Chemical Composition of Essential Oils from Different Parts of Zingiber kerrii Craib and Their Antibacterial, Antioxidant, and Tyrosinase Inhibitory Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Essential Oil Extraction
2.3.1. Solid-Phase Microextraction (SPME)
2.3.2. Hydrodistillation Extraction (HD)
2.3.3. Organic Solvent Extraction (OS)
2.4. Rhizome Extraction
2.5. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.6. Total Phenolic Content Assay
2.7. DPPH Free Radical Scavenging Assay
2.8. ABTS Radical Cation Scavenging Assay
2.9. Ferric Reducing Antioxidant Power (FRAP) Assay
2.10. Cytotoxicity Assay
2.11. Antibacterial Microdilution Assay
2.12. Inhibition of Tyrosinase Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Essential Oils Composition
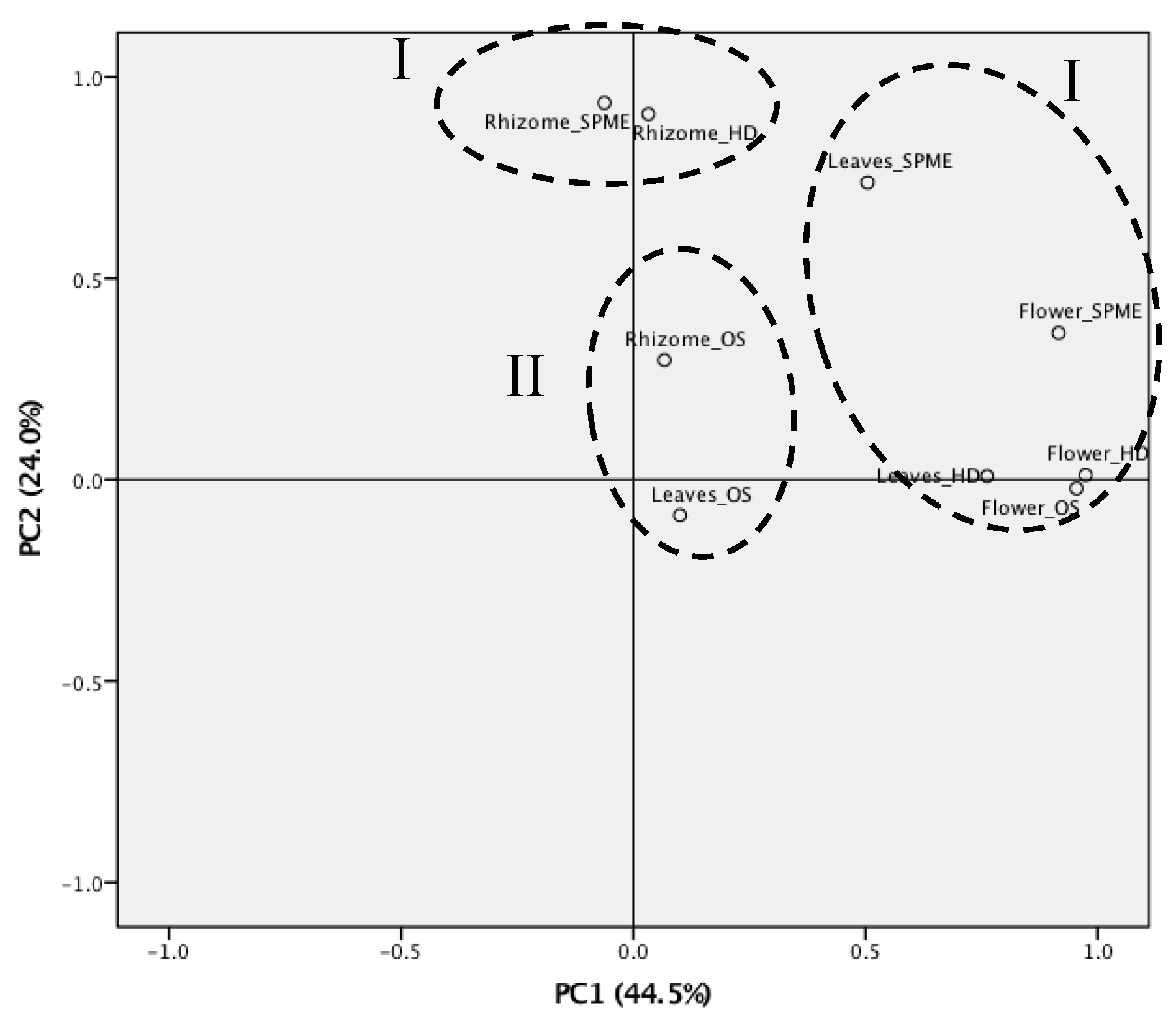
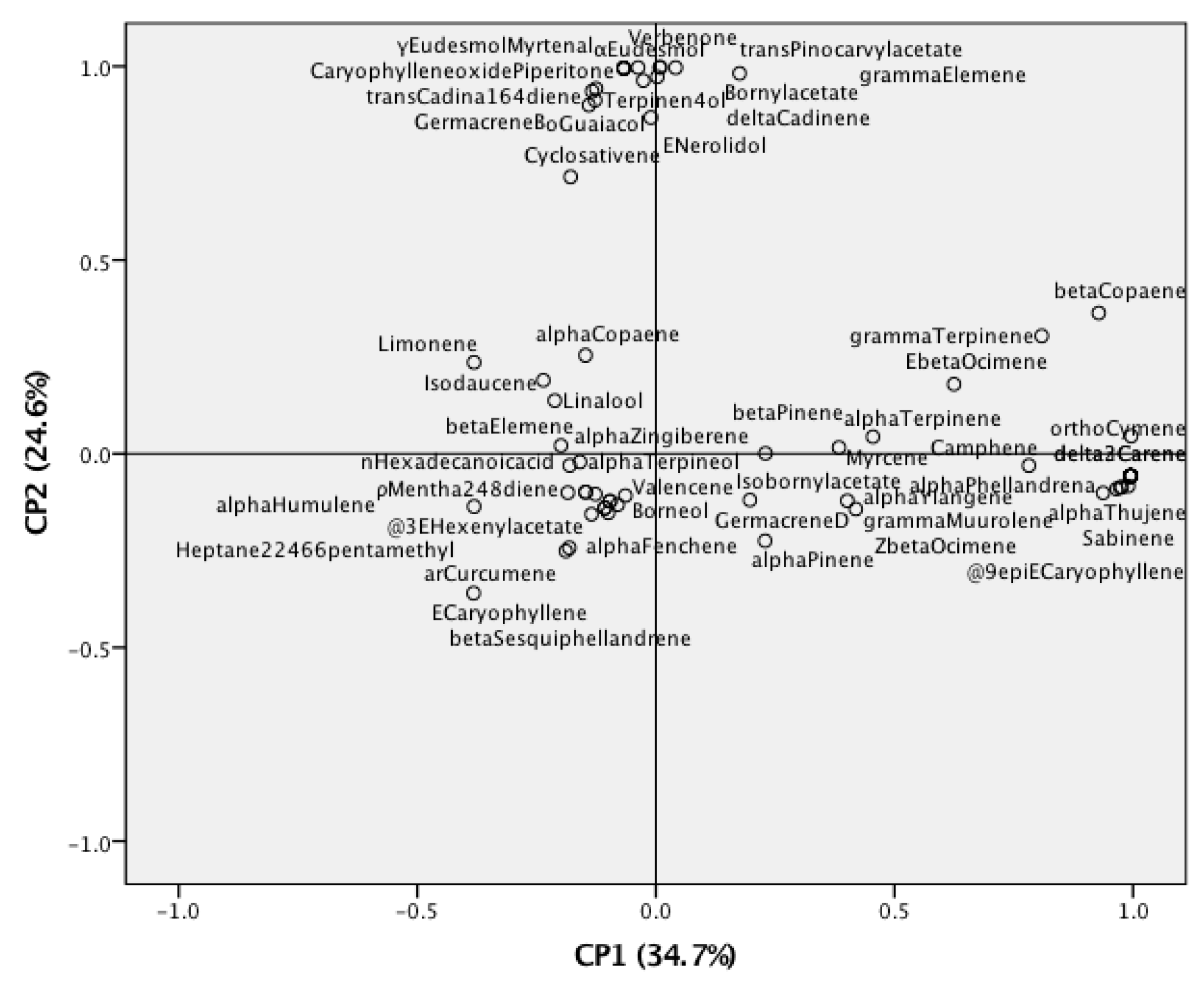
3.2. Statistical Analysis of Z. kerrii Volatile Components
3.3. Antioxidant Activities and Total Phenolic Content
3.4. In Vitro Cytotoxicity
3.5. Antibacterial Activity
3.6. Tyrosinase Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sukari, M.A.; Sharif, N.W.M.; Yap, A.L.C.; Tang, S.W.; Neoh, B.K.; Rahmani, M.; Ee, G.C.L.; Taufiq-Yap, Y.H.; Yusof, U.K. Chemical constituents variations of essential oils from rhizomes of four Zingiberaceae species. Malays. J. Anal. Sci. 2008, 12, 638–644. [Google Scholar]
- Thongam, B.; Sarangthem, N.; Konsam, B. Zingiber kerrii (Zingiberaceae): A new record for India from Manipur. Taiwania 2013, 58, 291–294. [Google Scholar]
- Triboun, P.; Chantaranothai, P.; Larsen, K. Taxonomic changes regarding three species of Zingiber (Zingiberaceae) from Thailand. Acta Phytotax. Sin. 2007, 45, 403–404. [Google Scholar]
- Busatta, C.; Barbosa, J.; Cardoso, R.I.; Paroul, N.; Rodrigues, M.; Oliveira, D.; Oliveira, J.V.; Cansian, R.L. Chemical profiles of essential oils of marjoram (Origanum majorana) and oregano (Origanum vulgare) obtained by hydrodistillation and supercritical CO2. J. Essent. Oil Res. 2017, 29, 367–374. [Google Scholar] [CrossRef]
- Moradi, M.; Kaykhaii, M.; Ghiasvand, A.R.; Shadabi, S.; Salehinia, A. Comparison of headspace solid-phase microextraction, headspace single-drop microextraction and hydrodistillation for chemical screening of volatiles in Myrtus communis L. Phytochem. Anal. 2011, 23, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Pripdeevech, P.; Moonggoot, S.; Popluechai, S.; Chukeatirote, E. Analysis of volatile constituents of fermented tea with Bacillus subtilis by SPME-GC-MS. Chiang Mai J. Sci. 2014, 41, 395–402. [Google Scholar]
