Molecular Characterization, Nutritional and Insulin Regulation of Elovl6 in Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. RNA Isolation and cDNA Synthesis
2.3. Cloning, Sequence, and Phylogenetic Analysis of Elovl6
2.4. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.5. Cloning of Elovl6 Promoter from Rainbow Trout
2.6. Construction of Transcription Factor Plasmids, Cell Culturing, Transfection, and Luciferase Assay
2.7. Primary Hepatocyte Isolation and Incubation
2.8. Statistical Analysis
3. Results
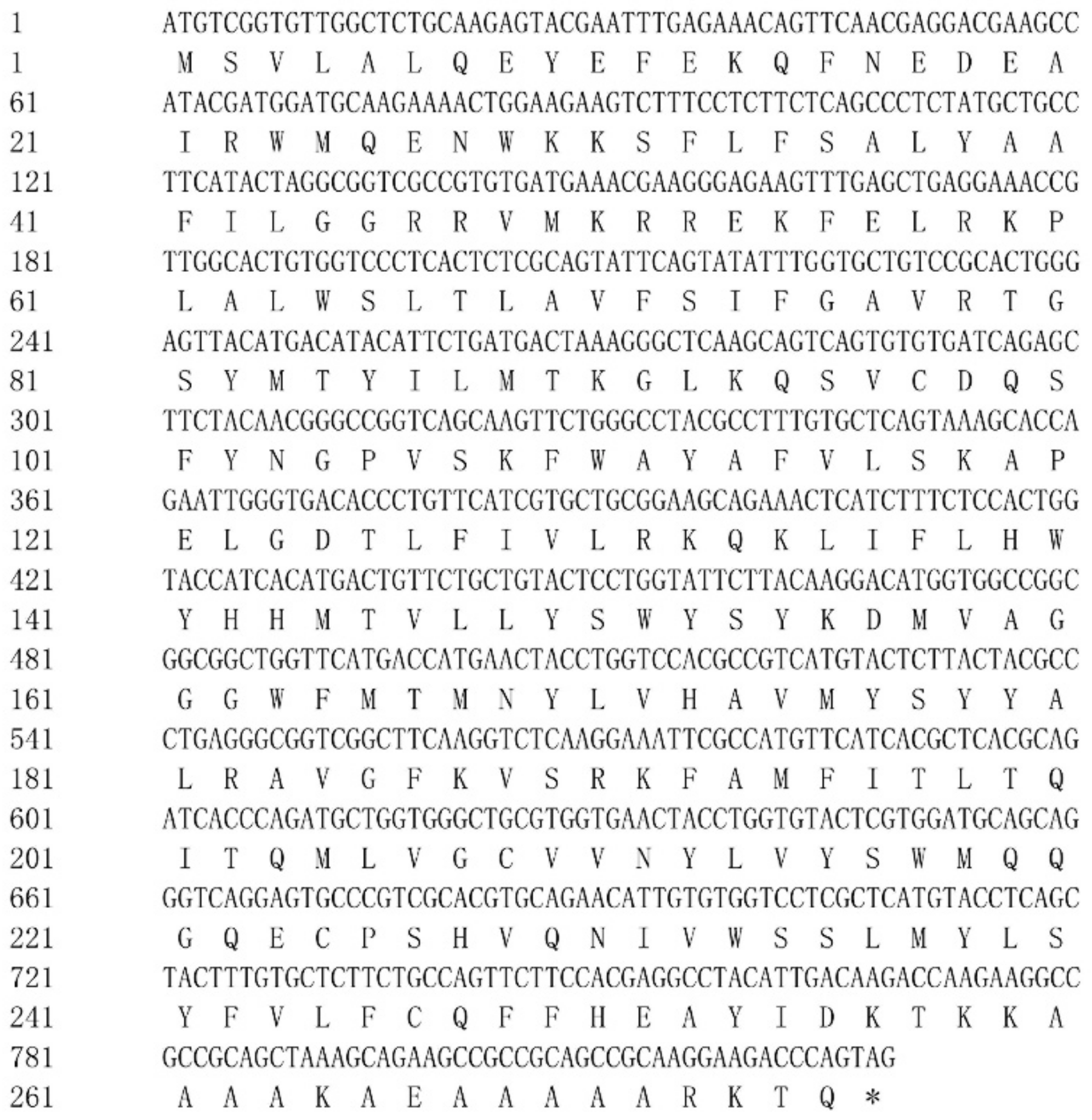
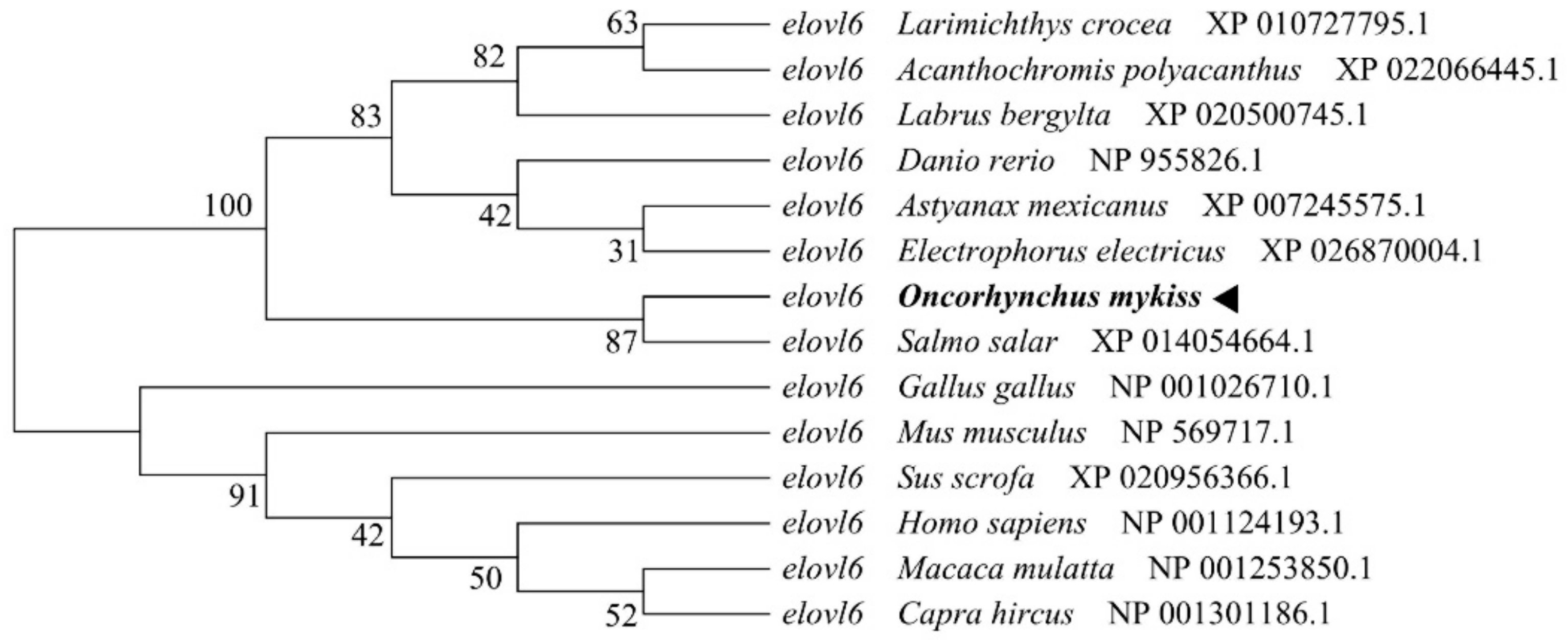
3.1. Molecular Characterization of Rainbow Trout Elovl6
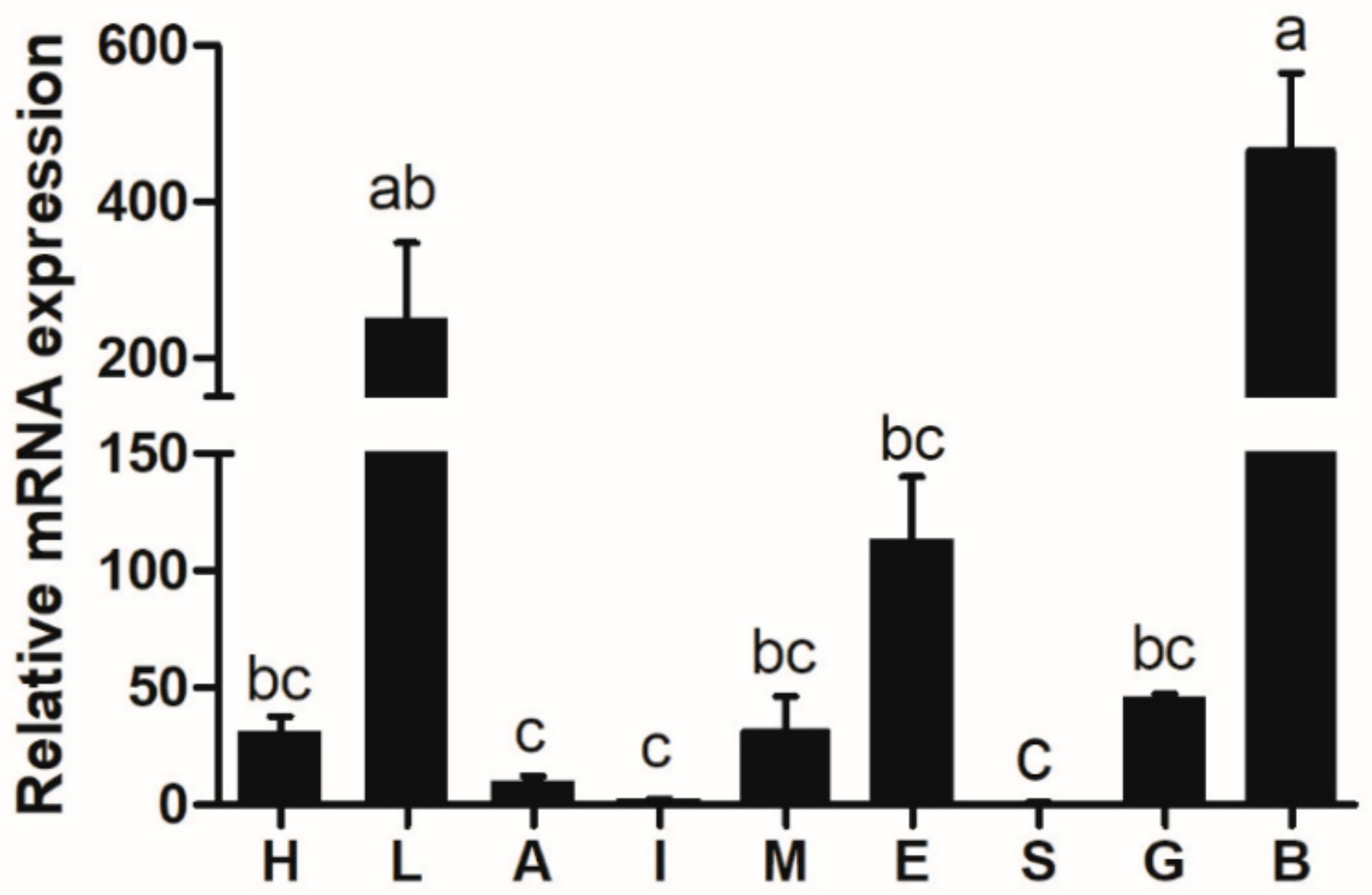
3.2. Tissue Distribution of Rainbow Trout Elovl6
3.3. Transcriptional Expression of Elovl6 in Response to Fatty Acids
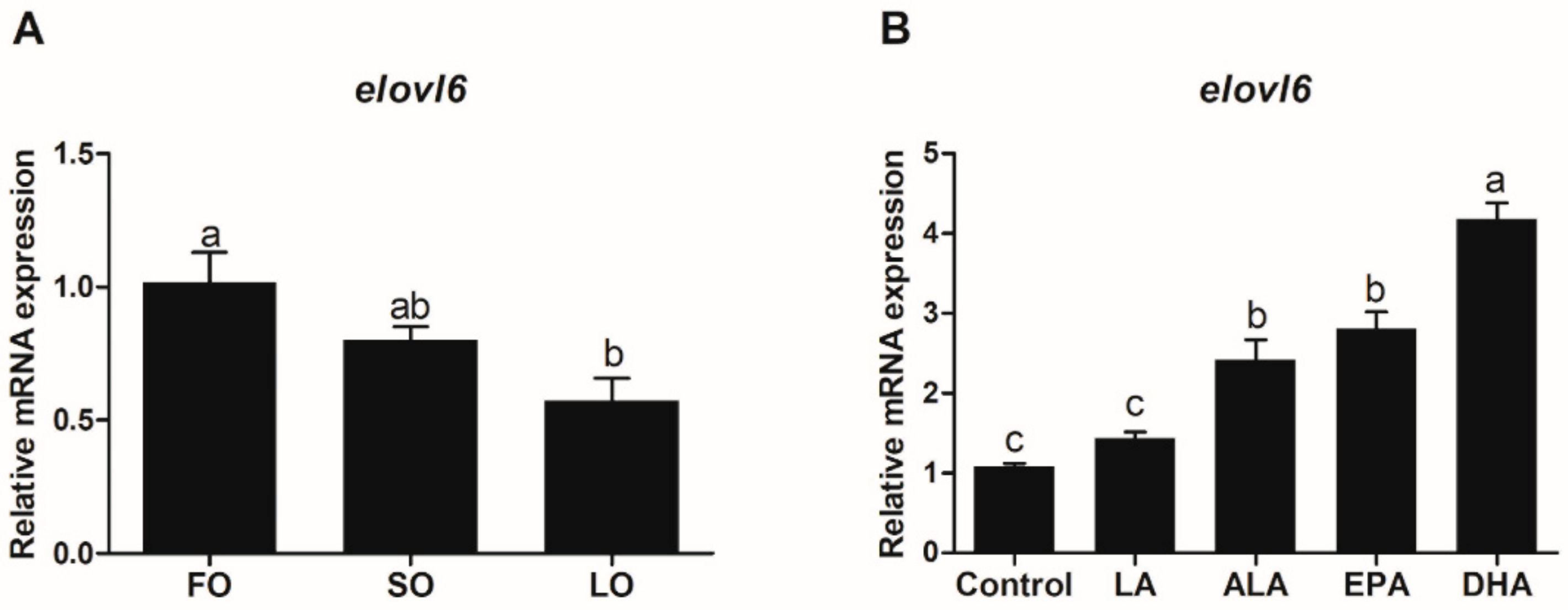
3.3.1. Expression of Elovl6 in Response to Dietary Fatty Acids
3.3.2. Expression of Elovl6 in Hepatocytes in Response to Fatty Acids
3.4. Transcriptional Expression of Elovl6 in Hepatocytes in Response to Insulin
3.5. Transcriptional Regulation of the Elovl6 in Response to Fatty Acids or Insulin
3.5.1. Regulation of the Elovl6 by Transcription Factors
3.5.2. Transcriptional Expression of Creb1 and Foxo1 in Response to Dietary Fatty Acids
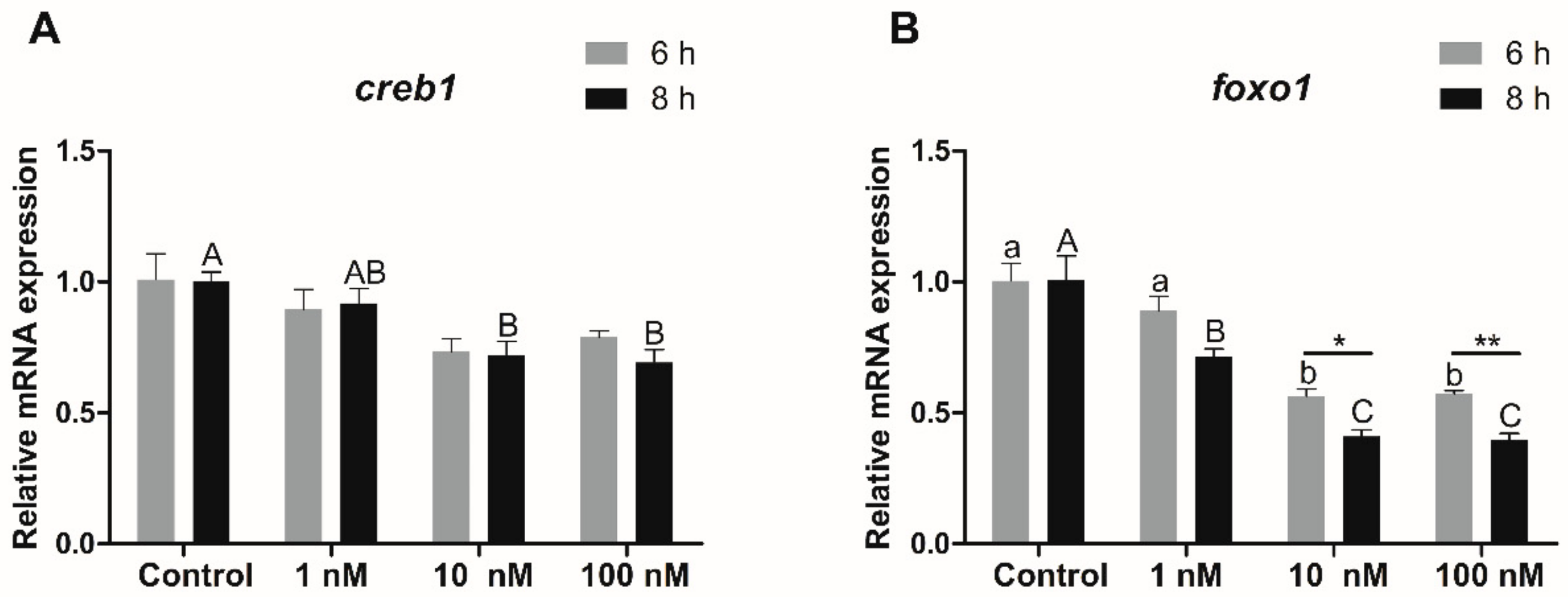
3.5.3. Transcriptional Expression of Creb1 and Foxo1 in Hepatocytes in Response to Insulin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metab. Clin. Exp. 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Moon, Y.-A.; Shah, N.A.; Mohapatra, S.; Warrington, J.A.; Horton, J.D. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 2001, 276, 45358–45366. