Phytomediated Photo-Induced Green Synthesis of Silver Nanoparticles Using Matricaria chamomilla L. and Its Catalytic Activity against Rhodamine B
Abstract
:1. Introduction
2. Experimental
2.1. Material and Methods
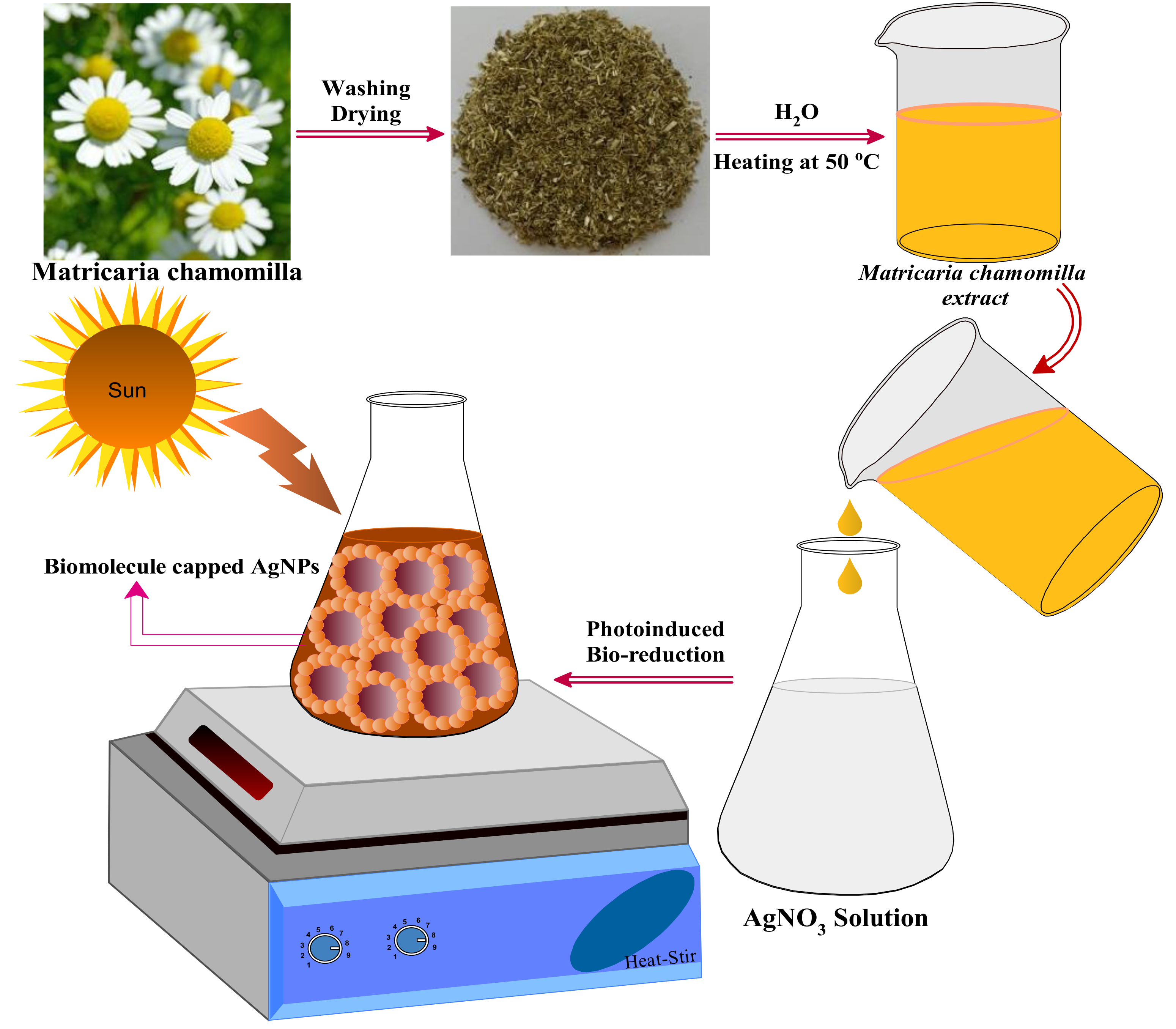
2.1.1. Preparation of Plant Extract
2.1.2. Biomolecule-Assisted Synthesis of AgNPs
2.2. Characterization of AgNPs
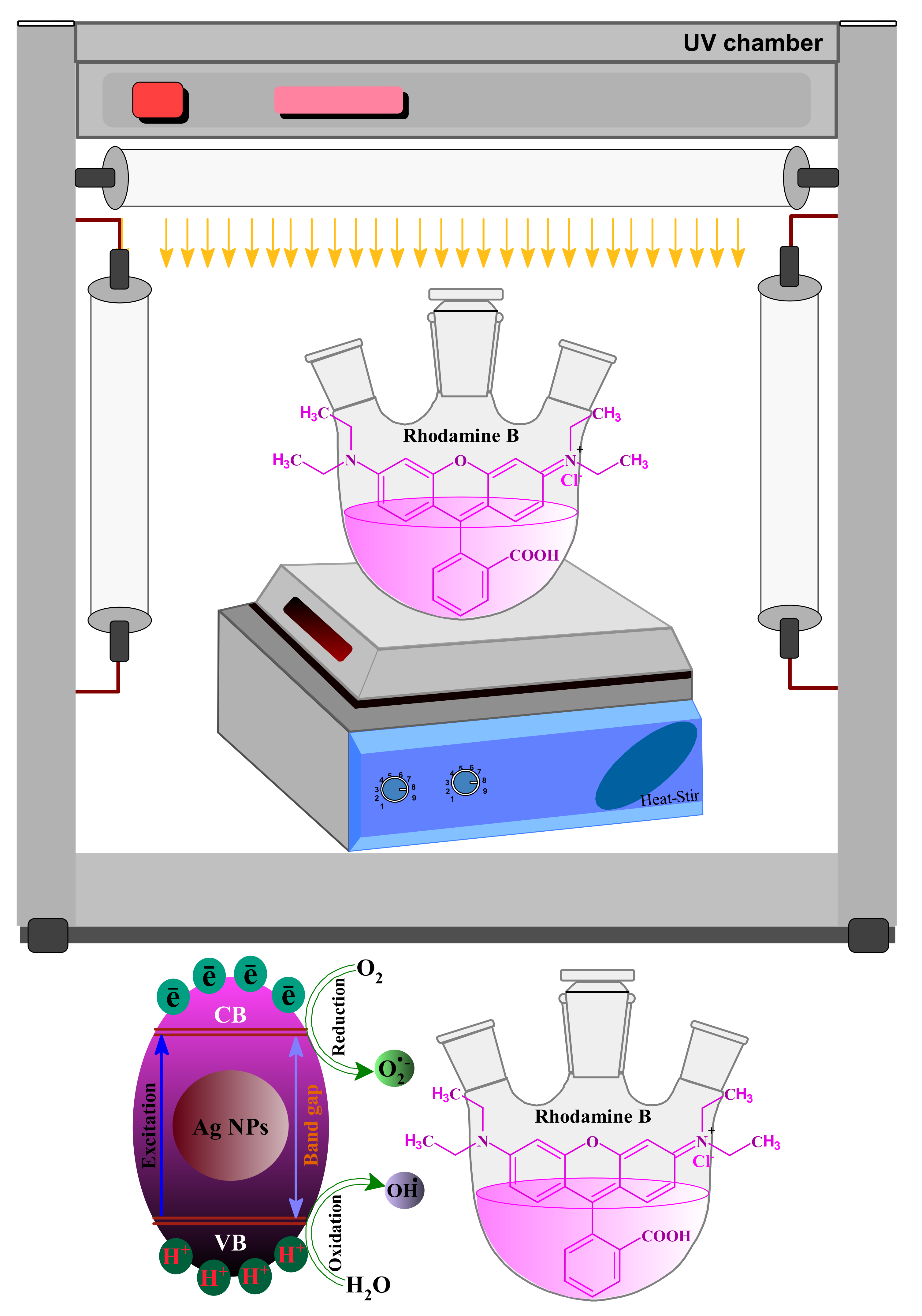
2.3. Photocatalytic Activity
3. Results and Discussion
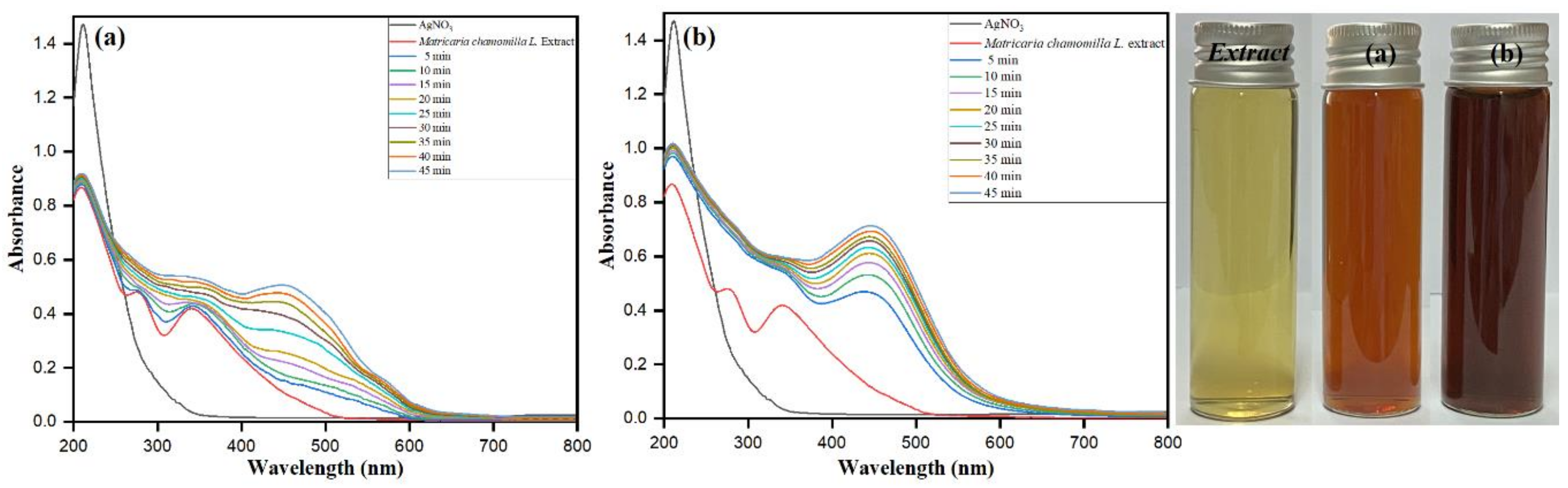
3.1. UV–Visible Spectroscopy
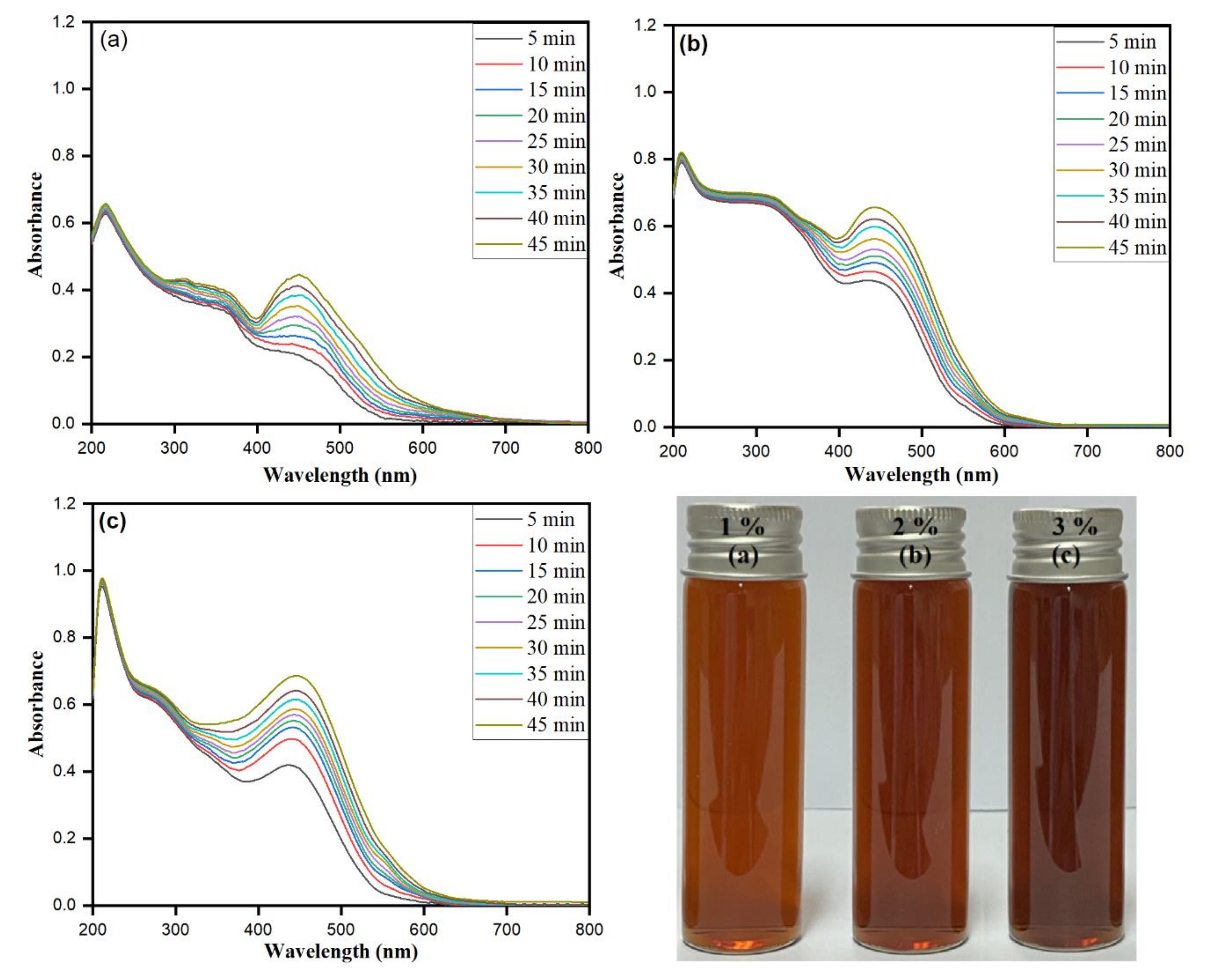
3.2. AgNO3 Concentration Optimization
3.3. Matricaria chamomilla L. Extract Inoculum Dose
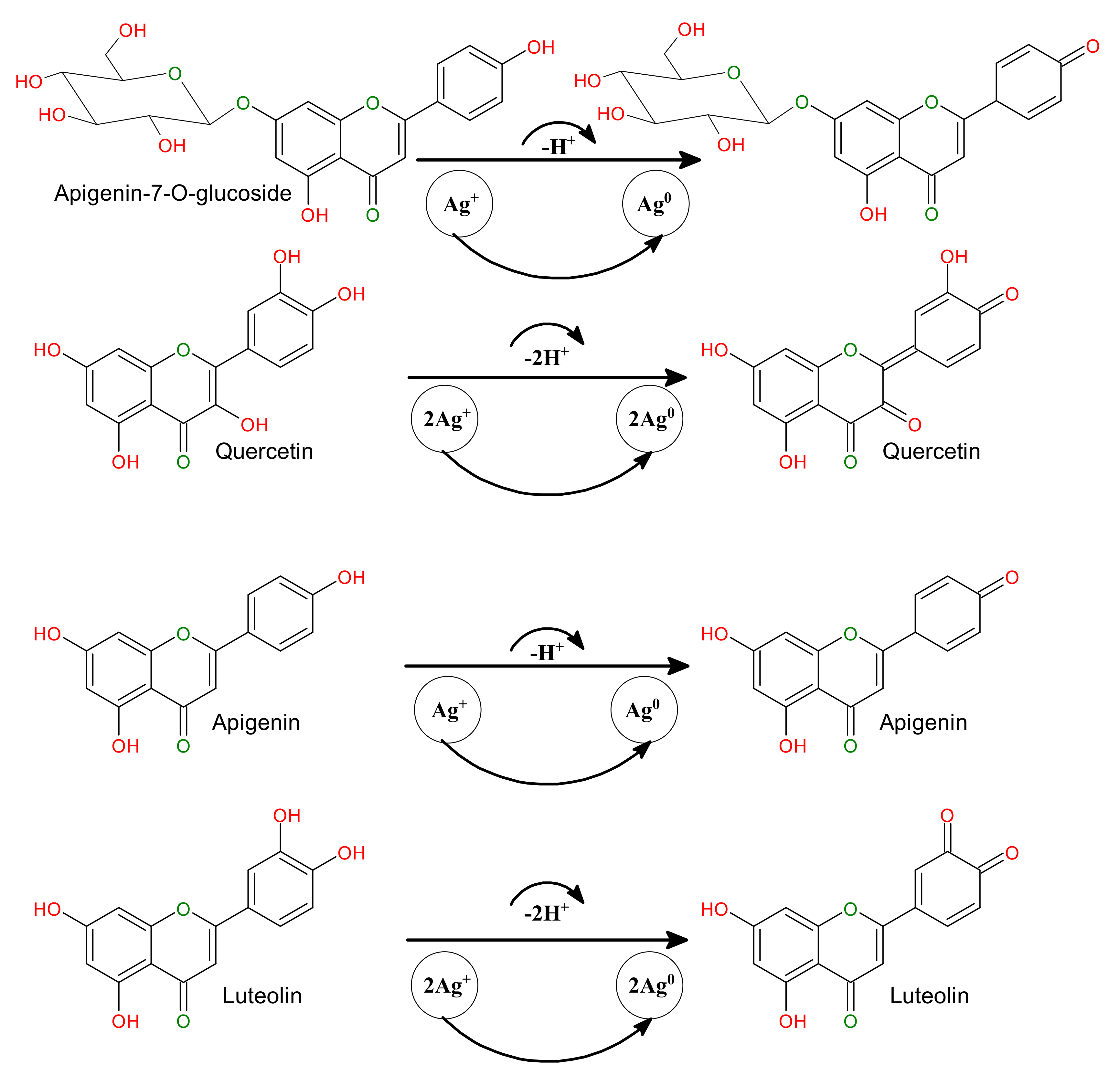
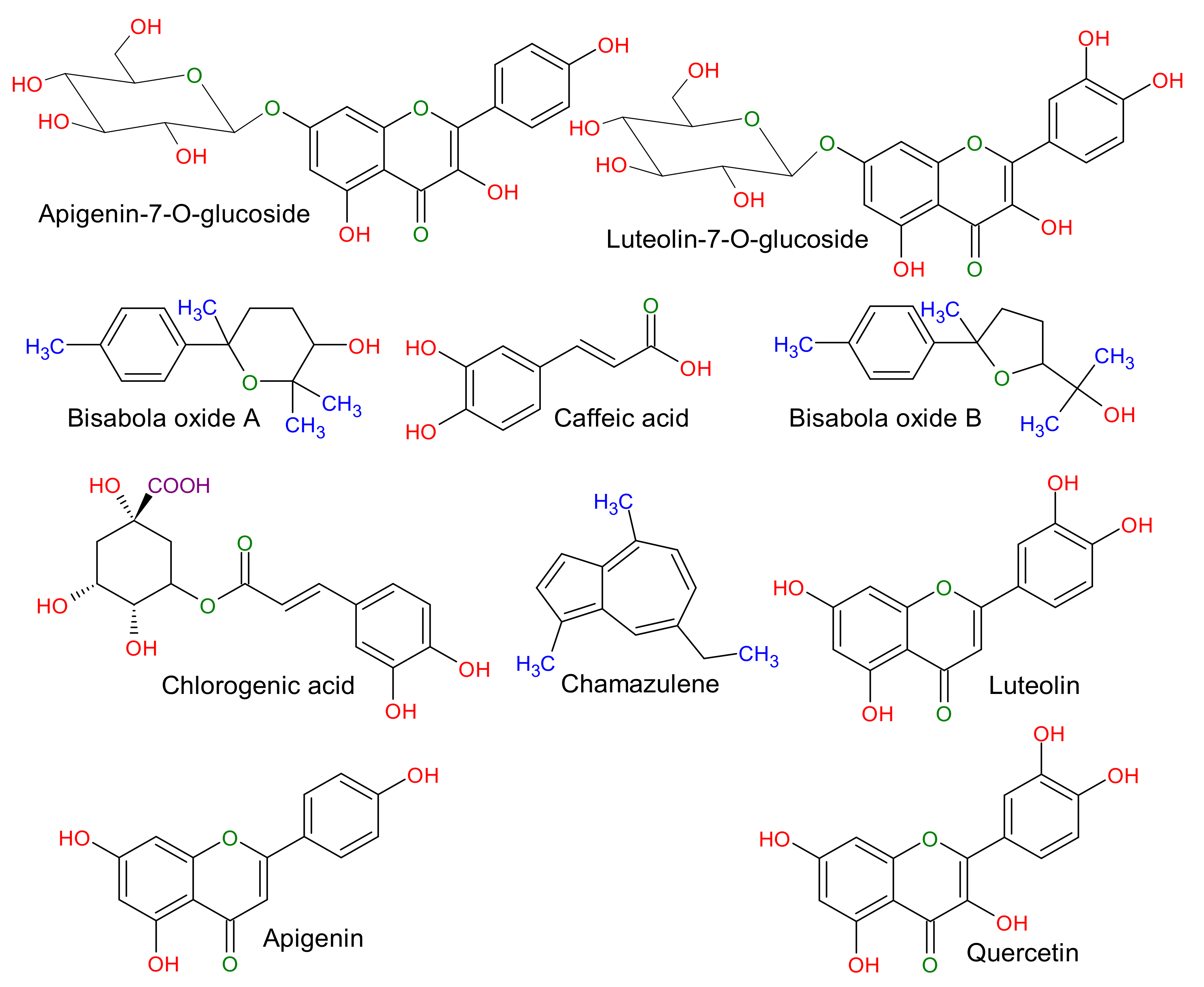
3.4. The Mechanism behind the Biomolecule-Assisted Synthesis of AgNPs
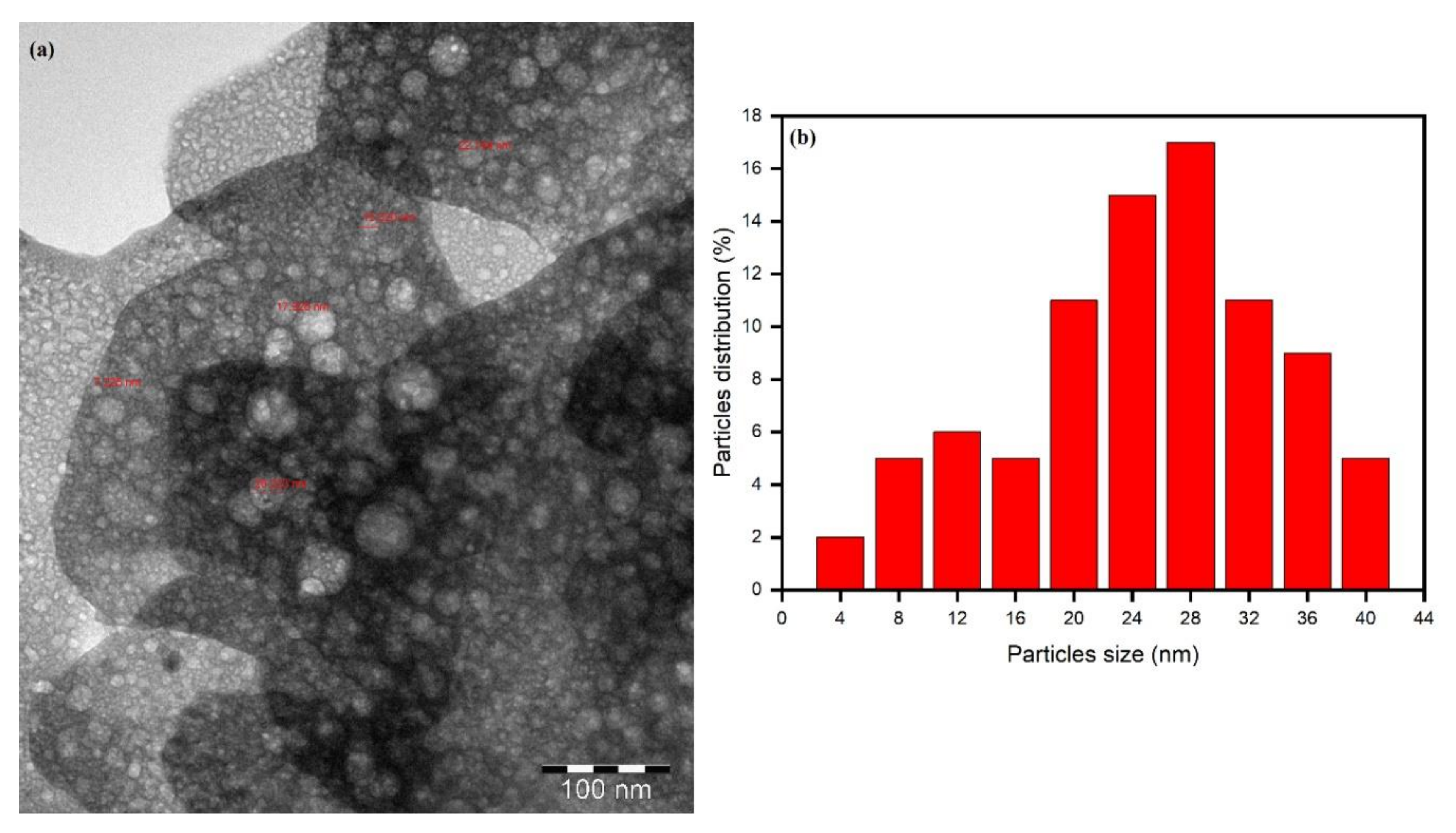
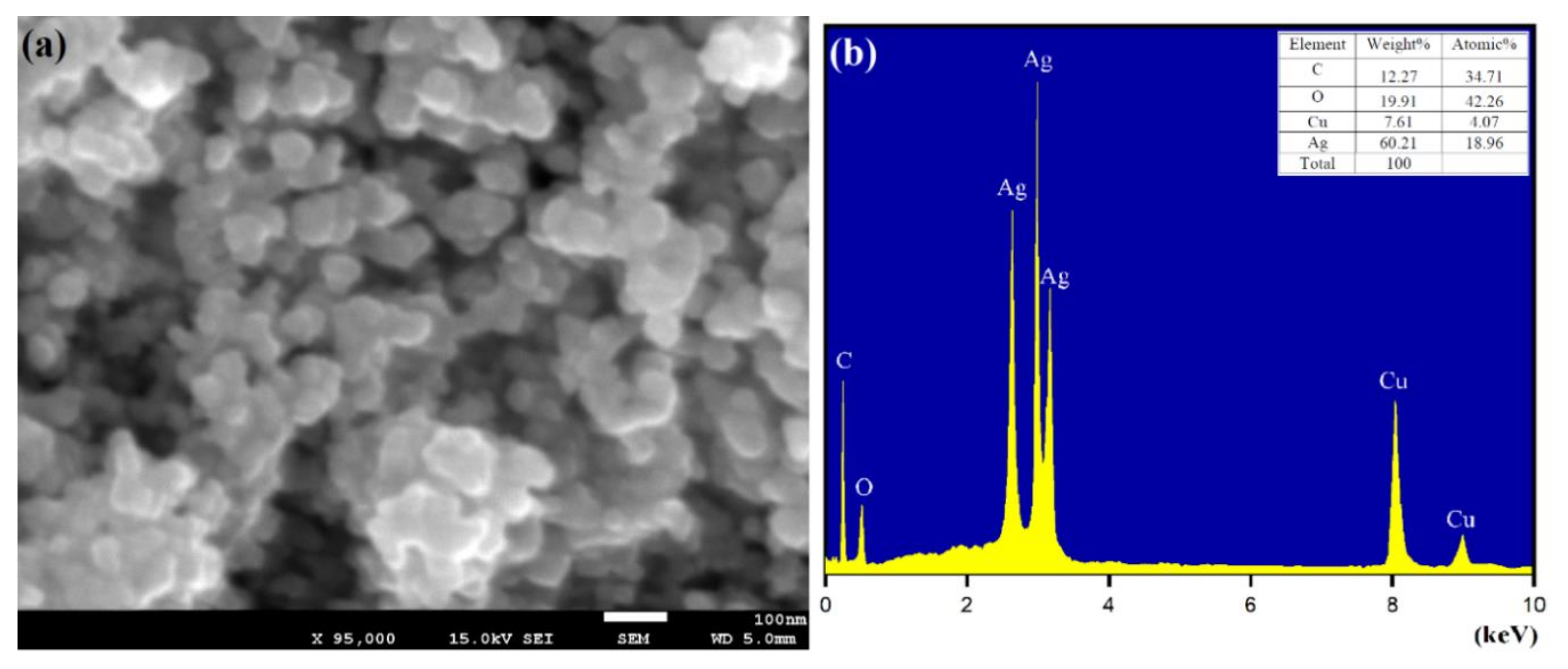
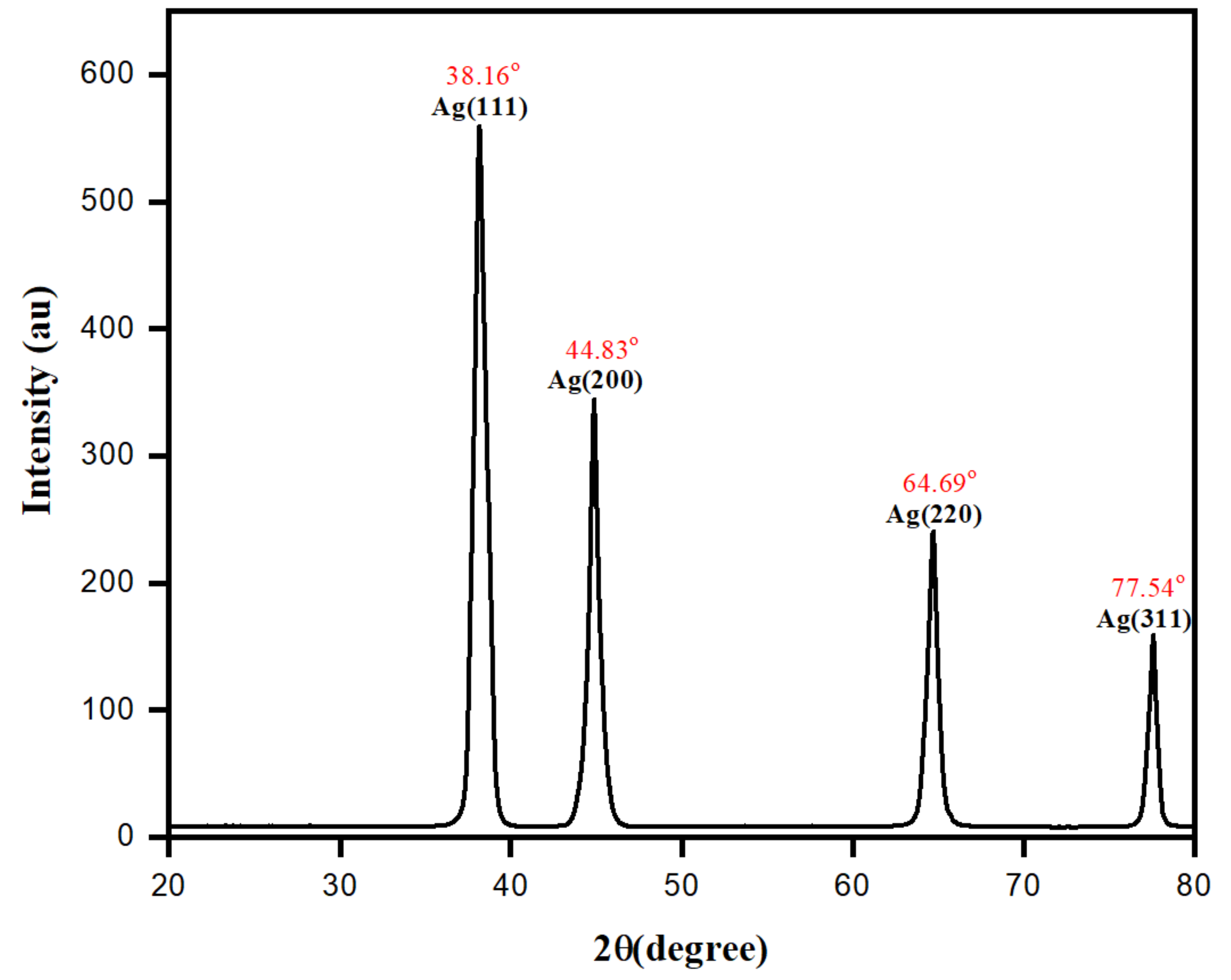
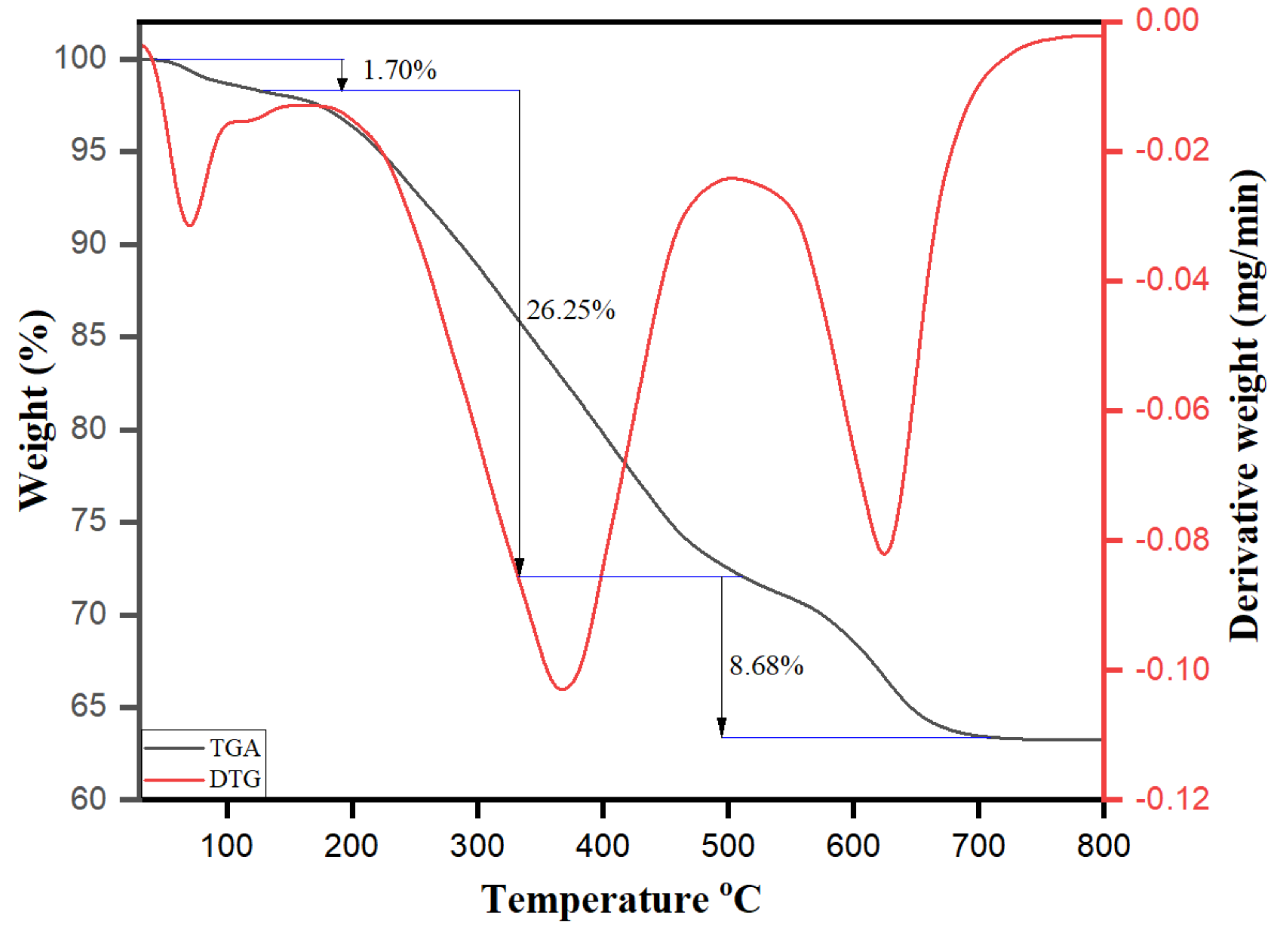
3.5. Morphological Characterization of Biomolecule-Capped AgNPs
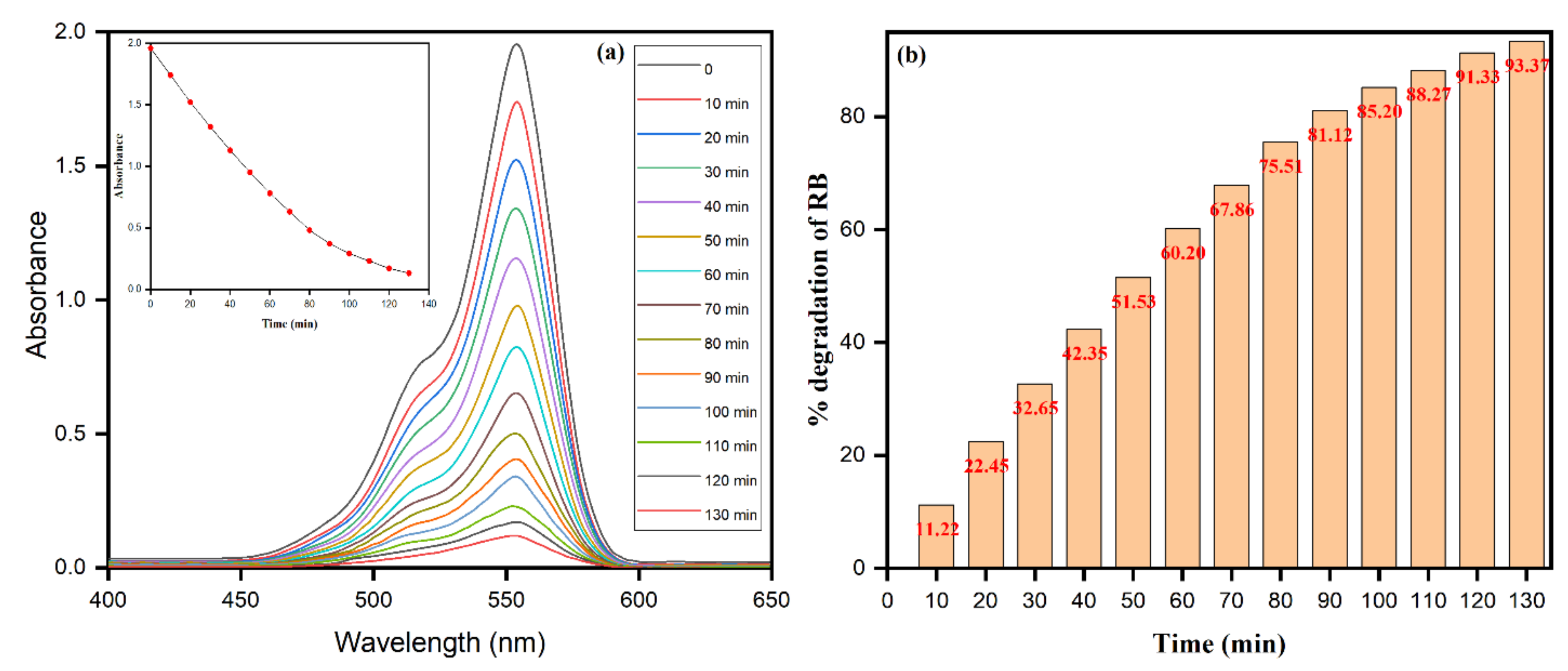
3.6. Photocatalytic Dye Degradation of Rhodamine B
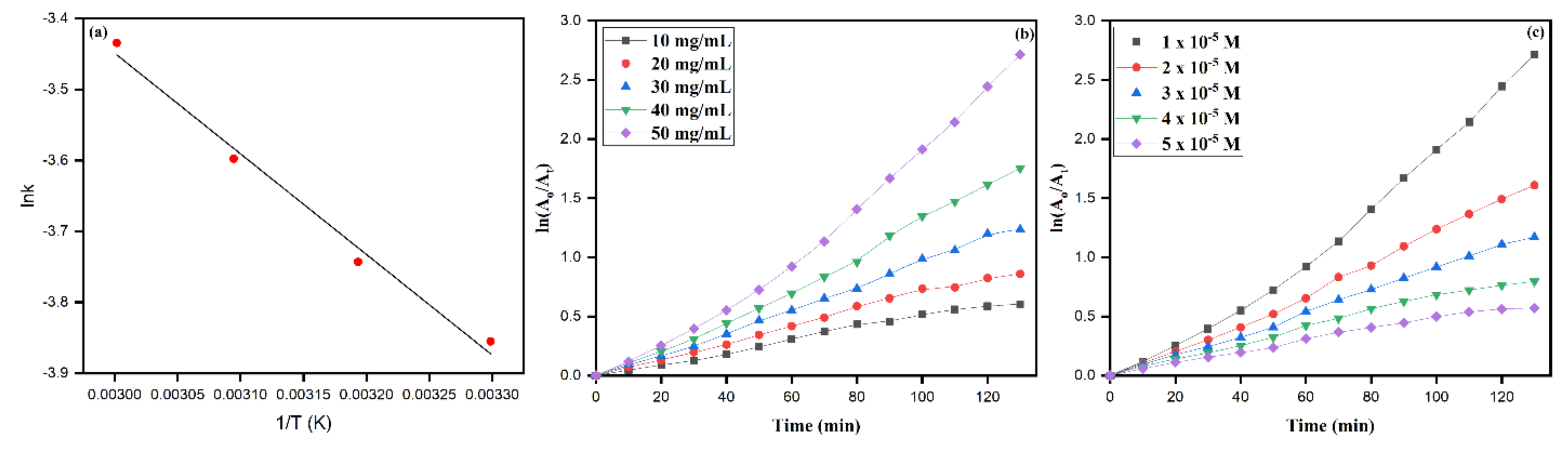
3.7. Effect of Temperature
3.8. Effect of pH
3.9. Role of Catalyst Dosage and Initial RB Dye Concentration
3.10. The Plausible Mechanism behind the Photodegradation of RB Dye
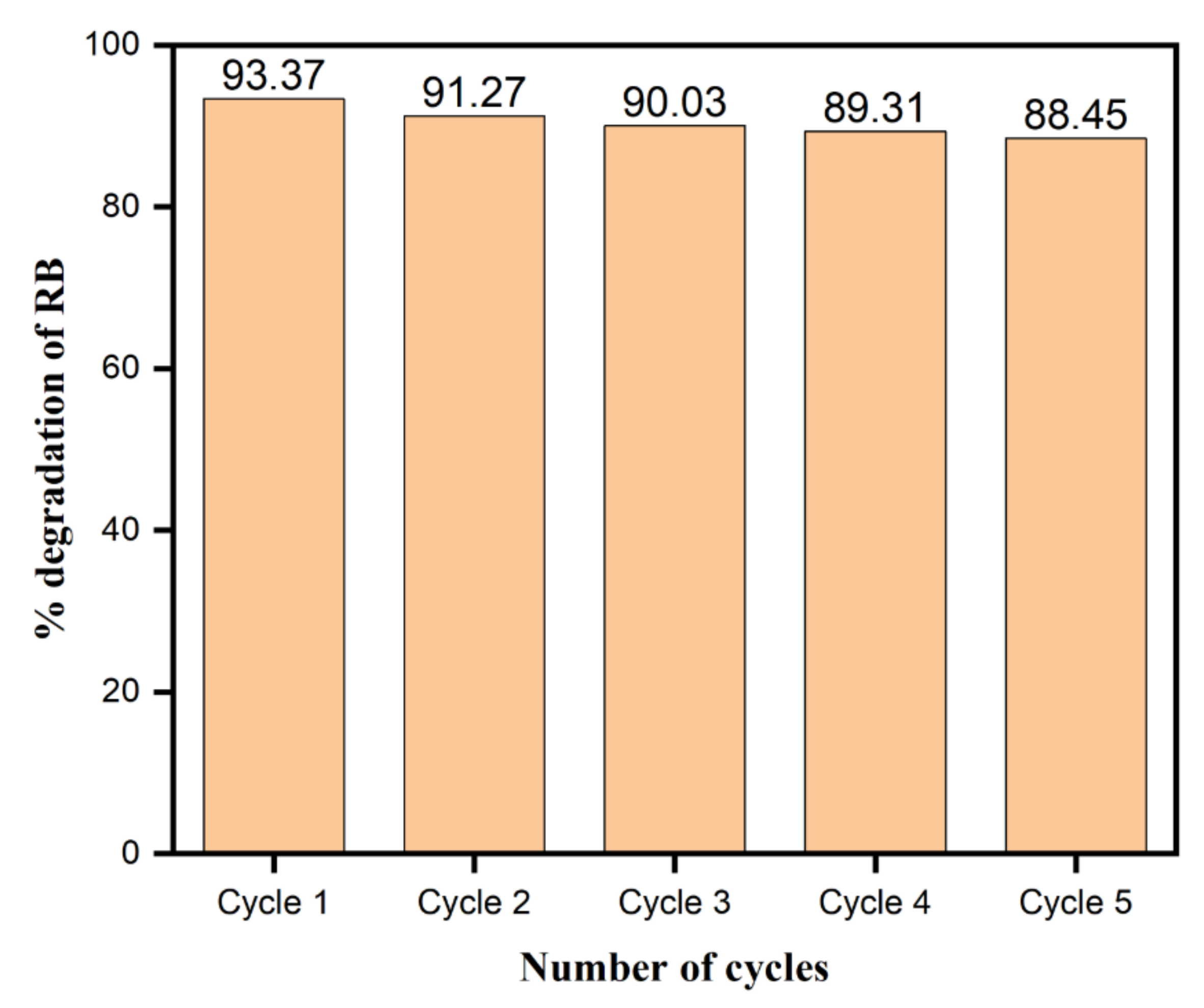
3.11. Stability and Reusability of the Phytochemical-Assisted AgNPs as a Catalyst
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Xiao, Y.; Zhang, W.; Lang, M. In-Situ formation of silver nanoparticles stabilized by amphiphilic star-shaped copolymer and their catalytic application. Appl. Surf. Sci. 2012, 258, 2655–2660. [Google Scholar] [CrossRef]
