Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer
Abstract
1. Introduction
2. Therapeutic Effects of Phenolics in Preclinical Cancer Research
2.1. BenzoicAacids
2.1.1. Vanillic Acid
2.1.2. Gentisic Acid
2.1.3. Protocatechuic Acid
2.1.4. Gallic Acid
2.1.5. Syringic Acid
2.2. Cinnamic Acids
2.2.1. Caffeic Acid
2.2.2. Ferulic Acid
2.2.3. p-Coumaric Acid
2.2.4. Sinapic Acid
3. Use of Phenolics in Clinical Research
4. Conclusions and Future Aspects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Apoptosis Inducing Factor | AIF |
| Apoptosis Protease Activating Factor-1 | Apaf-1 |
| B-Cell Lymphoma 2 | Bcl-2 |
| Bcl-2 Antagonist and Killer | Bak |
| Bcl-2 Associated Death Promoter | Bad |
| Bcl2-Associated X Protein | Bax |
| Bcl-2-Like Protein 11 | Bim |
| BH3 Interacting-Domain Death Agonist | Bid |
| Breast cancer resistance protein | BCRP |
| Catalase | CAT |
| Cadherin | CDH |
| C-Jun N-Terminal Kinase | JNK |
| Cyclin Dependent Kinases | CDKs |
| Epidermal Growth Factor Receptor | EGFR |
| Extracellular matrix | ECM |
| Extracellular signal-regulated kinase | ERK |
| Glutathione | GSH |
| Glutathione Peroxidase | GPx |
| Hypoxia Inducing Factor | HIF-1 |
| Lipid Hydroperoxides | LOOH |
| Macrophage Inhibitory Cytokine | MIC-1 |
| Matrix Metalloproteinases | MMP |
| Mechanistic target of rapamycin | mTOR |
| Mitogen-activated protein kinase | MEK |
| Mitogen-Activated Protein Kinase | MAPK |
| Multidrug resistance protein | MRP |
| Nicotinamide Adenine Dinucleotide Phosphate Hydrogen | NADPH |
| Nicotinamide Adenine Dinucleotide Phosphate Oxidases | Nox |
| N-methyl-N’-nitro-N-nitrosoguanidine | MNNG |
| Nuclear Factor Erythroid 2-Related Factor 2 | NRF2 |
| Nuclear factor-kappa B | NF- κB |
| Organic Anion Transporter-3 | OAT3 |
| P-glycoprotein | P-gp |
| Phosphoinositide 3-Kinase | PI3K |
| Poly (ADP-Ribose) Polymerase | PARP |
| Protein Kinase B | Akt |
| Protein Tyrosine Phosphatase Receptor | PTP-κ |
| Reactive Oxygen Species | ROS |
| Rhodamine-123 Second Mitochondrial Derived Activator of Caspase | Rh-123 Smac |
| Superoxide Dismutase | SOD |
| Tissue inhibitor of metalloproteinase | TIMP-1 |
| Thiobarbituric Acid Reactive Substances | TBARS |
| Vascular Endothelial Growth Factor | VEGF |
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ls, R.; Nja, S. Anticancer Properties of Phenolic Acids in Colon Cancer – A Review. J. Nutr. Food Sci. 2016, 06, 10–4172. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C.J.N. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA) A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Rahman, M.J.; Costa de Camargo, A.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted camelina and sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Chander, M. Anticancer Efficacy of Some Plant Phenolics - A Recent Scenario. IJCMAS 2018, 7, 1746–1768. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, S.; Yang, S. Vanillic Acid Suppresses HIF-1alpha Expression via Inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK Pathways in Human Colon Cancer HCT116 Cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef] [PubMed]
- Taner, G.; Ozkan Vardar, D.; Aydin, S.; Aytac, Z.; Basaran, A.; Basaran, N. Use of in vitro assays to assess the potential cytotoxic, genotoxic and antigenotoxic effects of vanillic and cinnamic acid. Drug Chem. Toxicol. 2017, 40, 183–190. [Google Scholar] [CrossRef]
- Velli, S.K.; Sundaram, J.; Murugan, M.; Balaraman, G.; Thiruvengadam, D. Protective effect of vanillic acid against benzo(a)pyrene induced lung cancer in Swiss albino mice. J. Biochem. Mol. Toxicol. 2019, 33, e22382. [Google Scholar] [CrossRef]
