TSPAN8 as a Novel Emerging Therapeutic Target in Cancer for Monoclonal Antibody Therapy
Abstract
1. Introduction
2. Clinical Relevance of Tetraspanins in Cancer
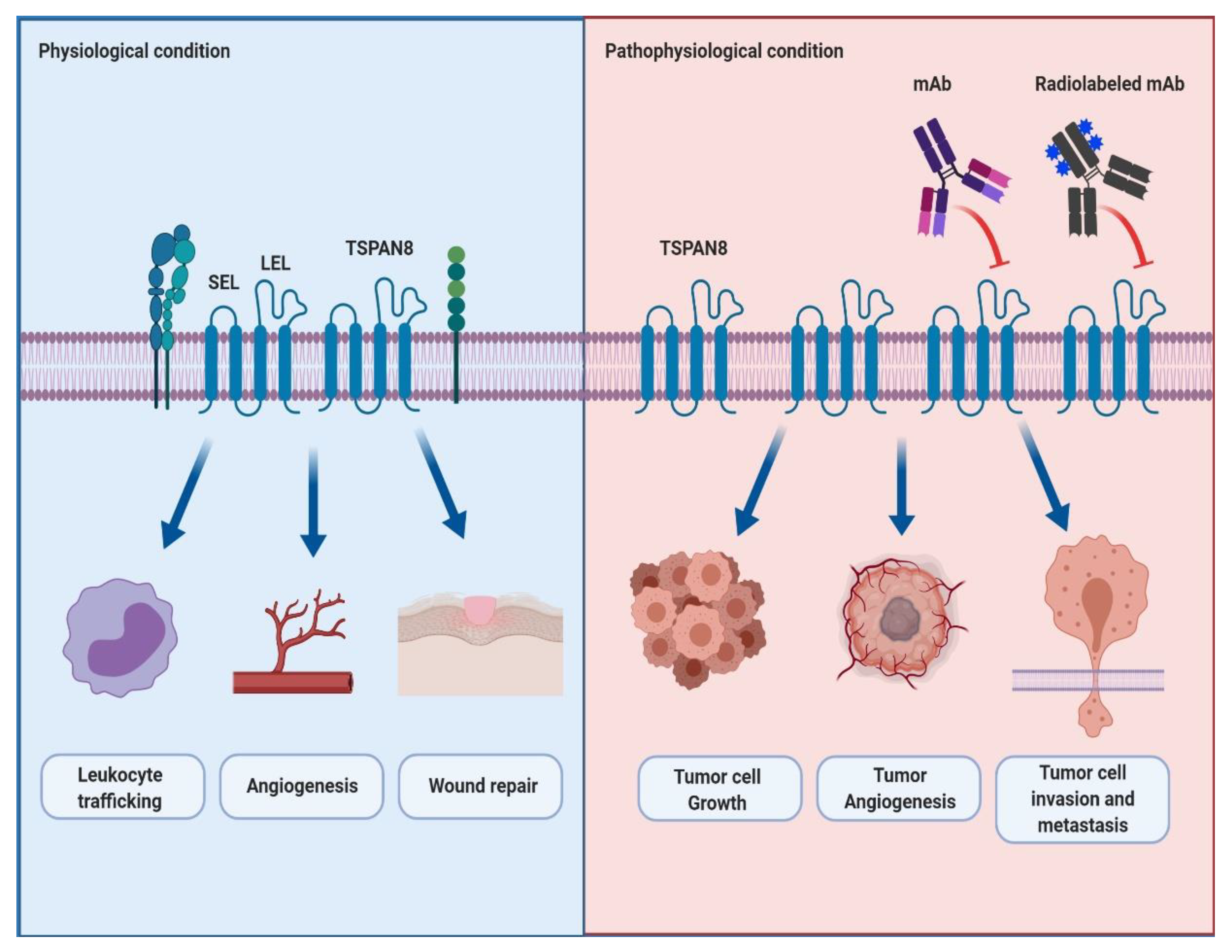
3. The Structure and Physiological Role of TSPAN8 in Cells
4. The Role of TSPAN8 in Cancer Progression and Metastasis
4.1. Pancreatic Cancer
4.2. Colon Cancers
4.3. Gastric Cancers
4.4. Liver Cancers
4.5. Lung Cancers
4.6. Breast Cancers
4.7. Ovarian Cancers
4.8. Gliomas
4.9. Melanomas
4.10. Esophageal Cancers
4.11. Nasopharyngeal Cancers
4.12. Cancer Stem Cells
5. TSPAN8 as an Emerging Therapeutic Target in Cancer for Antibody Therapy
5.1. Murine Monoclonal Antibody
5.2. Human Monoclonal Antibody
5.3. Radioisotope-Conjugate Antibody
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDR | Complementarity Determining Region |
| EOC | Epithelial Ovarian Cancer |
| IgG | Immunoglobulin |
| LEL | Large Extracellular Loop |
| mCRC | Metastatic colorectal cancer |
| SEL | Small Extracellular Loop |
| TSPAN8 | Tetraspanin 8 |
| TEM | Tetraspanin-enriched microdomain |
References
- Singh, S.; Kumar, N.K.; Dwiwedi, P.; Charan, J.; Kaur, R.; Sidhu, P.; Chugh, V.K. Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol. 2018, 13, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J.M. Antibodies to watch in 2020. MAbs 2020, 12, 1703531. [Google Scholar] [CrossRef] [PubMed]
- Monoclonal antibody approved for metastatic breast cancer. Oncology 1998, 12, 1727.