- Pripdeevech, P.; Rothwell, J.; D’Souza, P.E.; Panuwet, P. Differentiation of volatile profiles of Thai Oolong tea No. 12 provenances by SPME-GC-MS combined with principal component analysis. Int. J. Food Prop. 2017, 20, S2450–S2462. [Google Scholar] [CrossRef]
- Abbasi, H.; Rezaei, K.; Rashidi, L. Extraction of essential oils from the seeds of pomegranate using organic solvents and supercritical CO2. J. Am. Oil Chem. Soc. 2008, 85, 83–89. [Google Scholar] [CrossRef]
- Rehman, S.U.; Latief, R.; Bhat, K.A.; Khuroo, M.A.; Shawl, A.S.; Chandra, S. Comparative analysis of the aroma chemicals of Melissa officinalis using hydrodistillation and HS-SPME techniques. Arab. J. Chem. 2017, 10, S2485–S2490. [Google Scholar] [CrossRef]
- Mesomo, M.C.; Corazza, M.L.; Ndiaye, P.M.; Santa, O.R.D.; Cardozo, L.; Scheer, A.D.P. Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale R.): Chemical composition and antibacterial activity. J. Supercrit. Fluids 2013, 80, 44–49. [Google Scholar] [CrossRef]
- Sivasothy, Y.; Chong, W.K.; Hamid, A.; Eldeen, I.M.; Sulaiman, S.F.; Awang, K. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food Chem. 2011, 124, 514–517. [Google Scholar]
- Rana, V.S.; Ahluwalia, V.; Shakil, N.A.; Prasad, L. Essential oil composition, antifungal, and seedling growth inhibitory effects of zerumbone from Zingiber zerumbet Smith. J. Essent. Oil Res. 2017, 29, 320–329. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Chowdhury, J.U.; Begum, J. Volatile constituents of essential oils isolated from leaf and rhizome of Zingiber cassumunar Roxb. Bangladesh J. Pharmacol. 2008, 3, 69–73. [Google Scholar] [CrossRef]
- Onyenekwe, P.C.; Hashimoto, S. The composition of the essential oil of dried Nigerian ginger (Zingiber officinale Roscoe). Eur. Food Res. Technol. 1999, 209, 407–410. [Google Scholar] [CrossRef]
- Danciu, C.; Vlaia, L.; Fetea, F.; Hancianu, M.; Coricovac, D.E.; Ciurlea, S.A.; Şoica, C.M.; Marincu, I.; Vlaia, V.; Dehelean, C.A.; et al. Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Biol. Res. 2015, 48, 1–9. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef]
- Nag, A.; Bandyopadhyay, M.; Mukherjee, A. Antioxidant activities and cytotoxicity of Zingiber zerumbet (L.) Smith rhizome. J. Pharmacogn. Phytochem. 2013, 2, 102–108. [Google Scholar]
- Koga, A.Y.; Beltrame, F.L.; Pereira, A.V. Several aspects of Zingiber zerumbet: A review. Rev. Bras. Farmacogn. 2016, 26, 385–391. [Google Scholar] [CrossRef]