- Anbalagan, V.; Raju, K.; Shanmugam, M. Assessment of Lipid Peroxidation and Antioxidant Status in Vanillic Acid Treated 7,12-Dimethylbenz[a]anthracene Induced Hamster Buccal Pouch Carcinogenesis. J. Clin. Diagn Res. 2017, 11, BF01¨CBF04. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Skala, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Skorski, T.; Bialas, A.J.; Sakowicz, T.; Kowalczyk, T.; Radek, M.; et al. Transformed Root Extract of Leonurus sibiricus Induces Apoptosis through Intrinsic and Extrinsic Pathways in Various Grades of Human Glioma Cells. Pathol. Oncol. Res. 2017, 23, 679–687. [Google Scholar] [CrossRef]
- Bhavani, P.; Subramanian, P.; Kanimozhi, S. Preventive Efficacy of Vanillic Acid on Regulation of Redox Homeostasis, Matrix Metalloproteinases and Cyclin D1 in Rats Bearing Endometrial Carcinoma. Indian J. Clin. Biochem. 2017, 32, 429–436. [Google Scholar] [CrossRef]
- Ozpinar, A.; Elmaci, İ.A.; Altinoz, M. Gentisic Acid, a Quinonoid Aspirin Metabolite in Cancer Prevention and Treatment. New Horizons in Management of Brain Tumors and Systemic Cancers. J. Cancer Res. Oncobiol. 2018, 1, 109. [Google Scholar]
- Cavalcante, F.M.L.; Almeida, I.V.; Dusman, E.; Mantovani, M.S.; Vicentini, V.E.P. Cytotoxicity, mutagenicity, and antimutagenicity of the gentisic acid on HTC cells. Drug Chem. Toxicol. 2018, 41, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Demirel Sezer, E.; Oktay, L.M.; Karadadas, E.; Memmedov, H.; Selvi Gunel, N.; Sozmen, E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, Through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Altinoz, M.A.; Elmaci, I.; Cengiz, S.; Emekli-Alturfan, E.; Ozpinar, A. From epidemiology to treatment: Aspirin’s prevention of brain and breast-cancer and cardioprotection may associate with its metabolite gentisic acid. Chem. Biol. Interact. 2018, 291, 29–39. [Google Scholar] [CrossRef]
- Yip, E.C.; Chan, A.S.; Pang, H.; Tam, Y.K.; Wong, Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006, 22, 293–302. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic Acid and its potential roles as complementary medicine. Evid. Based Complement. Alternat. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, Z.; Wang, Y.; Lei, J.; Yu, J. Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phytother. Res. 2018, 32, 2256–2263. [Google Scholar] [CrossRef]
- Quinn, L.; Gray, S.G.; Meaney, S.; Finn, S.; Kenny, O.; Hayes, M. Sinapinic and protocatechuic acids found in rapeseed: Isolation, characterisation and potential benefits for human health as functional food ingredients. Irish J. Agric. Food Res. 2017, 56, 104–119. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Huang, J.J.; Cheung, P.C.K. Mechanistic Study of the In Vitro and In Vivo Inhibitory Effects of Protocatechuic Acid and Syringic Acid on VEGF-Induced Angiogenesis. J. Agric. Food Chem. 2018, 66, 6742–6751. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Chen, J.H.; Chou, F.P.; Wang, C.J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-kappaB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, N.; Park, J.Y.; Lee, S.; Cho, E.-J.; Lee, S.; Kang, K.S.; Hwang, G.S.; Kim, S.-N.; Kim, H.Y.; Shibamoto, T. Protective effects of protocatechuic acid against cisplatin-induced renal damage in rats. J. Funct. Foods 2015, 19, 20–27. [Google Scholar] [CrossRef]