- Bardelli, A.; Siena, S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J. Clin. Oncol. 2010, 28, 1254–1261. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Huang, S.; Yuan, S.; Dong, M.; Su, J.; Yu, C.; Shen, Y.; Xie, X.; Yu, Y.; Yu, X.; Chen, S.; et al. The phylogenetic analysis of tetraspanins projects the evolution of cell-cell interactions from unicellular to multicellular organisms. Genomics 2005, 86, 674–684. [Google Scholar] [CrossRef]
- Garcia-Espana, A.; Chung, P.J.; Sarkar, I.N.; Stiner, E.; Sun, T.T.; Desalle, R. Appearance of new tetraspanin genes during vertebrate evolution. Genomics 2008, 91, 326–334. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef]
- Murru, L.; Moretto, E.; Martano, G.; Passafaro, M. Tetraspanins shape the synapse. Mol. Cell Neurosci. 2018, 91, 76–81. [Google Scholar] [CrossRef]
- Li, Q.; Yang, X.H.; Xu, F.; Sharma, C.; Wang, H.X.; Knoblich, K.; Rabinovitz, I.; Granter, S.R.; Hemler, M.E. Tetraspanin CD151 plays a key role in skin squamous cell carcinoma. Oncogene 2013, 32, 1772–1783. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin proteins promote multiple cancer stages. Nat. Rev. Cancer 2014, 14, 49–60. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wang, S.; Zhang, X.; Gao, Y.; Niu, W.; Dong, N.; Shi, X.; Geng, Y.; Ma, Q.; Li, M.; et al. Tspan5 is an independent favourable prognostic factor and suppresses tumour growth in gastric cancer. Oncotarget 2016, 7, 40160–40173. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Shin, S.H.; Yim, S.H.; Lee, K.Y.; Kang, H.M.; Kim, T.M.; Chung, Y.J. CD63 as a biomarker for predicting the clinical outcomes in adenocarcinoma of lung. Lung Cancer 2007, 57, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, M.; Taki, T.; Ieki, Y.; Adachi, M.; Huang, C.L.; Koh, T.; Kodama, K.; Doi, O.; Miyake, M. Reduced motility related protein-1 (MRP-1/CD9) gene expression as a factor of poor prognosis in non-small cell lung cancer. Cancer Res. 1995, 55, 6040–6044. [Google Scholar]
- Miyake, M.; Nakano, K.; Itoi, S.I.; Koh, T.; Taki, T. Motility-related protein-1 (MRP-1/CD9) reduction as a factor of poor prognosis in breast cancer. Cancer Res. 1996, 56, 1244–1249. [Google Scholar]
- Mori, M.; Mimori, K.; Shiraishi, T.; Haraguchi, M.; Ueo, H.; Barnard, G.F.; Akiyoshi, T. Motility related protein 1 (MRP1/CD9) expression in colon cancer. Clin. Cancer Res. 1998, 4, 1507–1510. [Google Scholar]
- Sho, M.; Adachi, M.; Taki, T.; Hashida, H.; Konishi, T.; Huang, C.L.; Ikeda, N.; Nakajima, Y.; Kanehiro, H.; Hisanaga, M.; et al. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int. J. Cancer 1998, 79, 509–516. [Google Scholar] [CrossRef]
- Soyuer, S.; Soyuer, I.; Unal, D.; Ucar, K.; Yildiz, O.G.; Orhan, O. Prognostic significance of CD9 expression in locally advanced gastric cancer treated with surgery and adjuvant chemoradiotherapy. Pathol. Res. Pract. 2010, 206, 607–610. [Google Scholar] [CrossRef]