- Yih, T.; Al-Fandi, M. Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 2006, 97, 1184–1190. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, S.; Zhang, Z.; Liu, Y.; Pang, M. Template-free synthesis and metalation of hierarchical covalent organic framework spheres for photothermal therapy. Chem. Commun. 2019, 55, 14315–14318. [Google Scholar] [CrossRef]
- Shi, Y.; Deng, X.; Bao, S.; Liu, B.; Liu, B.; Ma, P.A.; Cheng, Z.; Pang, M.; Lin, J. Self-Templated Stepwise Synthesis of Monodispersed Nanoscale Metalated Covalent Organic Polymers for In Vivo Bioimaging and Photothermal Therapy. Chem. Asian J. 2017, 12, 2183–2188. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.M.R.; Guha, A.K.; Das, A.R.; Mandal, A.B. Silver-nano biohybride material: Synthesis, characterization and application in water purification. Bioresour. Technol. 2012, 124, 495–499. [Google Scholar] [CrossRef]
- Rastogi, P.K.; Ganesan, V.; Krishnamoorthi, S. Microwave assisted polymer stabilized synthesis of silver nanoparticles and its application in the degradation of environmental pollutants. Mater. Sci. Eng. B 2012, 177, 456–461. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Tan, S.; Gao, J.; Fu, Y.; Liu, Z. Hydrothermal synthesis of Ag nanoparticles on the nanocellulose and their antibacterial study. Inorg. Chem. Commun. 2019, 100, 44–50. [Google Scholar] [CrossRef]
- Cai, Y.; Piao, X.; Gao, W.; Zhang, Z.; Nie, E.; Sun, Z. Large-scale and facile synthesis of silver nanoparticles via a microwave method for a conductive pen. RSC Adv. 2017, 7, 34041–34048. [Google Scholar] [CrossRef] [Green Version]
- Francesko, A.; Fossas, M.C.; Petkova, P.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Sonochemical synthesis and stabilization of concentrated antimicrobial silver-chitosan nanoparticle dispersions. J. Appl. Polym. Sci. 2017, 134, 45136. [Google Scholar] [CrossRef]