- Saifullah, B.; Buskaran, K.; Shaikh, R.B.; Barahuie, F.; Fakurazi, S.; Mohd Moklas, M.A.; Hussein, M.Z. Graphene Oxide(-)PEG(-)Protocatechuic Acid Nanocomposite Formulation with Improved Anticancer Properties. Nanomaterials 2018, 8, 820. [Google Scholar] [CrossRef]
- Gani, S.A.; Muhammad, S.A.; Kura, A.U.; Barahuie, F.; Hussein, M.Z.; Fakurazi, S. Effect of protocatechuic acid-layered double hydroxide nanoparticles on diethylnitrosamine/phenobarbital-induced hepatocellular carcinoma in mice. PLoS ONE 2019, 14, e0217009. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, C.W.; Kim, J.H.; Lee, J.C.; An, W.G. Extract of Rhus verniciflua Stokes Induces p53-Mediated Apoptosis in MCF-7 Breast Cancer Cells. Evid. Based Complement. Alternat. Med. 2019, 2019, 9407340. [Google Scholar] [CrossRef]
- He, Z.; Chen, A.Y.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1alpha/VEGF signaling pathway in ovarian cancer cells. Oncol. Rep. 2016, 35, 291–297. [Google Scholar] [CrossRef]
- Paolini, A.; Curti, V.; Pasi, F.; Mazzini, G.; Nano, R.; Capelli, E. Gallic acid exerts a protective or an anti-proliferative effect on glioma T98G cells via dose-dependent epigenetic regulation mediated by miRNAs. Int. J. Oncol. 2015, 46, 1491–1497. [Google Scholar] [CrossRef]
- Heidarian, E.; Keloushadi, M.; Ghatreh-Samani, K.; Valipour, P. The reduction of IL-6 gene expression, pAKT, pERK1/2, pSTAT3 signaling pathways and invasion activity by gallic acid in prostate cancer PC3 cells. Biomed. Pharmacother. 2016, 84, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Demiroglu-Zergeroglu, A.; Candemir, G.; Turhanlar, E.; Sagir, F.; Ayvali, N. EGFR-dependent signalling reduced and p38 dependent apoptosis required by Gallic acid in Malignant Mesothelioma cells. Biomed. Pharmacother. 2016, 84, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.S.; Chou, C.T.; Liao, W.C.; Shieh, P.; Kuo, D.H.; Kuo, C.C.; Jan, C.R.; Liang, W.Z. The effect of gallic acid on cytotoxicity, Ca2+ homeostasis and ROS production in DBTRG-05MG human glioblastoma cells and CTX TNA2 rat astrocytes. Chem. Biol. Interact. 2016, 252, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. Gallic acid induces HeLa cell death via increasing GSH depletion rather than ROS levels. Oncol. Rep. 2017, 37, 1277–1283. [Google Scholar] [CrossRef]
- Lee, H.L.; Lin, C.S.; Kao, S.H.; Chou, M.C. Gallic acid induces G1 phase arrest and apoptosis of triple-negative breast cancer cell MDA-MB-231 via p38 mitogen-activated protein kinase/p21/p27 axis. Anticancer Drugs 2017, 28, 1150–1156. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chiu, Y.M.; Ho, T.Y.; Hsieh, C.T.; Shieh, D.C.; Lee, Y.J.; Tsay, G.J.; Wu, Y.Y. Gallic Acid Induces Apoptosis in Human Gastric Adenocarcinoma Cells. Anticancer Res. 2018, 38, 2057–2067. [Google Scholar]
- Weng, Y.-P.; Hung, P.-F.; Ku, W.-Y.; Chang, C.-Y.; Wu, B.-H.; Wu, M.-H.; Yao, J.-Y.; Yang, J.-R.; Lee, C.-H. The inhibitory activity of gallic acid against DNA methylation: Application of gallic acid on epigenetic therapy of human cancers. Oncotarget 2017, 9, 361–374. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kee, H.J.; Jin, L.; Ryu, Y.; Sun, S.; Kim, G.R.; Jeong, M.H. Inhibition of class IIa histone deacetylase activity by gallic acid, sulforaphane, TMP269, and panobinostat. Biomed. Pharmacother. 2018, 101, 145–154. [Google Scholar] [CrossRef]
- Pang, J.S.; Yen, J.H.; Wu, H.T.; Huang, S.T. Gallic Acid Inhibited Matrix Invasion and AP-1/ETS-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 2017, 18, 1354. [Google Scholar] [CrossRef]