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Kato, T.; Atsumi, A.; Yamamoto, T.; Inoue, N.; Ishikawa, M.; Okada, S.; Ishigaki, N.; et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat. Med. 2007, 13, 1193–1202. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H. Elovl6: A new player in fatty acid metabolism and insulin sensitivity. J. Mol. Med. 2009, 87, 379–384. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Yoshikawa, T.; Amemiya-Kudo, M.; Hasty, A.H.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; et al. Cloning and characterization of a mammalian fatty acyl-CoA elongase as a lipogenic enzyme regulated by SREBPs. J. Lipid Res. 2002, 43, 911–920. [Google Scholar] [PubMed]
- Matsuzaka, T.; Atsumi, A.; Matsumori, R.; Nie, T.; Shinozaki, H.; Suzuki-Kemuriyama, N.; Kuba, M.; Nakagawa, Y.; Ishii, K.; Shimada, M.; et al. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology 2012, 56, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pang, Y.; Xiang, X.; Du, J.; Mai, K.; Ai, Q. Molecular cloning, characterization, and nutritional regulation of Elovl6 in large yellow croaker (Larimichthys crocea). Int. J. Mol. Sci. 2019, 20, 1801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Matsuzaka, T.; Nakano, Y.; Motomura, K.; Tang, N.; Yokoo, T.; Okajima, Y.; Han, S.-I.; Takeuchi, Y.; Aita, Y.; et al. Elovl6 deficiency improves glycemic control in diabetic db/db mice by expanding β-cell mass and increasing insulin secretory capacity. Diabetes 2017, 66, 1833–1846. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.; Yan, J.; Jiang, J.; Cao, X.; Gao, J. Molecular characterization of elongase of very long-chain fatty acids 6 (elovl6) genes in Misgurnus anguillicaudatus and their potential roles in adaptation to cold temperature. Gene 2018, 666, 134–144. [Google Scholar] [CrossRef]