- Nasretdinova, G.R.; Fazleeva, R.R.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Ziganshina, A.Y.; Yanilkin, V.V. Electrochemical synthesis of silver nanoparticles in solution. Electrochem. Commun. 2015, 50, 69–72. [Google Scholar] [CrossRef]
- Gupta, S.; Prakash, R. Photochemically assisted formation of silver nanoparticles by dithizone, and its application in amperometric sensing of cefotaxime. J. Mater. Chem. C 2014, 2, 6859–6866. [Google Scholar] [CrossRef]
- Dhayagude, A.C.; Das, A.; Joshi, S.S.; Kapoor, S. γ-Radiation induced synthesis of silver nanoparticles in aqueous poly (N-vinylpyrrolidone) solution. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 148–156. [Google Scholar] [CrossRef]
- Ahmad, N.; Malik, M.A.; Al-Nowaiser, F.M.; Khan, Z. A kinetic study of silver nanoparticles formation from paracetamol and silver (I) in aqueous and micellar media. Colloids Surf. B Biointerfaces 2010, 78, 109–114. [Google Scholar] [CrossRef] [PubMed]
- AL-Thabaiti, N.S.; Malik, M.A.; Khan, Z. Protein interactions with silver nanoparticles: Green synthesis, and biophysical approach. Int. J. Biol. Macromol. 2017, 95, 421–428. [Google Scholar] [CrossRef]
- Siddiquee, M.A.; Parray, M.; Mehdi, S.H.; Alzahrani, K.A.; Alshehri, A.A.; Malik, M.A.; Patel, R. Green synthesis of silver nanoparticles from Delonix regia leaf extracts: In-vitro cytotoxicity and interaction studies with bovine serum albumin. Mater. Chem. Phys. 2020, 242, 122493. [Google Scholar] [CrossRef]
- Saeed, S.; Iqbal, A.; Ashraf, M.A. Bacterial-mediated synthesis of silver nanoparticles and their significant effect against pathogens. Environ. Sci. Pollut. Res. 2020, 27, 37347–37356. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M.; Alharbi, R.M.; Alkhulaifi, M.M. Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J. Biol. Sci. 2019, 26, 1207–1215. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Jemilugba, O.T.; Parani, S.; Mavumengwana, V.; Oluwafemi, O.S. Green synthesis of silver nanoparticles using Combretum erythrophyllum leaves and its antibacterial activities. Colloid Interface Sci. Commun. 2019, 31, 100191. [Google Scholar] [CrossRef]