- Chirangini, P.; Sharma, G.J. In vitro propagation and microrhizome induction in Zingiber cassumunar (Roxb.) an antioxidant-rich medicinal plant. J. Food Agric. Environ. 2005, 3, 139–142. [Google Scholar]
- Masuda, T.; Jitoe, A.; Mabry, T.J. Isolation and structure determination of cassumunarins A, B, and C: New anti-inflammatory antioxidants from a tropical ginger, Zingiber cassumunar. J. Am. Oil Chem. Soc. 1995, 72, 1053–1057. [Google Scholar] [CrossRef]
- Bua-in, S.; Paisooksantivatana, Y. Essential oil and antioxidant activity of Cassumunar ginger (Zingiberaceae: Zingiber montanum (Koenig) Link ex Dietr.) collected from various parts of Thailand. Kasetsart J. 2009, 43, 467–475. [Google Scholar]
- Manochaia, B.; Paisooksantivatanaa, Y.; Choib, H.; Hong, J.H. Variation in DPPH scavenging activity and major volatile oil components of Cassumunar ginger, Zingiber montanum (Koenig), in response to water deficit and light intensity. Sci. Hortic. 2010, 126, 462–466. [Google Scholar] [CrossRef]
- Phu, N.D.; Thy, L.H.P.; Lam, T.D.; Yen, V.H.; Lan, N.T.N. Extraction of jasmin essential oil by hydrodistillation method and applications on formulation of natural facial cleansers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 1–6. [Google Scholar]
- Berker, K.I.; Olgun, F.A.O.; Ozyurt, D.; Demirata, B.; Apak, R. Modified folin−ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Li, T.X.; Kong, Y.L.; Yang, M.H. Antioxidant aromatic butenolides from an insect-associated Aspergillus iizukae. Phytochem. Lett. 2016, 16, 134–140. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Sapodilla seed coat as a multifunctional ingredient for cosmetic applications. Process Biochem. 2011, 46, 2215–2218. [Google Scholar] [CrossRef]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Abe, S.; Hirose, S.; Nishitani, M.; Yoshida, I.; Tsukayama, M.; Tsuji, A.; Yuasa, K. Citrus peel polymethoxyflavones, sudachitin and nobiletin, induce distinct cellular responses in human keratinocyte HaCaT cells. Biosci. Biotechnol. Biochem. 2018, 82, 2064–2071. [Google Scholar] [CrossRef]
- Septisetyani, E.P.; Ningrum, R.A.; Romadhani, Y.; Wisnuwardhani, P.H.; Santoso, A. Optimization of sodium dodecyl sulphate as a formazan solvent and comparison of 3-(4,-5- dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with wst-1 assay in mcf-7 cells. Indonesian J. Pharm. 2014, 25, 245–254. [Google Scholar] [CrossRef]
- Zanette, C.; Pelin, M.; Crosera, M.; Adami, G.; Bovenzi, M.; Larese, F.F.; Florio, C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line. Toxicol. In Vitro 2011, 25, 1053–1060. [Google Scholar] [CrossRef]