- Ho, H.H.; Chang, C.S.; Ho, W.C.; Liao, S.Y.; Lin, W.L.; Wang, C.J. Gallic acid inhibits gastric cancer cells metastasis and invasive growth via increased expression of RhoB, downregulation of AKT/small GTPase signals and inhibition of NF-kappaB activity. Toxicol. Appl. Pharmacol. 2013, 266, 76–85. [Google Scholar] [CrossRef]
- Aikebaier, M.; Amier, A.; Hureshitanmu, K.; Xuejun, L. VDAC1 Mediated Anticancer Activity of Gallic Acid in Human Lung Adenocarcinoma A549 Cells. Anti-Cancer Agents Med. Chem. 2018, 18, 255–262. [Google Scholar]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in nonsmall cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [PubMed]
- Aborehab, N.M.; Osama, N. Effect of Gallic acid in potentiating chemotherapeutic effect of Paclitaxel in HeLa cervical cancer cells. Cancer Cell Int. 2019, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carranza, J.N.; Diaz, J.F.; Redondo-Horcajo, M.; Barasoain, I.; Alvarez, L.; Lastres, P.; Romero-Estrada, A.; Aller, P.; Gonzalez-Maya, L. Gallic acid sensitizes paclitaxel-resistant human ovarian carcinoma cells through an increase in reactive oxygen species and subsequent downregulation of ERK activation. Oncol. Rep. 2018, 39, 3007–3014. [Google Scholar] [PubMed]
- Wang, R.; Ma, L.; Weng, D.; Yao, J.; Liu, X.; Jin, F. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep. 2016, 35, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Rosman, R.; Saifullah, B.; Maniam, S.; Dorniani, D.; Hussein, M.Z.; Fakurazi, S. Improved Anticancer Effect of Magnetite Nanocomposite Formulation of GALLIC Acid (Fe(3)O(4)-PEG-GA) Against Lung, Breast and Colon Cancer Cells. Nanomaterials 2018, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.H.; Galal, A.F.; Shalby, A.B.; Abd-Rabou, A.A.; Mehaya, F.M. Improving Anti-Cancer Potentiality and Bioavailability of Gallic Acid by Designing Polymeric Nanocomposite Formulation. Asian Pac. J. Cancer Prev. 2018, 19, 3137–3146. [Google Scholar] [CrossRef]
- Ha, S.J.; Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Song, K.M.; Chang, P.S.; Jeong, C.H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Skala, E.; Toma, M.; Wielanek, M.; Pytel, D.; Wieczfinska, J.; Szemraj, J.; Sliwinski, T. Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells. Cytotechnology 2019, 71, 165–180. [Google Scholar]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: The suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef]
- Gheena, S.; Ezhilarasan, D. Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019, 38, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Velu, P.; Vinothkumar, V.; Babukumar, S.; Ramachandhiran, D. Chemopreventive effect of syringic acid on 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesis. Toxicol. Mech. Methods 2017, 27, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Periyannan, V.; Veerasamy, V. Syringic acid may attenuate the oral mucosal carcinogenesis via improving cell surface glycoconjugation and modifying cytokeratin expression. Toxicol. Rep. 2018, 5, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Abaza, M.S.; Al-Attiyah, R.; Bhardwaj, R.; Abbadi, G.; Koyippally, M.; Afzal, M. Syringic acid from Tamarix aucheriana possesses antimitogenic and chemo-sensitizing activities in human colorectal cancer cells. Pharm. Biol. 2013, 51, 1110–1124. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Mauricio, A.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation--A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar]