- Wu, D.H.; Liu, L.; Chen, L.H.; Ding, Y.Q. KAI1 gene expression in colonic carcinoma and its clinical significances. World J. Gastroenterol. 2004, 10, 2245–2249. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Wang, G.L.; Wang, Y.; Zhu, Y.Y.; Zhu, J. Clinicopathological significance of overexpression of TSPAN1, Ki67 and CD34 in gastric carcinoma. Tumori 2008, 94, 531–538. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.Y.; Zhang, X.J.; Wang, G.L.; Li, X.Y.; He, S.; Zhang, J.B.; Zhu, J.W. TSPAN1 protein expression: A significant prognostic indicator for patients with colorectal adenocarcinoma. World J. Gastroenterol. 2009, 15, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.Q.; Lei, X.F.; Yao, J.L.; Wang, Y.J.; Zhang, W. Tetraspanin 1 is involved in survival, proliferation and carcinogenesis of pancreatic cancer. Oncol. Rep. 2015, 34, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, C.; Otomo, R.; Miyazaki, M.; Matsushima-Hibiya, Y.; Kohno, T.; Iwakawa, R.; Takeshita, F.; Okayama, H.; Ichikawa, H.; Saya, H.; et al. TSPAN2 is involved in cell invasion and motility during lung cancer progression. Cell Rep. 2014, 7, 527–538. [Google Scholar] [CrossRef]
- Wang, X.; Lin, M.; Zhao, J.; Zhu, S.; Xu, M.; Zhou, X. TSPAN7 promotes the migration and proliferation of lung cancer cells via epithelial-to-mesenchymal transition. OncoTargets Ther. 2018, 11, 8815–8822. [Google Scholar] [CrossRef] [PubMed]
- Anami, K.; Oue, N.; Noguchi, T.; Sakamoto, N.; Sentani, K.; Hayashi, T.; Naito, Y.; Oo, H.Z.; Yasui, W. TSPAN8, identified by Escherichia coli ampicillin secretion trap, is associated with cell growth and invasion in gastric cancer. Gastric Cancer 2016, 19, 370–380. [Google Scholar] [CrossRef]
- Fang, T.; Lin, J.; Wang, Y.; Chen, G.; Huang, J.; Chen, J.; Zhao, Y.; Sun, R.; Liang, C.; Liu, B. Tetraspanin-8 promotes hepatocellular carcinoma metastasis by increasing ADAM12m expression. Oncotarget 2016, 7, 40630–40643. [Google Scholar] [CrossRef]
- Zhu, R.; Gires, O.; Zhu, L.; Liu, J.; Li, J.; Yang, H.; Ju, G.; Huang, J.; Ge, W.; Chen, Y.; et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat. Commun. 2019, 10, 2863. [Google Scholar] [CrossRef]
- Park, C.S.; Kim, T.K.; Kim, H.G.; Kim, Y.J.; Jeoung, M.H.; Lee, W.R.; Go, N.K.; Heo, K.; Lee, S. Therapeutic targeting of tetraspanin8 in epithelial ovarian cancer invasion and metastasis. Oncogene 2016, 35, 4540–4548. [Google Scholar] [CrossRef]
- Lin, X.; Bi, Z.; Hu, Q.; Li, Q.; Liu, J.; Luo, M.L.; Xiang, Y.; Yao, H. TSPAN8 serves as a prognostic marker involving Akt/MAPK pathway in nasopharyngeal carcinoma. Ann. Transl. Med. 2019, 7, 470. [Google Scholar] [CrossRef]
- Feng, T.; Sun, L.; Qi, W.; Pan, F.; Lv, J.; Guo, J.; Zhao, S.; Ding, A.; Qiu, W. Prognostic significance of Tspan9 in gastric cancer. Mol. Clin. Oncol. 2016, 5, 231–236. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zhao, S.; Yuan, X.Y.; Liu, Y.; Zhang, K.; Wang, J.; Zhu, J.; Ma, R. miR-4732-5p promotes breast cancer progression by targeting TSPAN13. J. Cell Mol. Med. 2019, 23, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.; Li, L.; Qin, Y.R.; Liu, H.; Jiang, C.; Zeng, T.T.; Li, M.Q.; Xie, D.; Li, Y.; et al. TSPAN15 interacts with BTRC to promote oesophageal squamous cell carcinoma metastasis via activating NF-kappaB signaling. Nat. Commun. 2018, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.B.; Zhang, X.C.; Chen, P.; Ma, L.M.; Shen, Z.Q. miR378a3p inhibits cellular proliferation and migration in glioblastoma multiforme by targeting tetraspanin 17. Oncol. Rep. 2019, 42, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Ke, A.W.; Shi, G.M.; Zhou, J.; Wu, F.Z.; Ding, Z.B.; Hu, M.Y.; Xu, Y.; Song, Z.J.; Wang, Z.J.; Wu, J.C.; et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology 2009, 49, 491–503. [Google Scholar] [CrossRef]