- Wikaningtyas, P.; Sukandar, E.Y. The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens. Asian Pac. J. Trop. Biomed. 2016, 6, 16–19. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Tadeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Yamamoto, K.; Tsuru, D. Extracellular tyrosinase from Streptomyces sp. KY-453: Purification and some enzymatic properties. J. Biochem. 1985, 97, 1747–1754. [Google Scholar]
- Sasidharan, I.; Venugopal, V.V.; Menon, A.N. Essential oil composition of two unique ginger (Zingiber officinale Roscoe) cultivars from Sikkim. Nat. Prod. Res. 2012, 19, 1759–1764. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; Heluani, C.S.; Lampasona, M.P.; Catalan, C.A.N. Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol. 2008, 46, 3295–3302. [Google Scholar] [CrossRef]
- Ming, J.C.; Vera, R.; Chalchat, J.C. Chemical composition of the essential oil from rhizomes, leaves and flowers of Zingiber zerumber Smith form Reunion Island. J. Essent. Oil Res. 2001, 15, 202–205. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured publishing Corporation: Carol Stream, IL, USA, 2009; pp. 1–804. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic compounds: Function properties, impact of processing and bioavailability. In Phenolic Compounds—Biological Activity; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Hasan, H.A.; Raauf, A.M.R.; Razik, B.M.A.; Hassan, B.A.R. Chemical composition and antimicrobial activity of the crude extracts isolated from Zingiber officinale by different solvents. Pharm. Anal. Acta 2012, 3, 1–5. [Google Scholar]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, K.S.; Yokozawa, T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem. Toxicol. 2008, 46, 2466–2471. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009, 116, 1–7. [Google Scholar] [CrossRef]
| Compound | LRI Cal. | LRI Lit. | Rhizomes (%) | Flowers (%) | Leaves (%) | Identification Methods | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPME | HD | OS | SPME | HD | OS | SPME | HD | OS | ||||
| α-Thujene | 931 | 930 | 0.5 ± 0.04 | nd | nd | nd | nd | nd | 0.3 ± 0.1 | nd | nd | MS, LRI, AD |
| α-Pinene | 939 | 939 | 22.1 ± 0.6 | 24.3 ± 1.6 | 3.2 ± 0.3 | 22.2 ± 1.8 | nd | nd | 39.7 ± 0.7 | 1.7 ± 0.1 | 3.0 ± 1.9 | MS, LRI, AD |
| α-Fenchene | 953 | 952 | nd | nd | nd | 0.5 ± 0.2 | nd | nd | nd | nd | nd | MS, LRI, AD |
| Camphene | 954 | 954 | 2.6 ± 0.4 | 1.5 ± 0.3 | 0.2 ± 0.04 | 0.4 ± 0.1 | nd | nd | nd | nd | nd | MS, LRI, AD |
| Sabinene | 979 | 975 | 12.3 ± 1.7 | nd | nd | 1.3 ± 0.7 | nd | nd | 1.6 ± 0.5 | nd | nd | MS, LRI, AD |