- Murad, L.D.; Soares Nda, C.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Nasr Bouzaiene, N.; Kilani Jaziri, S.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Manica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; de Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; Chen, L.; Yang, Y.; Cao, S.; Ye, Y.; Wang, X.; Mu, J.; Li, Z.; Li, L. Blockage of TGFbeta-SMAD2 by demethylation-activated miR-148a is involved in caffeic acid-induced inhibition of cancer stem cell-like properties in vitro and in vivo. FEBS Open Bio 2015, 5, 466–475. [Google Scholar] [CrossRef]
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzebska-Stojko, Z.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules 2017, 22, 1554. [Google Scholar] [CrossRef]
- Marin, E.H.; Paek, H.; Li, M.; Ban, Y.; Karaga, M.K.; Shashidharamurthy, R.; Wang, X. Caffeic acid phenethyl ester exerts apoptotic and oxidative stress on human multiple myeloma cells. Invest. New Drugs 2019, 37, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Han, N.Z.; Liu, S.S. Caffeic acid phenethyl ester inhibits the progression of ovarian cancer by regulating NF-κB signaling. Biomed. Pharmacother. 2018, 99, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yang, S.W.; Chang, K.P.; Feng, T.H.; Chang, K.S.; Tsui, K.H.; Shin, Y.S.; Chen, C.C.; Chao, M.; Juang, H.H. Caffeic Acid Phenethyl Ester Induces N-myc Downstream Regulated Gene 1 to Inhibit Cell Proliferation and Invasion of Human Nasopharyngeal Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1397. [Google Scholar] [CrossRef] [PubMed]
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition. Integr. Cancer Ther. 2018, 17, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.E.; Abu, N.; Yeap, S.K.; Lim, K.L.; Romli, M.F.; Sharifuddin, S.A.; Long, K.; Alitheen, N.B. Apoptosis and metastasis inhibitory potential of pineapple vinegar against mouse mammary gland cells in vitro and in vivo. Nutr. Metab. 2019, 16, 49. [Google Scholar] [CrossRef]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicano, T.M.; Saturnino, C.; Malzert-Freon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. 2019, 10, 4280–4290. [Google Scholar] [CrossRef]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef]
- Sarwar, T.; Zafaryab, M.; Husain, M.A.; Ishqi, H.M.; Rehman, S.U.; Rizvi, M.M.; Tabish, M. Redox cycling of endogenous copper by ferulic acid leads to cellular DNA breakage and consequent cell death: A putative cancer chemotherapy mechanism. Toxicol. Appl. Pharmacol. 2015, 289, 251–261. [Google Scholar] [CrossRef]
- Eroglu, C.; Secme, M.; Bagci, G.; Dodurga, Y. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumour Biol. 2015, 36, 9437–9446. [Google Scholar] [CrossRef]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Yang, G.W.; Jiang, J.S.; Lu, W.Q. Ferulic Acid Exerts Anti-Angiogenic and Anti-Tumor Activity by Targeting Fibroblast Growth Factor Receptor 1-Mediated Angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef] [PubMed]
- Fahrioglu, U.; Dodurga, Y.; Elmas, L.; Secme, M. Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene 2016, 576, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wu, Q.; Yang, S.H. Ferulic acid promoting apoptosis in human osteosarcoma cell lines. Pak. J. Med. Sci. 2017, 33, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gong, X.; Jiang, R.; Li, H.; Du, W.; Kuang, G. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. American J. Trans. Res. 2016, 8, 968. [Google Scholar]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dodurga, Y.; Eroglu, C.; Secme, M.; Elmas, L.; Avci, C.B.; Satiroglu-Tufan, N.L. Anti-proliferative and anti-invasive effects of ferulic acid in TT medullary thyroid cancer cells interacting with URG4/URGCP. Tumour Biol. 2016, 37, 1933–1940. [Google Scholar] [CrossRef]