- Matsumoto, N.; Morine, Y.; Utsunomiya, T.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Iwahashi, S.; Saito, Y.; Yamada, S.; Ishikawa, D.; et al. Role of CD151 expression in gallbladder carcinoma. Surgery 2014, 156, 1212–1217. [Google Scholar] [CrossRef]
- Kwon, M.J.; Park, S.; Choi, J.Y.; Oh, E.; Kim, Y.J.; Park, Y.H.; Cho, E.Y.; Kwon, M.J.; Nam, S.J.; Im, Y.H.; et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br. J. Cancer 2012, 106, 923–930. [Google Scholar] [CrossRef]
- Medrano, M.; Communal, L.; Brown, K.R.; Iwanicki, M.; Normand, J.; Paterson, J.; Sircoulomb, F.; Krzyzanowski, P.; Novak, M.; Doodnauth, S.A.; et al. Interrogation of Functional Cell-Surface Markers Identifies CD151 Dependency in High-Grade Serous Ovarian Cancer. Cell Rep. 2017, 18, 2343–2358. [Google Scholar] [CrossRef]
- Tokuhara, T.; Hasegawa, H.; Hattori, N.; Ishida, H.; Taki, T.; Tachibana, S.; Sasaki, S.; Miyake, M. Clinical significance of CD151 gene expression in non-small cell lung cancer. Clin. Cancer Res. 2001, 7, 4109–4114. [Google Scholar]
- Ang, J.; Lijovic, M.; Ashman, L.K.; Kan, K.; Frauman, A.G. CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: A new prognostic indicator? Cancer Epidemiol. Biomark. Prev. 2004, 13, 1717–1721. [Google Scholar]
- Kang, B.W.; Lee, D.; Chung, H.Y.; Han, J.H.; Kim, Y.B. Tetraspanin CD151 expression associated with prognosis for patients with advanced gastric cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1835–1843. [Google Scholar] [CrossRef]
- Kwon, H.J.; Min, S.Y.; Park, M.J.; Lee, C.; Park, J.H.; Chae, J.Y.; Moon, K.C. Expression of CD9 and CD82 in clear cell renal cell carcinoma and its clinical significance. Pathol. Res. Pract. 2014, 210, 285–290. [Google Scholar] [CrossRef]
- Uchida, S.; Shimada, Y.; Watanabe, G.; Li, Z.G.; Hong, T.; Miyake, M.; Imamura, M. Motility-related protein (MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph node metastasis in oesophageal squamous cell carcinoma. Br. J. Cancer 1999, 79, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Taki, T.; Ieki, Y.; Huang, C.L.; Higashiyama, M.; Miyake, M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996, 56, 1751–1755. [Google Scholar] [PubMed]
- Huang, C.I.; Kohno, N.; Ogawa, E.; Adachi, M.; Taki, T.; Miyake, M. Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am. J. Pathol. 1998, 153, 973–983. [Google Scholar] [CrossRef]
- Su, J.S.; Arima, K.; Hasegawa, M.; Franco, O.E.; Umeda, Y.; Yanagawa, M.; Sugimura, Y.; Kawamura, J. Decreased expression of KAI1 metastasis suppressor gene is a recurrence predictor in primary pTa and pT1 urothelial bladder carcinoma. Int. J. Urol. 2004, 11, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hashida, H.; Takabayashi, A.; Tokuhara, T.; Hattori, N.; Taki, T.; Hasegawa, H.; Satoh, S.; Kobayashi, N.; Yamaoka, Y.; Miyake, M. Clinical significance of transmembrane 4 superfamily in colon cancer. Br. J. Cancer 2003, 89, 158–167. [Google Scholar] [CrossRef]
- Zhang, N.; Zuo, L.; Zheng, H.; Li, G.; Hu, X. Increased Expression of CD81 in Breast Cancer Tissue is Associated with Reduced Patient Prognosis and Increased Cell Migration and Proliferation in MDA-MB-231 and MDA-MB-435S Human Breast Cancer Cell Lines In Vitro. Med. Sci. Monit. 2018, 24, 5739–5747. [Google Scholar] [CrossRef]
- Paiva, B.; Gutierrez, N.C.; Chen, X.; Vidriales, M.B.; Montalban, M.A.; Rosinol, L.; Oriol, A.; Martinez-Lopez, J.; Mateos, M.V.; Lopez-Corral, L.; et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia 2012, 26, 1862–1869. [Google Scholar] [CrossRef]