| β-Pinene | 983 | 979 | 17.2 ± 0.7 | 33.1 ± 2.5 | 7.7 ± 1.1 | 8.6 ± 1.1 | nd | nd | 6.3 ± 0.5 | 1.8 ± 0.3 | 2.6 ± 0.9 | MS, LRI, AD |
| 2,2,4,6,6-Pentamethyl heptane | 985 | - | nd | nd | nd | nd | nd | nd | nd | nd | 2.4 ± 1.4 | LRI, AD |
| Myrcene | 996 | 990 | 1.5 ± 0.5 | 2.0 ± 0.4 | 0.4 ± 0.1 | 0.9 ± 0.2 | nd | nd | 0.4 ± 0.1 | nd | nd | MS, LRI, AD |
| 3-(E)-Hexenyl acetate | 1002 | 1002 | nd | nd | nd | nd | nd | nd | nd | nd | 1.7 ± 1.1 | MS, LRI, AD |
| δ-2-Carene | 1006 | 1002 | 0.5 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Phellandrena | 1010 | 1002 | 0.2 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| δ-3-Carene | 1015 | 1011 | 0.7 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Terpinene | 1021 | 1017 | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.1 ± 0.05 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| o-Cymene | 1029 | 1026 | 2.7 ± 0.3 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Limonene | 1033 | 1029 | nd | 4.0 ± 0.6 | 1.8 ± 0.2 | 1.7 ± 0.3 | nd | nd | 1.8 ± 0.2 | 1.4 ± 0.6 | 0.5 ± 0.3 | MS, LRI, AD |
| Sylvestrene | 1034 | 1030 | 3.0 ± 0.3 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| (Z)-β-Ocimene | 1041 | 1037 | 0.4 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| (E)-β-Ocimene | 1051 | 1050 | 8.1 ± 0.3 | 7.8 ± 0.5 | 4.1 ± 0.8 | 1.7 ± 0.1 | nd | nd | 1.5 ± 0.5 | 1.2 ± 0.3 | nd | MS, LRI, AD |
| γ-Terpinene | 1063 | 1059 | 1.7 ± 0.3 | nd | 0.7 ± 0.1 | 0.9 ± 0.1 | nd | nd | nd | nd | nd | MS, LRI, AD |
| ρ-Mentha-2,4(8)-diene | 1083 | 1088 | nd | 0.8 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| o-Guaiacol | 1086 | 1089 | nd | nd | 1.8 ± 0.3 | nd | nd | 0.6 ± 0.1 | nd | nd | nd | MS, LRI, AD |
| Terpinolene | 1094 | 1088 | 0.58 ± 0.18 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Linalool | 1106 | 1096 | nd | nd | 1.9 ± 0.2 | nd | nd | 1.1 ± 0.1 | nd | 4.0 ± 0.5 | 3.3 ± 0.4 | MS, LRI, AD |
| allo-Ocimene | 1133 | 1132 | 0.6 ± 0.2 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Borneol | 1170 | 1169 | nd | 0.8 ± 0.2 | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Terpinen-4-ol | 1182 | 1177 | 1.8 ± 0.6 | 3.8 ± 0.3 | 11.5 ± 0.6 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Terpineol | 1196 | 1188 | nd | 0.8 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Myrtenal | 1201 | 1195 | nd | nd | 2.3 ± 0.6 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Verbenone | 1213 | 1205 | nd | nd | 0.4 ± 0.1 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Piperitone | 1248 | 1252 | nd | nd | 0.2 ± 0.02 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Isobornyl acetate | 1289 | 1285 | 0.2 ± 0.1 | 0.5 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Indole | 1290 | 1291 | nd | nd | nd | nd | nd | 1.7 ± 0.9 | nd | nd | nd | MS, LRI, AD |