- Yue, S.J.; Zhang, P.X.; Zhu, Y.; Li, N.G.; Chen, Y.Y.; Li, J.J.; Zhang, S.; Jin, R.Y.; Yan, H.; Shi, X.Q.; et al. A Ferulic Acid Derivative FXS-3 Inhibits Proliferation and Metastasis of Human Lung Cancer A549 Cells via Positive JNK Signaling Pathway and Negative ERK/p38, AKT/mTOR and MEK/ERK Signaling Pathways. Molecules 2019, 24, 2165. [Google Scholar] [CrossRef]
- Sudhagar, S.; Sathya, S.; Anuradha, R.; Gokulapriya, G.; Geetharani, Y.; Lakshmi, B.S. Inhibition of epidermal growth factor receptor by ferulic acid and 4-vinylguaiacol in human breast cancer cells. Biotechnol. Lett. 2018, 40, 257–262. [Google Scholar] [CrossRef]
- Muthusamy, G.; Gunaseelan, S.; Prasad, N.R. Ferulic acid reverses P-glycoprotein-mediated multidrug resistance via inhibition of PI3K/Akt/NF-kappaB signaling pathway. J. Nutr. Biochem. 2019, 63, 62–71. [Google Scholar] [CrossRef]
- Ezhuthupurakkal, P.B.; Ariraman, S.; Arumugam, S.; Subramaniyan, N.; Muthuvel, S.K.; Kumpati, P.; Rajamani, B.; Chinnasamy, T. Anticancer potential of ZnO nanoparticle-ferulic acid conjugate on Huh-7 and HepG2 cells and diethyl nitrosamine induced hepatocellular cancer on Wistar albino rat. Nanomedicine 2018, 14, 415–428. [Google Scholar] [CrossRef]
- Zheng, Y.; You, X.; Chen, L.; Huang, J.; Wang, L.; Wu, J.; Guan, S. Biotherapeutic Nanoparticles of Poly(Ferulic Acid) Delivering Doxorubicin for Cancer Therapy. J. Biomed. Nanotechnol. 2019, 15, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, K.; Demir, S.; Turan, I.; Mentese, A.; Orem, A.; Sonmez, M.; Aliyazicioglu, Y. Rosa canina Extract has Antiproliferative and Proapoptotic Effects on Human Lung and Prostate Cancer Cells. Nutr. Cancer 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boz, H.J.I. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. IJFST 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chem. Biol. Interact. 2018, 291, 16–28. [Google Scholar] [CrossRef]
- Sharma, S.H.; Chellappan, D.R.; Chinnaswamy, P.; Nagarajan, S. Protective effect of p-coumaric acid against 1,2 dimethylhydrazine induced colonic preneoplastic lesions in experimental rats. Biomed. Pharmacother. 2017, 94, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Supplementation of p-coumaric acid exhibits chemopreventive effect via induction of Nrf2 in a short-term preclinical model of colon cancer. Eur. J. Cancer Prev. 2019, 28, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shan, S.; Li, H.; Shi, J.; Zhang, X.; Li, Z. Reversal Effects of Bound Polyphenol from Foxtail Millet Bran on Multidrug Resistance in Human HCT-8/Fu Colorectal Cancer Cell. J. Agric. Food Chem. 2018, 66, 5190–5199. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Aliyazicioglu, R.; Kilinc, K.; Ozer Yaman, S.; Akbulut Cakiroglu, K.; Kanbolat, S.; Ayazoglu Demir, E.; Mentese, A.; Aliyazicioglu, Y.; et al. Dimethyl Sulfoxide Extract of Dianthus carmelitarum Induces S Phase Arrest and Apoptosis in Human Colon Cancer Cells. Nutr. Cancer 2019, 71, 1181–1188. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Zaki, M.A.; Rateb, M.E.; Mohammed, T.A.; Amin, E.; Abdelmohsen, U.R. Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum. Mar. Drugs 2018, 16, 253. [Google Scholar] [CrossRef]
- Eroglu, C.; Avci, E.; Vural, H.; Kurar, E. Anticancer mechanism of Sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene 2018, 671, 127–134. [Google Scholar] [CrossRef]