- Boyer, T.; Guihard, S.; Roumier, C.; Peyrouze, P.; Gonzales, F.; Berthon, C.; Quesnel, B.; Preudhomme, C.; Behal, H.; Duhamel, A.; et al. Tetraspanin CD81 is an adverse prognostic marker in acute myeloid leukemia. Oncotarget 2016, 7, 62377–62385. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, J.H.; Lee, J.S.; Yun, S.J.; Kim, W.J.; Ahn, H.; Park, J. Prognostic Significance of CREB-Binding Protein and CD81 Expression in Primary High Grade Non-Muscle Invasive Bladder Cancer: Identification of Novel Biomarkers for Bladder Cancer Using Antibody Microarray. PLoS ONE 2015, 10, e0125405. [Google Scholar] [CrossRef]
- Yau, J.C.; Dabbagh, L.K.; Formenti, K.S.; Coupland, R.W.; Burns, B.F.; Shaw, A.R. Expression of transmembrane 4 superfamily member, CD9, is related to improved progression-free survival in patients with diffuse non-Hodgkin’s lymphoma. Oncol. Rep. 1998, 5, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Kusukawa, J.; Ryu, F.; Kameyama, T.; Mekada, E. Reduced expression of CD9 in oral squamous cell carcinoma: CD9 expression inversely related to high prevalence of lymph node metastasis. J. Oral Pathol. Med. 2001, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Maruyama, A.; Okugawa, K.; Akazawa, K.; Baba, H.; Maehara, Y.; Mekada, E. Loss of motility-related protein 1 (MRP1/CD9) and integrin alpha3 expression in endometrial cancers. Cancer 2001, 92, 542–548. [Google Scholar] [CrossRef]
- Erovic, B.M.; Pammer, J.; Hollemann, D.; Woegerbauer, M.; Geleff, S.; Fischer, M.B.; Burian, M.; Frommlet, F.; Neuchrist, C. Motility-related protein-1/CD9 expression in head and neck squamous cell carcinoma. Head Neck 2003, 25, 848–857. [Google Scholar] [CrossRef]
- Mhawech, P.; Herrmann, F.; Coassin, M.; Guillou, L.; Iselin, C.E. Motility-related protein 1 (MRP-1/CD9) expression in urothelial bladder carcinoma and its relation to tumor recurrence and progression. Cancer 2003, 98, 1649–1657. [Google Scholar] [CrossRef][Green Version]
- De Bruyne, E.; Bos, T.J.; Asosingh, K.; Vande Broek, I.; Menu, E.; Van Valckenborgh, E.; Atadja, P.; Coiteux, V.; Leleu, X.; Thielemans, K.; et al. Epigenetic silencing of the tetraspanin CD9 during disease progression in multiple myeloma cells and correlation with survival. Clin. Cancer Res. 2008, 14, 2918–2926. [Google Scholar] [CrossRef]
- Zou, Q.; Xiong, L.; Yang, Z.; Lv, F.; Yang, L.; Miao, X. Expression levels of HMGA2 and CD9 and its clinicopathological significances in the benign and malignant lesions of the gallbladder. World J. Surg. Oncol. 2012, 10, 92. [Google Scholar] [CrossRef]
- Fabian, J.; Opitz, D.; Althoff, K.; Lodrini, M.; Hero, B.; Volland, R.; Beckers, A.; de Preter, K.; Decock, A.; Patil, N.; et al. MYCN and HDAC5 transcriptionally repress CD9 to trigger invasion and metastasis in neuroblastoma. Oncotarget 2016, 7, 66344–66359. [Google Scholar] [CrossRef]
- Touzet, L.; Dumezy, F.; Roumier, C.; Berthon, C.; Bories, C.; Quesnel, B.; Preudhomme, C.; Boyer, T. CD9 in acute myeloid leukemia: Prognostic role and usefulness to target leukemic stem cells. Cancer Med. 2019, 8, 1279–1288. [Google Scholar] [CrossRef]
- Zoller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55. [Google Scholar] [CrossRef]