| Bornyl acetate | 1301 | 1288 | nd | nd | 0.6 ± 0.1 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| (E)-Pinocarvyl acetate | 1303 | 1298 | nd | nd | 0.5 ± 0.2 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| δ-Elemene | 1340 | 1338 | 0.3 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Cubebene | 1353 | 1348 | 5.3 ± 0.6 | nd | 0.6 ± 0.1 | nd | nd | nd | 0.1 ± 0.06 | nd | nd | MS, LRI, AD |
| Cyclosativene | 1370 | 1371 | nd | 2.3 ± 0.4 | 2.8 ± 0.6 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Ylangene | 1374 | 1375 | 3.9 ± 0.8 | nd | nd | 0.7 ± 0.1 | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Copaene | 1380 | 1376 | 0.2 ± 0.1 | 1.3 ± 0.3 | 2.0 ± 0.5 | nd | nd | nd | 3.2 ± 0.6 | nd | nd | MS, LRI, AD |
| β-Cubebene | 1388 | 1388 | 0.89 ± 0.15 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| β-Elemene | 1397 | 1390 | nd | 1.7 ± 0.3 | 3.0 ± 0.6 | 0.5 ± 0.1 | nd | nd | 3.8 ± 0.4 | 12.2 ± 1.3 | 3.6 ± 0.6 | MS, LRI, AD |
| Longifolene | 1413 | 1407 | 3.6 ± 0.6 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| (E)-Caryophyllene | 1424 | 1419 | nd | 4.2 ± 0.4 | 3.2 ± 0.3 | 58.1 ± 1.3 | 94.8 ± 0.9 | 74.2 ± 1.5 | 21.2 ± 0.8 | 24.2 ± 1.6 | 6.0 ± 0.8 | MS, LRI, AD |
| β-Copaene | 1432 | 1432 | 0.6 ± 0.3 | nd | 0.2 ± 0.1 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| γ-Elemene | 1437 | 1436 | 0.2 ± 0.1 | nd | 0.4 ± 0.1 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Humulene | 1456 | 1454 | nd | nd | 0.6 ± 0.1 | 0.8 ± 0.3 | 2.3 ± 0.5 | 2.0 ± 0.03 | 0.4 ± 0.05 | 1.4 ± 0.7 | nd | MS, LRI, AD |
| (E)-β-Farnesene | 1460 | 1456 | nd | nd | nd | nd | nd | nd | 0.1 ± 0.05 | nd | nd | MS, LRI, AD |
| 9-epi-(E)-Caryophyllene | 1463 | 1466 | 0.5 ± 0.1 | nd | nd | nd | nd | nd | 0.5 ± 0.5 | nd | nd | MS, LRI, AD |
| (E)-Cadina-1(6),4-diene | 1474 | 1476 | nd | 1.3 ± 0.7 | 3.0 ± 0.4 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| γ-Muurolene | 1480 | 1479 | 0.8 ± 0.2 | nd | nd | nd | nd | nd | 0.2 ± 0.1 | nd | nd | MS, LRI, AD |
| α-Curcumene | 1488 | 1480 | nd | nd | nd | nd | nd | nd | 3.5 ± 0.7 | 4.2 ± 0.4 | 2.9 ± 0.4 | MS, LRI, AD |
| Germacrene D | 1484 | 1485 | 0.2 ± 0.1 | nd | nd | nd | nd | 0.4 ± 0.1 | nd | nd | nd | MS, LRI, AD |
