3 deficient mice | attenuated inflammation and fibrosis | [90] |
| Connective tissue diseases | SLE | anti-Gal-3 antibody injected into skin | induction of lupus-like histologic changes | [73] |
| Neoplasms | glioma | analysis of preneoplastic lesions | expressed in preneoplastic lesions | [43] |
| ovarian cancer | ovarian cancer xenografted mice. | galectin-3 maintains ovarian cancer stem cells | [91] | |
| oral cancer | Gal-3 deficient mice | no significant difference | [92] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. https://doi.org/10.3390/biom10030389
Hara A, Niwa M, Noguchi K, Kanayama T, Niwa A, Matsuo M, Hatano Y, Tomita H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules. 2020; 10(3):389. https://doi.org/10.3390/biom10030389
Chicago/Turabian StyleHara, Akira, Masayuki Niwa, Kei Noguchi, Tomohiro Kanayama, Ayumi Niwa, Mikiko Matsuo, Yuichiro Hatano, and Hiroyuki Tomita. 2020. "Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases" Biomolecules 10, no. 3: 389. https://doi.org/10.3390/biom10030389
APA StyleHara, A., Niwa, M., Noguchi, K., Kanayama, T., Niwa, A., Matsuo, M., Hatano, Y., & Tomita, H. (2020). Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules, 10(3), 389. https://doi.org/10.3390/biom10030389