- Bonnet, M.; Maisonial-Besset, A.; Zhu, Y.; Witkowski, T.; Roche, G.; Boucheix, C.; Greco, C.; Degoul, F. Targeting the Tetraspanins with Monoclonal Antibodies in Oncology: Focus on Tspan8/Co-029. Cancers 2019, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Szala, S.; Kasai, Y.; Steplewski, Z.; Rodeck, U.; Koprowski, H.; Linnenbach, A.J. Molecular cloning of cDNA for the human tumor-associated antigen CO-029 and identification of related transmembrane antigens. Proc. Natl. Acad. Sci. USA 1990, 87, 6833–6837. [Google Scholar] [CrossRef] [PubMed]
- Claas, C.; Seiter, S.; Claas, A.; Savelyeva, L.; Schwab, M.; Zoller, M. Association between the rat homologue of CO-029, a metastasis-associated tetraspanin molecule and consumption coagulopathy. J. Cell Biol. 1998, 141, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Champy, M.F.; Le Voci, L.; Selloum, M.; Peterson, L.B.; Cumiskey, A.M.; Blom, D. Reduced body weight in male Tspan8-deficient mice. Int. J. Obes. 2011, 35, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Erb, U.; Hackert, T.; Zoller, M.; Yue, S. Distorted leukocyte migration, angiogenesis, wound repair and metastasis in Tspan8 and Tspan8/CD151 double knockout mice indicate complementary activities of Tspan8 and CD51. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gesierich, S.; Paret, C.; Hildebrand, D.; Weitz, J.; Zgraggen, K.; Schmitz-Winnenthal, F.H.; Horejsi, V.; Yoshie, O.; Herlyn, D.; Ashman, L.K.; et al. Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: Impact on cell motility. Clin. Cancer Res. 2005, 11, 2840–2852. [Google Scholar] [CrossRef]
- Yue, S.; Mu, W.; Zoller, M. Tspan8 and CD151 promote metastasis by distinct mechanisms. Eur. J. Cancer 2013, 49, 2934–2948. [Google Scholar] [CrossRef]
- Claas, C.; Wahl, J.; Orlicky, D.J.; Karaduman, H.; Schnolzer, M.; Kempf, T.; Zoller, M. The tetraspanin D6.1A and its molecular partners on rat carcinoma cells. Biochem. J. 2005, 389, 99–110. [Google Scholar] [CrossRef][Green Version]
- Gesierich, S.; Berezovskiy, I.; Ryschich, E.; Zoller, M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006, 66, 7083–7094. [Google Scholar] [CrossRef]
- Greco, C.; Bralet, M.P.; Ailane, N.; Dubart-Kupperschmitt, A.; Rubinstein, E.; Le Naour, F.; Boucheix, C. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 2010, 70, 7674–7683. [Google Scholar] [CrossRef]
- Guo, Q.; Xia, B.; Zhang, F.; Richardson, M.M.; Li, M.; Zhang, J.S.; Chen, F.; Zhang, X.A. Tetraspanin CO-029 inhibits colorectal cancer cell movement by deregulating cell-matrix and cell-cell adhesions. PLoS ONE 2012, 7, e38464. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, Y.; Zheng, W.; Lu, S. CO-029 is overexpressed in gastric cancer and mediates the effects of EGF on gastric cancer cell proliferation and invasion. Int. J. Mol. Med. 2015, 35, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Y.; Suo, Z. TSPAN8 promotes gastric cancer growth and metastasis via ERK MAPK pathway. Int. J. Clin. Exp. Med. 2015, 8, 8599–8607. [Google Scholar] [PubMed]
- Li, L.; Yang, D.; Cui, D.; Li, Y.; Nie, Z.; Wang, J.; Liang, L. Quantitative proteomics analysis of the role of tetraspanin-8 in the drug resistance of gastric cancer. Int. J. Oncol. 2018, 52, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Kanetaka, K.; Sakamoto, M.; Yamamoto, Y.; Yamasaki, S.; Lanza, F.; Kanematsu, T.; Hirohashi, S. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J. Hepatol. 2001, 35, 637–642. [Google Scholar] [CrossRef]