- Shi, Q.Y.; Yang, Z.G.; Yao, Q.Q.; Cheng, Y.X.; Yang, Q.; Wei, B.H. Full-length cDNA cloning of Elovl6 and its tentative study in Chinese mitten crab (Eriocheir sinensis). J. Fish. China 2016, 40, 844–855. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Monroig, Ó.; Wang, T.; Yuan, Y.; Carlos, N.J.; Hontoria, F.; Liao, K.; Tocher, D.R.; Mai, K.; Xu, W.; et al. Functional characterization and differential nutritional regulation of putative Elovl5 and Elovl4 elongases in large yellow croaker (Larimichthys crocea). Sci. Rep. 2017, 7, 2303. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Oh, A.R.; Lee, H.J.; Ahn, Y.H.; Cha, J.Y. Hepatic Elovl6 gene expression is regulated by the synergistic action of ChREBP and SREBP-1c. Biochem. Biophys. Res. Commun. 2016, 478, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Matsuzaka, T.; Tahara-Hanaoka, S.; Shibuya, K.; Shimano, H.; Nakahashi-Oda, C.; Shibuya, A. Elovl6 regulates mechanical damage-induced keratinocyte death and skin inflammation. Cell Death Dis. 2018, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Matsuzaka, T.; Karasawa, T.; Sekiya, M.; Okada, N.; Igarashi, M.; Matsumori, R.; Ishii, K.; Nakagawa, Y.; Iwasaki, H.; et al. Macrophage Elovl6 deficiency ameliorates foam cell formation and reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, A.; Westerberg, R.; Jacobsson, A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog. Lipid Res. 2006, 45, 237–249. [Google Scholar] [CrossRef]
- Green, C.D.; Ozguden-Akkoc, C.G.; Wang, Y.; Jump, D.B.; Olson, L.K. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species. J. Lipid Res. 2010, 51, 1871–1877. [Google Scholar] [CrossRef]