- Luna-Sánchez, J.; Jiménez-Pérez, J.; Carbajal-Valdez, R.; Lopez-Gamboa, G.; Pérez-González, M.; Correa-Pacheco, Z. Green synthesis of silver nanoparticles using Jalapeño Chili extract and thermal lens study of acrylic resin nanocomposites. Thermochim. Acta 2019, 678, 178314. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A. Rapid and green synthesis of silver nanoparticles using Diospyros lotus extract: Evaluation of their biological and catalytic activities. Polyhedron 2019, 171, 172–180. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.D.; Zaheer, Z.; Aazam, E.S. Sennoside A drug capped biogenic fabrication of silver nanoparticles and their antibacterial and antifungal activities. Saudi Pharm. J. 2020, 28, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Cantisani, M.; Ingle, A.; Galdiero, M.; Rai, M. Silver Nanoparticles As Novel Antibacterial and Antiviral Agents. In Handbook of Nanobiomedical Research: Fundamentals, Applications and Recent Developments: Volume 1. Materials for Nanomedicine; World Scientific: Singapore, 2014; pp. 565–594. [Google Scholar]
- Harish, B.; Uppuluri, K.B.; Anbazhagan, V. Synthesis of fibrinolytic active silver nanoparticle using wheat bran xylan as a reducing and stabilizing agent. Carbohydr. Polym. 2015, 132, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C. Active silver nanoparticles for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, D.K.; Mohan, S.; Hasan, S.H. Photo-induced biosynthesis of silver nanoparticles using aqueous extract of Erigeron bonariensis and its catalytic activity against Acridine Orange. J. Photochem. Photobiol. B Biol. 2016, 155, 39–50. [Google Scholar] [CrossRef]