- Saenglee, S.; Jogloy, S.; Patanothai, A.; Leid, M.; Senawong, T. Cytotoxic effects of peanut phenolics possessing histone deacetylase inhibitory activity in breast and cervical cancer cell lines. Pharmacol. Rep. 2016, 68, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Gierach, G.L.; Freedman, N.D.; Andaya, A.; Hollenbeck, A.R.; Park, Y.; Schatzkin, A.; Brinton, L.A. Coffee intake and breast cancer risk in the NIH-AARP diet and health study cohort. Int. J. Cancer 2012, 131, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Stephens, T.; Hino, P.D.; Law, R.M.; Yatskayer, M.; Foltis, P.; Pillai, S.; Pinnell, S.R. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J. Cosmet. Dermatol. 2008, 7, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.P.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Morton, L.W.; Puddey, I.B.; Beilin, L.J.; Croft, K.D. Gallic Acid Metabolites Are Markers of Black Tea Intake in Humans. J. Agric. Food Chem. 2000, 48, 2276–2280. [Google Scholar] [CrossRef]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Busselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef]
- Jasek, K.; Kubatka, P.; Samec, M.; Liskova, A.; Smejkal, K.; Vybohova, D.; Bugos, O.; Biskupska-Bodova, K.; Bielik, T.; Zubor, P.; et al. DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions. Biomolecules 2019, 9, 289. [Google Scholar] [CrossRef]
- Moreno-Jimenez, M.R.; Lopez-Barraza, R.; Cervantes-Cardoza, V.; Perez-Ramirez, I.F.; Reyna-Rojas, J.A.; Gallegos-Infante, J.A.; Estrella, I.; Rojas-Contreras, J.A.; Gonzalez-Laredo, R.F.; Rocha-Guzman, N.E. Mechanisms associated to apoptosis of cancer cells by phenolic extracts from two canned common beans varieties (Phaseolus vulgaris L.). J. Food Biochem. 2019, 43, e12680. [Google Scholar] [CrossRef]
- Eskra, J.N.; Dodge, A.; Schlicht, M.J.; Bosland, M.C. Effects of Black Raspberries and Their Constituents on Rat Prostate Carcinogenesis and Human Prostate Cancer Cell Growth In Vitro. Nutr. Cancer 2019, 1–14. [Google Scholar] [CrossRef]
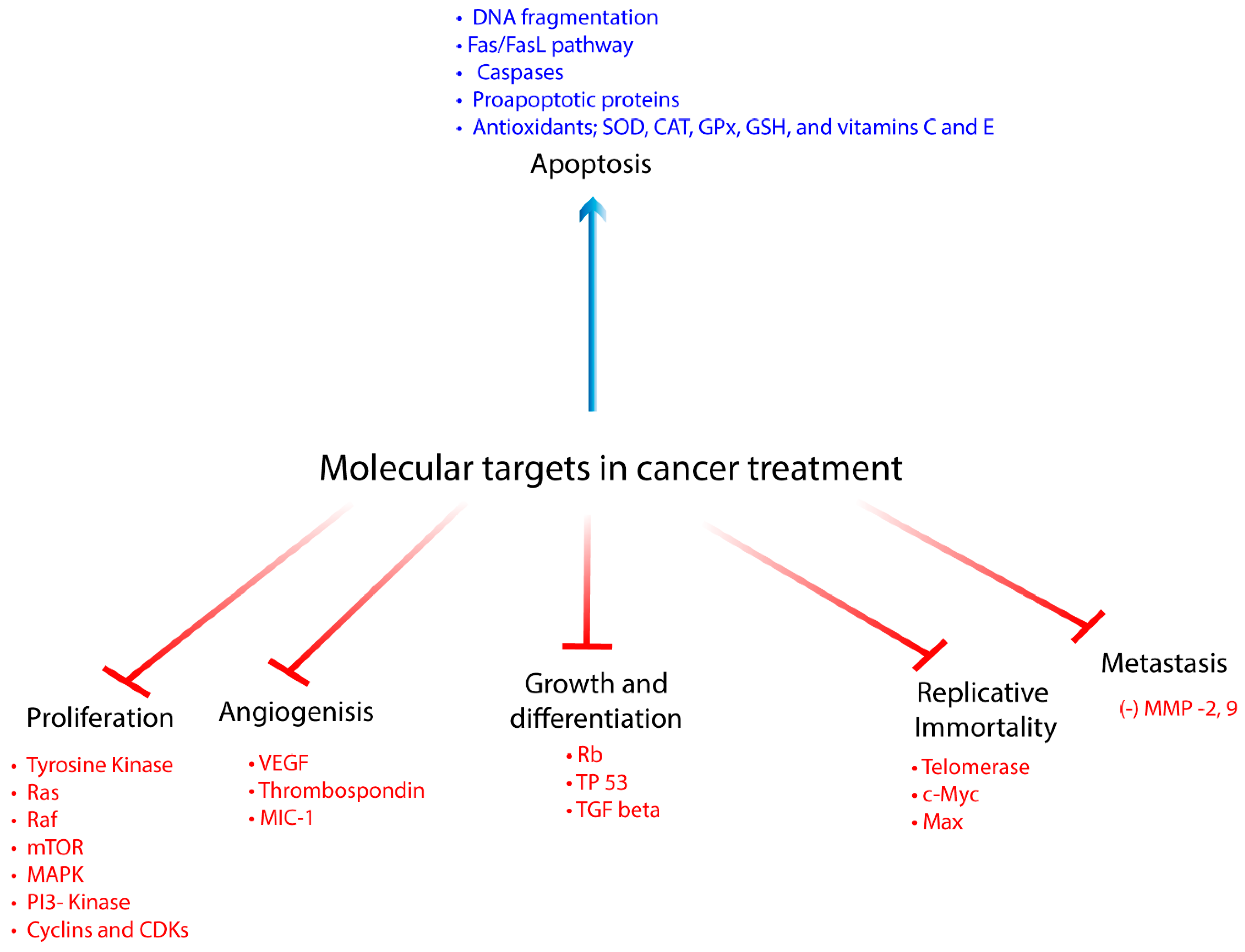

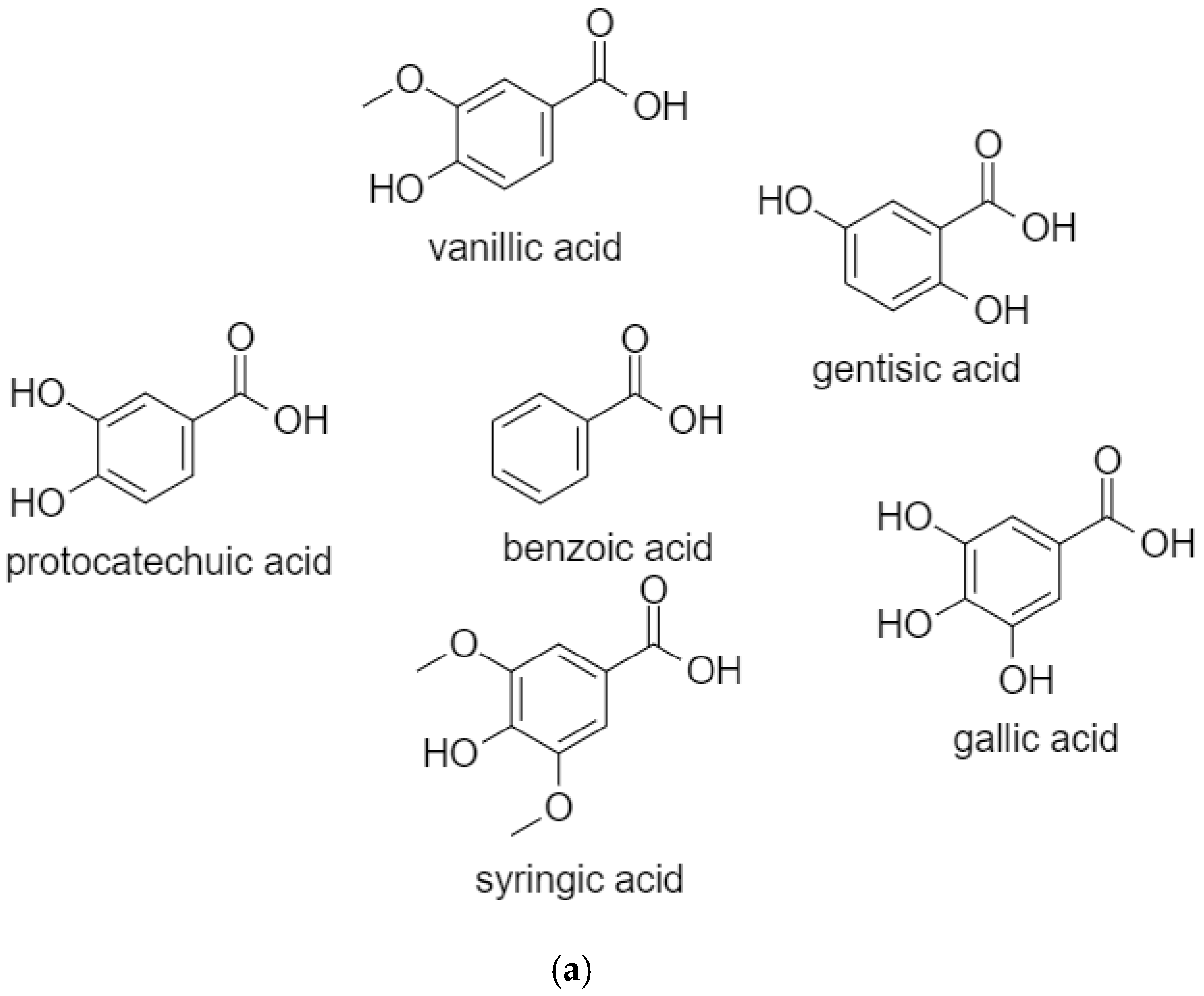

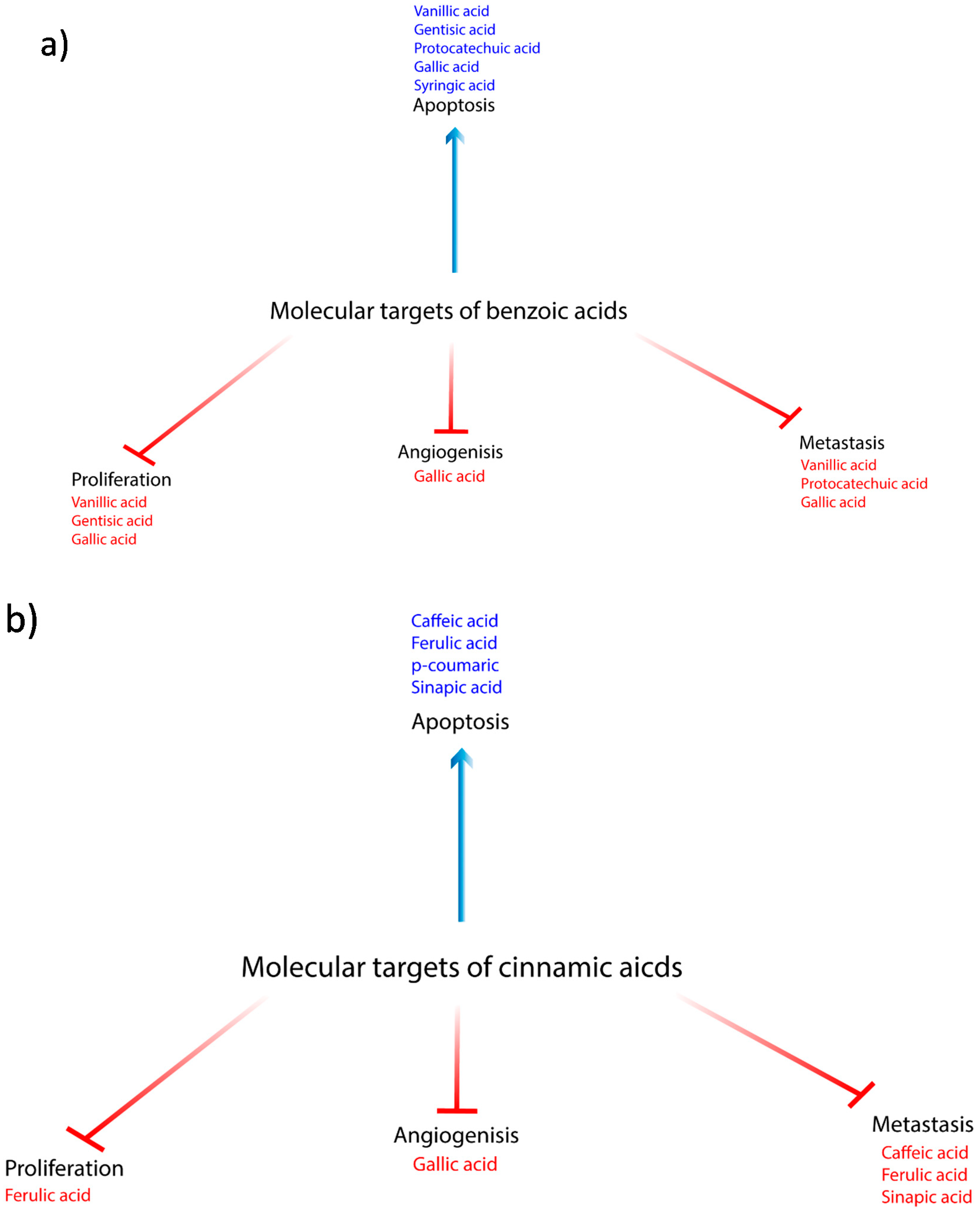
| Compound | Source | Anticancer Effect | Cancer Type | Type of Study | Mechanism | References |
|---|---|---|---|---|---|---|
| Vanillic Acid | Angelica sinensis and green tea | (-) growth and proliferation | Colon | in vitro | (-) mTOR/p70S6K/4E-BP1 | [8] |
| Vanillic Acid | (+) apoptosis and antioxidant | Endometrial rat model | in vivo | (+) SOD, CAT, GPx, GSH, and vitamins C and E, (-) TBARS, LOOH | [13] | |
| Vanillic Acid | (-) metastasis | Endometrial rat model | in vivo | (-) Cyclin D1, MMP -2, -9 | [13] | |
| Gentisic acid | citric fruits, grapes, artichoke, sesame, and olives | (+) apoptosis and antioxidant | Glioblastoma | in vitro | direct free radical scavenging activity indirect agonist of NRF2 | [14] |
| Protocatechuic acid | plum, star anise, melissa, rosemary, cinnamon, sudan mallow, St. John’s wort, berries, cauliflower, and lentils | (+) apoptosis and antioxidant | Leukemia Gastric | in vitro | (+) ROS, DNA fragmentation, Bax, RB phosphorylation, Fas/FasL pathway, (-) Bcl-2, loss of mitochondrial membrane potential | [19] |
| Protocatechuic acid | (-) metastasis | Gastric | in vitro | (-) MMP-2 | [21] | |
| Gallic acid | chestnut green chicory, blackberry, raspberry, walnuts, chocolate, wine, green tea, and vinegar | (-) proliferation | Mesothelioma | in vitro | (-) VEGF and EGFR | [32] |