- Shi, H.; Wu, M.; Zhu, J.; Zhang, C.; Yao, D.; Luo, J.; Loor, J.J. Fatty acid elongase 6 plays a role in the synthesis of long-chain fatty acids in goat mammary epithelial cells. J. Dairy Sci. 2017, 100, 4987–4995. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, T.; Wang, S.; He, B.; Zhang, Y.; Piao, D.; Yu, C.; Wu, N.; Han, P. The effect of LXRα, ChREBP and Elovl6 in liver and white adipose tissue on medium- and long-chain fatty acid diet-induced insulin resistance. Diabetes Res. Clin. Pract. 2013, 102, 183–192. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Kuba, M.; Koyasu, S.; Yamamoto, Y.; Motomura, K.; Arulmozhiraja, S.; Ohno, H.; Sharma, R.; Shimura, T.; Okajima, Y.; et al. Hepatocyte Elovl6 determines ceramide acyl-chain length and hepatic insulin sensitivity in mice. Hepatology 2019. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Kim, J.B.; Sarraf, P.; Wright, M.; Yao, K.M.; Mueller, E.; Solanes, G.; Lowell, B.B.; Spiegelman, B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Investig. 1998, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guichard, C.; Ferré, P.; Foufelle, F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. USA 1999, 96, 12737–12742. [Google Scholar] [CrossRef] [PubMed]
- Kumadaki, S.; Matsuzaka, T.; Kato, T.; Yahagi, N.; Yamamoto, T.; Okada, S.; Kobayashi, K.; Takahashi, A.; Yatoh, S.; Suzuki, H.; et al. Mouse Elovl6 promoter is an SREBP target. Biochem. Biophys. Res. Commun. 2008, 368, 261–266. [Google Scholar] [CrossRef]
- Ortega-Martínez, S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.; Fukushima, A.; Viengchareun, S.; Lombès, M.; Kishi, F.; Miyauchi, A.; Kanematsu, M.; Doi, J.; Kajimura, J.; Nakai, R.; et al. Involvement of SIK2/TORC2 signaling cascade in the regulation of insulin-induced PGC-1α and UCP-1 gene expression in brown adipocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1430–E1439. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Hedrick, S.; Morantte, I.; Koo, S.-H.; Galimi, F.; Montminy, M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-γ. Nature 2003, 426, 190–193. [Google Scholar] [CrossRef]
- Sun, J.; Deng, W.; Gou, N.-N.; Ji, H.; Du, Z.-Y.; Chen, L.-Q. CIDEA and CIDEC are regulated by CREB and are not induced during fasting in grass carp Ctenopharyngodon idella adipocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 234, 50–57. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, S.; Copps, K.; Dong, X.; Kollipara, R.; Rodgers, J.T.; Depinho, R.A.; Puigserver, P.; White, M.F. FOXO1 integrates insulin signaling with mitochondrial function in the liver. Nat. Med. 2009, 15, 1307–1311. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, Y.-B.; Zhou, J.; Kang, D.-M. Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FOXO1 expression. Biochem. Biophys. Res. Commun. 2016, 469, 319–325. [Google Scholar] [CrossRef]
- O-Sullivan, I.; Zhang, W.; Wasserman, D.H.; Liew, C.W.; Liu, J.; Paik, J.; DePinho, R.A.; Stolz, D.B.; Kahn, C.R.; Schwartz, M.W.; et al. FOXO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 2015, 6, 7079. [Google Scholar] [CrossRef] [PubMed]
- Mirdamadi, Y.; Thielitz, A.; Wiede, A.; Goihl, A.; Papakonstantinou, E.; Hartig, R.; Zouboulis, C.C.; Reinhold, D.; Simeoni, L.; Bommhardt, U.; et al. Insulin and insulin-like growth factor-1 can modulate the phosphoinositide-3-kinase/Akt/FOXO1 pathway in SZ95 sebocytes in vitro. Mol. Cell. Endocrinol. 2015, 415, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Dong, H.H. FOXO integration of insulin signaling with glucose and lipid metabolism. J. Endocrinol. 