- Amaladhas, T.P.; Usha, M.; Naveen, S. Sunlight induced rapid synthesis and kinetics of silver nanoparticles using leaf extract of Achyranthes aspera L. and their antimicrobial applications. Mat. Lett. 2013, 4, 779–785. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Sarangi, B.K.; Satpute, D.; Pandey, R.A. Synthesis and characterization of silver nanoparticles using Cynodon dactylon leaves and assessment of their antibacterial activity. Bioprocess Biosyst. Eng. 2013, 36, 999–1004. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Subramaniam, S.; Veerappan, G.; Hari, N.; Sivasubramanian, A.; Veerappan, A. β-Sitosterol-d-glucopyranoside isolated from Desmostachya bipinnata mediates photoinduced rapid green synthesis of silver nanoparticles. RSC Adv. 2014, 4, 59130–59136. [Google Scholar] [CrossRef]
- Kumar, V.; Bano, D.; Mohan, S.; Singh, D.K.; Hasan, S.H. Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater. Lett. 2016, 181, 371–377. [Google Scholar] [CrossRef]
- Kumar, V.; Gundampati, R.K.; Singh, D.K.; Jagannadham, M.V.; Sundar, S.; Hasan, S.H. Photo-induced rapid biosynthesis of silver nanoparticle using aqueous extract of Xanthium strumarium and its antibacterial and antileishmanial activity. J. Ind. Eng. Chem. 2016, 37, 224–236. [Google Scholar] [CrossRef]
- Singh, A.K.; Tiwari, R.; Kumar, V.; Singh, P.; Khadim, S.R.; Tiwari, A.; Srivastava, V.; Hasan, S.; Asthana, R. Photo-induced biosynthesis of silver nanoparticles from aqueous extract of Dunaliella salina and their anticancer potential. J. Photochem. Photobiol. B Biol. 2017, 166, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Salamon, I. Chamomile: A medicinal plant. HerbSpice Med. Plant Dig. 1992, 1, 1–5. [Google Scholar]