- Akiel, M.A.; Santhekadur, P.K.; Mendoza, R.G.; Siddiq, A.; Fisher, P.B.; Sarkar, D. Tetraspanin 8 mediates AEG-1-induced invasion and metastasis in hepatocellular carcinoma cells. FEBS Lett. 2016, 590, 2700–2708. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, L.; Lu, S.; Xiong, J.; Geng, Z. Overexpression of TSPAN8 Promotes Tumor Cell Viability and Proliferation in Nonsmall Cell Lung Cancer. Cancer Biother. Radiopharm. 2016, 31, 353–359. [Google Scholar] [CrossRef]
- Voglstaetter, M.; Thomsen, A.R.; Nouvel, J.; Koch, A.; Jank, P.; Navarro, E.G.; Gainey-Schleicher, T.; Khanduri, R.; Gross, A.; Rossner, F.; et al. Tspan8 is expressed in breast cancer and regulates E-cadherin/catenin signalling and metastasis accompanied by increased circulating extracellular vesicles. J. Pathol. 2019, 248, 421–437. [Google Scholar] [CrossRef]
- Pan, S.J.; Wu, Y.B.; Cai, S.; Pan, Y.X.; Liu, W.; Bian, L.G.; Sun, B.; Sun, Q.F. Over-expression of tetraspanin 8 in malignant glioma regulates tumor cell progression. Biochem. Biophys. Res. Commun. 2015, 458, 476–482. [Google Scholar] [CrossRef]
- Pan, S.J.; Zhan, S.K.; Pan, Y.X.; Liu, W.; Bian, L.G.; Sun, B.; Sun, Q.F. Tetraspanin 8-rictor-integrin alpha3 complex is required for glioma cell migration. Int. J. Mol. Sci. 2015, 16, 5363–5374. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, W.; Zhan, S.K.; Pan, Y.X.; Bian, L.G.; Sun, B.; Sun, Q.F.; Pan, S.J. GSK621 Targets Glioma Cells via Activating AMP-Activated Protein Kinase Signalings. PLoS ONE 2016, 11, e0161017. [Google Scholar] [CrossRef] [PubMed]
- Berthier-Vergnes, O.; El Kharbili, M.; de la Fouchardiere, A.; Pointecouteau, T.; Verrando, P.; Wierinckx, A.; Lachuer, J.; Le Naour, F.; Lamartine, J. Gene expression profiles of human melanoma cells with different invasive potential reveal TSPAN8 as a novel mediator of invasion. Br. J. Cancer 2011, 104, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Agaesse, G.; Barbollat-Boutrand, L.; Sulpice, E.; Bhajun, R.; El Kharbili, M.; Berthier-Vergnes, O.; Degoul, F.; de la Fouchardiere, A.; Berger, E.; Voeltzel, T.; et al. A large-scale RNAi screen identifies LCMR1 as a critical regulator of Tspan8-mediated melanoma invasion. Oncogene 2017, 36, 446–457. [Google Scholar] [CrossRef] [PubMed]
- El Kharbili, M.; Robert, C.; Witkowski, T.; Danty-Berger, E.; Barbollat-Boutrand, L.; Masse, I.; Gadot, N.; de la Fouchardiere, A.; McDonald, P.C.; Dedhar, S.; et al. Tetraspanin 8 is a novel regulator of ILK-driven beta1 integrin adhesion and signaling in invasive melanoma cells. Oncotarget 2017, 8, 17140–17155. [Google Scholar] [CrossRef] [PubMed]
- Agaesse, G.; Barbollat-Boutrand, L.; El Kharbili, M.; Berthier-Vergnes, O.; Masse, I. p53 targets TSPAN8 to prevent invasion in melanoma cells. Oncogenesis 2017, 6, e309. [Google Scholar] [CrossRef] [PubMed]
- El Kharbili, M.; Agaesse, G.; Barbollat-Boutrand, L.; Pommier, R.M.; de la Fouchardiere, A.; Larue, L.; Caramel, J.; Puisieux, A.; Berthier-Vergnes, O.; Masse, I. Tspan8-beta-catenin positive feedback loop promotes melanoma invasion. Oncogene 2019, 38, 3781–3793. [Google Scholar] [CrossRef]
- Zhou, Z.; Ran, Y.L.; Hu, H.; Pan, J.; Li, Z.F.; Chen, L.Z.; Sun, L.C.; Peng, L.; Zhao, X.L.; Yu, L.; et al. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin. Exp. Metastasis 2008, 25, 537–548. [Google Scholar] [CrossRef]