2017, 233, R67–R69. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.L.; Zhang, Y.; Bae, S.-H.; Farooqi, M.S.; Liang, G.; Hammer, R.E.; Goldstein, J.L.; Brown, M.S. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc. Natl. Acad. Sci. USA 2012, 109, 16184–16189. [Google Scholar] [CrossRef]
- Tian, J.; Goldstein, J.L.; Brown, M.S. Insulin induction of SREBP-1c in rodent liver requires LXRα-C/EBPβ complex. Proc. Natl. Acad. Sci. USA 2016, 113, 8182–8187. [Google Scholar] [CrossRef]
- Botolin, D.; Wang, Y.; Christian, B.; Jump, D.B. Docosahexaneoic acid (22: 6, n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk-and 26S proteasome-dependent pathways. J. Lipid Res. 2006, 47, 181–192. [Google Scholar] [CrossRef]
- Boughanem, H.; Cabrera-Mulero, A.; Millán-Gómez, M.; Garrido-Sánchez, L.; Cardona, F.; Tinahones, F.J.; Moreno-Santos, I.; Macías-González, M. Transcriptional analysis of FOXO1, C/EBP-α and PPAR-γ2 genes and their association with obesity-related insulin resistance. Genes 2019, 10, 706. [Google Scholar] [CrossRef]
- Wu, Y.L.; Peng, X.E.; Wang, D.; Chen, W.N.; Lin, X. Human liver fatty acid binding protein (hFABP1) gene is regulated by liver-enriched transcription factors HNF3β and C/EBPα. Biochimie 2012, 94, 384–392. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef]
- Suzuki, R.; Lee, K.; Jing, E.; Biddinger, S.B.; McDonald, J.G.; Montine, T.J.; Craft, S.; Kahn, C.R. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab. 2010, 12, 567–579. [Google Scholar] [CrossRef]
- Miao, J.; Haas, J.T.; Manthena, P.; Wang, Y.; Zhao, E.; Vaitheesvaran, B.; Kurland, I.J.; Biddinger, S.B. Hepatic insulin receptor deficiency impairs the SREBP-2 response to feeding and statins. J. Lipid Res. 2014, 55, 659–667. [Google Scholar] [CrossRef] [PubMed]