- Franke, R.; Schilcher, H. Chamomile: Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essential Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar]
- Newall, C.A.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines. A Guide for Health-Care Professionals; The Pharmaceutical Press: London, UK, 1996. [Google Scholar]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Jerković, J.; Zengin, G.; Gašić, U.; Tešić, Ž.; Mašković, P.; Soares, C.; Barroso, M.F. The influence of the extraction temperature on polyphenolic profiles and bioactivity of chamomile (Matricaria chamomilla L.) subcritical water extracts. Food Chem. 2019, 271, 328–337. [Google Scholar]
- Guzelmeric, E.; Ristivojević, P.; Vovk, I.; Milojković-Opsenica, D.; Yesilada, E. Quality assessment of marketed chamomile tea products by a validated HPTLC method combined with multivariate analysis. J. Pharm. Biomed. Anal. 2017, 132, 35–45. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Švehlíková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert). Phytochemistry 2004, 65, 2323–2332. [Google Scholar]
- Liu, M.-M.; Ma, R.-H.; Ni, Z.-J.; Thakur, K.; Cespedes-Acuña, C.L.; Jiang, L.; Wei, Z.-J. Apigenin 7-O-glucoside promotes cell apoptosis through the PTEN/PI3K/AKT pathway and inhibits cell migration in cervical cancer Hela cells. Food Chem. Toxicol. 2020, 146, 111843. [Google Scholar] [CrossRef]
- Bouzaiene, N.N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Baia, L.; Muresan, D.; Baia, M.; Popp, J.; Simon, S. Structural properties of silver nanoclusters–phosphate glass composites. Vib. Spectrosc. 2007, 43, 313–318. [Google Scholar] [CrossRef]
- Sastry, M.; Mayya, K.; Bandyopadhyay, K. pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1997, 127, 221–228. [Google Scholar] [CrossRef]