- Freysd’ottir, J. Production of monoclonal antibodies. Methods Mol. Med. 2000, 40, 267–279. [Google Scholar] [CrossRef]
- Koprowski, H.; Steplewski, Z.; Mitchell, K.; Herlyn, M.; Herlyn, D.; Fuhrer, P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somat. Cell Genet. 1979, 5, 957–971. [Google Scholar] [CrossRef]
- Ailane, N.; Greco, C.; Zhu, Y.; Sala-Valdes, M.; Billard, M.; Casal, I.; Bawa, O.; Opolon, P.; Rubinstein, E.; Boucheix, C. Effect of an anti-human Co-029/tspan8 mouse monoclonal antibody on tumor growth in a nude mouse model. Front. Physiol. 2014, 5, 364. [Google Scholar] [CrossRef]
- Stuhlmiller, G.M.; Borowitz, M.J.; Seigler, H.F. D6.1, a murine antimelanoma monoclonal antibody from congenitally athymic nude mice immunized with purified melanoma tumor-associated antigen. Hybridoma 1984, 3, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Park, C.S.; Jeoung, M.H.; Lee, W.R.; Go, N.K.; Choi, J.R.; Lee, T.S.; Shim, H.; Lee, S. Generation of a human antibody that inhibits TSPAN8-mediated invasion of metastatic colorectal cancer cells. Biochem. Biophys. Res. Commun. 2015, 468, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Press, O.W. Whither Radioimmunotherapy: To Be or Not To Be? Cancer Res. 2017, 77, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2019. MAbs 2019, 11, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Maisonial-Besset, A.; Witkowski, T.; Navarro-Teulon, I.; Berthier-Vergnes, O.; Fois, G.; Zhu, Y.; Besse, S.; Bawa, O.; Briat, A.; Quintana, M.; et al. Tetraspanin 8 (TSPAN 8) as a potential target for radio-immunotherapy of colorectal cancer. Oncotarget 2017, 8, 22034–22047. [Google Scholar] [CrossRef]
| Tetraspanins | Cancer Types Correlated with Poor Prognosis | Cancer Types Correlated with Good Prognosis | References |
|---|---|---|---|
| TSPAN1 (NET-1) | GC, CRC, PCC | [20,21,22] | |
| TSPAN2 | Lung ADC | [23] | |
| TSPAN5 (NET-4) | GC | [12] | |
| TSPAN7 (Talla1, TM4SF2, CD231) | NSCLC | [24] | |
| TSPAN8 (Co-029) | GC, HCC, BC, EOC, NPC | [25,26,27,28,29] | |
| TSPAN9 (NET-5, PP1057) | GC | [30] | |
| TSPAN13 (NET-6) | BC | [31] | |
| TSPAN15 (NET-7) | ESCC | [32] | |
| TSPAN17 | GBM | [33] | |
| TSPAN24 (CD151, Peta3) | HCC, GBC, BC, HGSC, NSCLC, PC, GC, CRC | [34,35,36,37,38,39,40] | |
| TSPAN27 (CD82, KAI1) | CCRCC | ESCC, NSCLC, PCC, BC, GBC, CRC | [17,41,42,43,44,45,46] |
| TSPAN28 (CD81, Tapa1) | BC, MM, AML | GBC | [47,48,49,50] |
| TSPAN29 (CD9, p24) | GC | NSCLC, BC, CRC, PCC, NHL, ESCC, OSCC, EM, HNSCC, UBC, MM, GBC, NB, AML | [14,15,16,17,18,42,51,52,53,54,55,56,57,58,59] |
| TSPAN30 (CD63) | Lung ADC | [13] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, K.; Lee, S. TSPAN8 as a Novel Emerging Therapeutic Target in Cancer for Monoclonal Antibody Therapy. Biomolecules 2020, 10, 388. https://doi.org/10.3390/biom10030388
Heo K, Lee S. TSPAN8 as a Novel Emerging Therapeutic Target in Cancer for Monoclonal Antibody Therapy. Biomolecules. 2020; 10(3):388. https://doi.org/10.3390/biom10030388
Chicago/Turabian StyleHeo, Kyun, and Sukmook Lee. 2020. "TSPAN8 as a Novel Emerging Therapeutic Target in Cancer for Monoclonal Antibody Therapy" Biomolecules 10, no. 3: 388. https://doi.org/10.3390/biom10030388
APA StyleHeo, K., & Lee, S. (2020). TSPAN8 as a Novel Emerging Therapeutic Target in Cancer for Monoclonal Antibody Therapy. Biomolecules, 10(3), 388. https://doi.org/10.3390/biom10030388