| Primer | Sequences 5′-3′ | Primer Information |
|---|---|---|
| ORF-F | ATGTCGGTGTTGGCTCTGCAAG | Elovl6-ORF |
| ORF-R | CTACTGGGTCTTCCTTGCGGC | Elovl6-ORF |
| qElovl6-F | TCAACGAGGACGAAGCCATACGA | Elovl6 q-PCR |
| qElovl6-R | CCCAGTGCGGACAGCACCAAATA | Elovl6 q-PCR |
| qCREB1-F | AGGAGTCAGTGGACAGTGTGA | CREB1 q-PCR |
| qCREB1-R | TGCTGGTCTGGTAGATAGGGC | CREB1 q-PCR |
| qFOXO1-F | AACTCCCACAGCCACAGCAA | FOXO1 q-PCR |
| qFOXO1-R | CGATGTCCTGTTCCAGGAAGG | FOXO1 q-PCR |
| β-actin-F | ATCAGGGAGTGATGGTTGGGATG | β-actin q-PCR |
| β-actin-R | CTCGTAGATGGGTACTGTGTGGG | β-actin q-PCR |
| E6-P-F | GTAAACTGTTGCTGGAGATTCCGGAC | Elovl6 promoter |
| E6-P-R | GTTCACTGTGCGCTTTCCTGTAAACG | Elovl6 promoter |
| PCS2+-SREBP1-F | ATGAACTTGTCTTTTGACGATCAG | Expression plasmid |
| PCS2+-SREBP1-R | CTAGGCAGAGGTGACAGTGGTGC | Expression plasmid |
| PCS2+-SREBP2-F | ATGGACAGTAACGTTAGTGGGGAG | Expression plasmid |
| PCS2+-SREBP2-R | TCAGGAGGCCGCGATGGTG | Expression plasmid |
| PCS2+-CEBPα-F | ATGGAGCAACCAAACCTCTACGAG | Expression plasmid |
| PCS2+-CEBPα-R | TCACTGGCAGTTGGCCAATG | Expression plasmid |
| PCS2+-CEBPβ-F | TGGAAGTGGCCGGTTTCTACG | Expression plasmid |
| PCS2+-CEBPβ-R | CTAACCGGTGGCAGAAAGCAAG | Expression plasmid |
| PCS2+-HNF1α-F | ATGGAGGGAGAGGAGAGGAAAGG | Expression plasmid |
| PCS2+-HNF1α-R | CTACTGTGCGGTAGAGACCATCTGT | Expression plasmid |
| PCS2+-PPARγ-F | ATGCATATGATGTGTAGCAATTTTA | Expression plasmid |
| PCS2+-PPARγ-R | CTAGTAGAGGTCTCTCATGATCTCCT | Expression plasmid |
| PCS2+-RXRα-F | ATGACGCTGGAAATTCTGACATATT | Expression plasmid |
| PCS2+-RXRα-R | TTATGTCATTTGGTGGGGCG | Expression plasmid |
| PCS2+-CREB1-F | ATGACCATGGAGTCGGGAGC | Expression plasmid |
| PCS2+-CREB1-R | CTACTCAGATTTATGGCAGTACAGGTC | Expression plasmid |