| δ-Selinene | 1486 | 1492 | 0.2 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| (Z)-β-Guaiene | 1489 | 1493 | 0.3 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Zingiberene | 1501 | 1493 | nd | nd | 2.8 ± 0.3 | 0.2 ± 0.1 | nd | nd | 4.4 ± 0.7 | 11.7 ± 1.0 | 3.8 ± 0.5 | MS, LRI, AD |
| Valencene | 1496 | 1496 | 0.3 ± 0.1 | nd | nd | nd | nd | nd | nd | 21.2 ± 1.3 | nd | MS, LRI, AD |
| Isodaucene | 1497 | 1500 | nd | 4.1 ± 0.4 | 2.7 ± 0.4 | nd | nd | nd | nd | nd | 4.2 ± 1.0 | MS, LRI, AD |
| α-Muurolene | 1504 | 1500 | 0.6 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| β-Bisabolene | 1514 | 1505 | nd | nd | nd | 0.5 ± 0.1 | nd | nd | 2.7 ± 0.6 | nd | nd | MS, LRI, AD |
| 7-epi-α-Selinene | 1520 | 1522 | 0.6 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| β-Sesquiphellandrene | 1527 | 1522 | nd | nd | nd | nd | nd | nd | 3.2 ± 0.5 | 4.8 ± 0.3 | 1.6 ± 0.6 | MS, LRI, AD |
| δ-Cadinene | 1529 | 1523 | 0.2 ± 0.1 | nd | 1.5 ± 0.4 | 0.3 ± 0.1 | nd | 0.4 ± 0.1 | nd | nd | nd | MS, LRI, AD |
| (E)-γ-Bisabolene | 1535 | 1531 | nd | nd | nd | nd | nd | nd | 0.63 ± 0.76 | nd | nd | MS, LRI, AD |
| Germacrene B | 1559 | 1561 | nd | 1.9 ± 0.9 | 4.2 ± 0.6 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| (E)-Nerolidol | 1568 | 1563 | 0.2 ± 0.1 | nd | 1.8 ± 0.6 | nd | nd | nd | nd | 1.9 ± 0.8 | nd | MS, LRI, AD |
| Caryophyllene oxide | 1586 | 1583 | nd | nd | 4.4 ± 0.7 | nd | nd | 0.8 ± 0.1 | nd | nd | 1.9 ± 1.3 | MS, LRI, AD |
| γ-Eudesmol | 1627 | 1632 | nd | nd | 2.1 ± 0.5 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| α-Eudesmol | 1648 | - | nd | nd | 4.1 ± 0.4 | nd | nd | nd | nd | nd | nd | LRI, AD |
| Cryptomeridiol | 1809 | 1813 | nd | nd | 5.0 ± 0.7 | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Cyclohexadecanolide | 1936 | 1933 | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS, LRI, AD |
| Sandaracopimaradiene | 1948 | - | nd | nd | 0.2 ± 0.1 | nd | nd | nd | nd | nd | nd | LRI, AD |
| n-Hexadecanoic acid | 1960 | 1959 | nd | nd | 8.7 ± 0.9 | nd | nd | 14.9 ± 0.4 | nd | nd | 55.7 ± 1.3 | MS, LRI, AD |
| Number of constituents | 37 | 19 | 36 | 16 | 2 | 9 | 20 | 13 | 14 | |||
| % of constituents identified | 95.5% | 96.9% | 90.8% | 99.1% | 97.2% | 97.1% | 95.4% | 91.8% | 93.1% | |||
| Yield (v/w) | 0.4% | 0.6% | 0.2% | 0.3% | 0.2% | 0.3% | ||||||
| Monoterpene hydrocarbons | 75.0% | 74.3% | 20.1% | 38.0% | - | 0.6% | 51.5% | 6.2% | - | |||
| Oxygenated monoterpenes | 2.0% | 5.9% | 17.6% | - | - | 1.1% | - | 4.0% | 3.3% | |||
| Sesquiterpene hydrocarbons | 18.4% | 16.7% | 26.9% | 61.1% | 97.2% | 77.0% | 43.9% | 79.7% | 22.1% | |||