| Gallic acid | (+) apoptosis and antioxidant | Cervical Prostate Colon GBM | in vitro | (+) ROS & GSH (-) p38 MAPK Changes in calcium ion homeostasis | [27,30,34] | |
| Gallic acid | (-) metastasis | Prostate Nasopharyngeal | in vitro | (-) MMP-1, -2, -9 | [27,31,40] | |
| Syringic acid | dates, olives, pumpkin, grapes, spices, acai, red wine, palm and honey | (+) apoptosis and antioxidant | Colon | in vitro | extrinsic, intrinsic, and mitochondrial pathways; (+) p53, Bax, Bak, Bad, Bid, Bim, Apaf1, AIF Smac, caspases-2, 3, 6, 7, 8 and 9, endoplasmic stress markers. cytochrome c, ROS (-) in the mitochondrial membrane potential, Bcl-2 | [50,54] |
| Syringic acid | (+) apoptosis and antioxidant | Hamster buccal pouch | in vivo | (-) TBARS, LOOH, (+) enzymatic (SOD, CAT and Gpx) and non-enzymatic (vitamin E and GSH) antioxidants | [53] | |
| Syringic acid | cell cycle | Colon | in vitro | arrest at S-phase, (-) cell cycle proteins CDK4, 6 and cyclins B, C, E1, H and (+) p19, p21Cip1/Waf1 and p27kip1 | [54] | |
| Caffeic acid | wheat, quinoa, triticale, barley, corn, oat, rye, rice, thyme, oregano millet, sage, and sorghum | antioxidant | Colon | in vitro | iron- chelating property (-) Fenton-induced oxidative damage and preventing the formation of free hydroxyl radicals | [55] |
| Caffeic acid | (-) metastasis | Lung Colon | in vitro | (-) cell adhesion | [3,57] | |
| Ferulic acid | wheat, buckwheat, rice, corn, oats, rye, orange, corn, herbs, spices, sorghum, millet, quinoa, and barley | (-) metastasis | Endothelial Breast | in vitro | (-) FGF, cell adhesion, MMP -2, -9 | [71,75] |
| Ferulic acid | Cell cycle arrest | Lung Colon Osteosarcoma | in vitro | G0/G1 arrest (-) CDK 2, 4 and 6, PI3K/Akt, Cyclins D1 and E | [3,57,73,74,77] | |
| Ferulic acid | (-) proliferation | Breast | in vitro | (-) EGF | [78] | |
| Ferulic acid | (+) apoptosis and antioxidant | Thyroid Lung Osteosarcoma | in vitro | (+) Bax, PARP, PUMA, NOXA, Bid, p53, PTEN, caspases-3 and -9, (-) CDK 4/6, CD 1, Bcl-2 | [73,74,76,77] | |
| p-Coumaric | wheat, barley oat, corn, rye, quinoa, rice, millet, honey sorghum barley grains and buckwheat | (+) apoptosis and antioxidant | Lung Prostate Colon | in vitro | (+) the ROS levels, Bax/Bcl-2 ratio, loss of mitochondrial membrane potential, Rh-123(-) MRP1, P-gp, and BCRP | [82,85,86,87] |
| p-Coumaric | anti-inflammatory | Colon | in vitro | (-) IL-6, COX-2, TNF-α, PGE2, p-p65 and p-IκBα | [84] | |
| Sinapic acid | cereal grains, rye, wheat triticale, barley, oat, rye, rice, rapeseed, kale, white cabbage, turnip, broccoli, citrus fruits, sage and thyme | (+) apoptosis and antioxidant | Prostate | in vitro | (+) activities of enzymatic and non- enzymatic antioxidants; SOD, CAT, and GSH (+) Bax, caspases -3, -7, -8, FAS, TIMP-1, cytochrome c | [21,90] |
| Sinapic acid | (-) metastasis | Prostate | in vitro | (-) MMP-2, -9, CDH 1, 2 | [90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. https://doi.org/10.3390/biom10020221
Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules. 2020; 10(2):221. https://doi.org/10.3390/biom10020221
Chicago/Turabian StyleAbotaleb, Mariam, Alena Liskova, Peter Kubatka, and Dietrich Büsselberg. 2020. "Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer" Biomolecules 10, no. 2: 221. https://doi.org/10.3390/biom10020221
APA StyleAbotaleb, M., Liskova, A., Kubatka, P., & Büsselberg, D. (2020). Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules, 10(2), 221. https://doi.org/10.3390/biom10020221