| PCS2+-P65-F | ATGGATGGAATGTATGGATGGGG | Expression plasmid |
| PCS2+-P65-R | TAAGTCTGATGTCCGGACACGAA | Expression plasmid |
| PCS2+-FOXO1-F | ATGGCAGAATTACCACCGCCG | Expression plasmid |
| PCS2+-FOXO1-R | CTAGCCAGACACCCAGCTGTGTGTG | Expression plasmid |
| Transcription Factor | Software | Position | Predicted Site |
|---|---|---|---|
| CEBPα/CEBPβ | JASPAR | −1417 | TATTGCACCATA |
| +52 | AATTGCAAAATA | ||
| RXRα | JASPAR | −1155 | TAACATTAAATAACTTTGG |
| P65 | TF binding | −1081 | GGAAACTCTC |
| SREBP1/SREBP2 | JASPAR | −935 | ATGGGGAGAT |
| FOXO1 | JASPAR | −531 | TGTAAACAAGA |
| HNF1α | JASPAR | −246 | GGGTAATTGTTTAC |
| CREB1 | JASPAR | −35 | GGACGTCA |
| PPARγ | JASPAR | +104 | TTGGTGGAGAGGGCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Pang, Y.; Zhao, Z.; Xiang, X.; Mai, K.; Ai, Q. Molecular Characterization, Nutritional and Insulin Regulation of Elovl6 in Rainbow Trout (Oncorhynchus mykiss). Biomolecules 2020, 10, 264. https://doi.org/10.3390/biom10020264
Li Y, Pang Y, Zhao Z, Xiang X, Mai K, Ai Q. Molecular Characterization, Nutritional and Insulin Regulation of Elovl6 in Rainbow Trout (Oncorhynchus mykiss). Biomolecules. 2020; 10(2):264. https://doi.org/10.3390/biom10020264
Chicago/Turabian StyleLi, Yongnan, Yuning Pang, Zengqi Zhao, Xiaojun Xiang, Kangsen Mai, and Qinghui Ai. 2020. "Molecular Characterization, Nutritional and Insulin Regulation of Elovl6 in Rainbow Trout (Oncorhynchus mykiss)" Biomolecules 10, no. 2: 264. https://doi.org/10.3390/biom10020264
APA StyleLi, Y., Pang, Y., Zhao, Z., Xiang, X., Mai, K., & Ai, Q. (2020). Molecular Characterization, Nutritional and Insulin Regulation of Elovl6 in Rainbow Trout (Oncorhynchus mykiss). Biomolecules, 10(2), 264. https://doi.org/10.3390/biom10020264