| Oxygenated sesquiterpenes | 0.2% | - | 17.3% | - | - | 1.7% | - | 1.9% | 1.9% | |||
| Diterpene hydrocarbons | - | - | 0.2% | - | - | - | - | - | - | |||
| Fatty acid esters | - | - | 8.7% | - | - | 14.9% | - | - | 55.7% | |||
| Other compounds | - | - | - | - | - | 1.7% | - | - | - | |||
| Sample | Total Phenolic Content (mg GAE/100 g Extract) | Antioxidant (IC50, μg/mL) | FRAP (mM AAE/g Extract) | Tyrosinase Inhibitory Activity (mg KAE/g Extract) | |
|---|---|---|---|---|---|
| DPPH | ABTS | ||||
| Z. kerrii | 2.2 ± 0.1 | 143.6 ± 2.3 | 169.7 ± 41.0 | 0.3 ± 0.01 | 22.7 ± 0.8 |
| Ascorbic acid | - | 1.6 ± 0.8 | 5.2 ± 0.8 | - | - |
| Concentration (µg/mL) | % Cell Viability |
|---|---|
| 12.5 | 98.7 ± 6.0 |
| 25 | 95.9 ± 6.9 |
| s50 | 99.9 ± 0.3 |
| 100 | 97.5 ± 16.0 |
| 200 | 23.1 ± 10.4 |
| Sample | Gram (+) Bacteria | Gram (−) Bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| B. cereus | B. subtilis | S. aureus | S. epidermidis | E. coli | S. typhimurium | Ps. aeruginosa | Serratia marcescens | |
| Z. kerrii | 1280 | 1280 | 640 | 1280 | - | 640 | 1280 | - |
| Vancomycin | 320 | 160 | 10 | 1280 | - | - | - | - |
| Gentamycin | - | - | - | - | 160 | 80 | 640 | 160 |
| Ampicillin | - | 320 | 5 | 320 | 80 | 640 | 1280 | 160 |
| DMSO | 1280 | 1280 | - | 1280 | 1280 | - | 1280 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintatum, A.; Laphookhieo, S.; Logie, E.; Berghe, W.V.; Maneerat, W. Chemical Composition of Essential Oils from Different Parts of Zingiber kerrii Craib and Their Antibacterial, Antioxidant, and Tyrosinase Inhibitory Activities. Biomolecules 2020, 10, 228. https://doi.org/10.3390/biom10020228
Pintatum A, Laphookhieo S, Logie E, Berghe WV, Maneerat W. Chemical Composition of Essential Oils from Different Parts of Zingiber kerrii Craib and Their Antibacterial, Antioxidant, and Tyrosinase Inhibitory Activities. Biomolecules. 2020; 10(2):228. https://doi.org/10.3390/biom10020228
Chicago/Turabian StylePintatum, Aknarin, Surat Laphookhieo, Emilie Logie, Wim Vanden Berghe, and Wisanu Maneerat. 2020. "Chemical Composition of Essential Oils from Different Parts of Zingiber kerrii Craib and Their Antibacterial, Antioxidant, and Tyrosinase Inhibitory Activities" Biomolecules 10, no. 2: 228. https://doi.org/10.3390/biom10020228
APA StylePintatum, A., Laphookhieo, S., Logie, E., Berghe, W. V., & Maneerat, W. (2020). Chemical Composition of Essential Oils from Different Parts of Zingiber kerrii Craib and Their Antibacterial, Antioxidant, and Tyrosinase Inhibitory Activities. Biomolecules, 10(2), 228. https://doi.org/10.3390/biom10020228