- García, M.A. Surface plasmons in metallic nanoparticles: Fundamentals and applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Abdelwahed, N.A. Application of statistical experimental design for optimization of silver nanoparticles biosynthesis by a nanofactory Streptomyces viridochromogenes. J. Microbiol. 2014, 52, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Smith, D.R.; Schultz, S. Local refractive index dependence of plasmon resonance spectra from individual nanoparticles. Nano Lett. 2003, 3, 485–491. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Chen, S.; Yu, H.; Ge, Z.; Zhang, X.; Zhang, P.; Tang, J. Facile preparation and synergistic antibacterial effect of three-component Cu/TiO2/CS nanoparticles. J. Mater. Chem. 2012, 22, 9092–9099. [Google Scholar] [CrossRef]
- Philip, D.; Unni, C.; Aromal, S.A.; Vidhu, V. Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Bulut, E.; Ozacar, M. Rapid, facile synthesis of silver nanostructure using hydrolyzable tannin. Ind. Eng. Chem. Res. 2009, 48, 5686–5690. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Mittal, P.; Bansal, P.; Khokra, S.L.; Kaushik, D. Pharmacological potential of Matricaria recutita-A review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 12–16. [Google Scholar]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Biosynthesis of silver nanoparticles using Olea europaea leaves extract and its antibacterial activity. Nanosci. Nanotechnol. 2012, 2, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.; Kumar, S.; Ahamed, M.; Alrokayan, S.A.; Alsalhi, M.; Alhoshan, M.; Aldwayyan, A. Structural and spectroscopic studies of thin film of silver nanoparticles. Appl. Surf. Sci. 2011, 257, 10607–10612. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ilisei, S.; Luca, C. Hydroxyapatite-silver nanoparticles coatings on porous polyurethane scaffold. Mater. Sci. Eng. C 2014, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 2016, 62, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Albeladi, S.S.R.; Malik, M.A.; Al-thabaiti, S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol. 2020, 9, 10031–10044. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Khosropour, A.R.; Razmjou, A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014, 4, 61394–61403. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Coll. Surf. A. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Muthuraj, V.; Vadivel, S. Constructing novel Ag nanoparticles anchored on MnO 2 nanowires as an efficient visible light driven photocatalyst. RSC Adv. 2016, 6, 61357–61366. [Google Scholar] [CrossRef]
- Tahir, K.; Ahmad, A.; Li, B.; Nazir, S.; Khan, A.U.; Nasir, T.; Khan, Z.U.H.; Naz, R.; Raza, M. Visible light photo catalytic inactivation of bacteria and photo degradation of methylene blue with Ag/TiO2 nanocomposite prepared by a novel method. J. Photochem. Photobiol. B Biol. 2016, 162, 189–198. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Wahab, H.; Hadi, H. Visible light N-TiO 2-induced photodegradation of Congo red: Characterization, kinetics and mechanistic study. Int. J. Environ. Sci. Technol. 2017, 14, 2135–2148. [Google Scholar] [CrossRef]
- Nagaraja, R.; Kottam, N.; Girija, C.; Nagabhushana, B. Photocatalytic degradation of Rhodamine B dye under UV/solar light using ZnO nanopowder synthesized by solution combustion route. Powder Technol. 2012, 215, 91–97. [Google Scholar] [CrossRef]
- Shaikh, W.; Chakraborty, S.; Islam, R. Photocatalytic degradation of rhodamine B under UV irradiation using Shorea robusta leaf extract-mediated bio-synthesized silver nanoparticles. Int. J. Environ. Sci. Technol. 2019, 17, 2059–2072. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Cheng, Y.-H.; Muthukrishnan, P.; Padmavathy, S.; Elangovan, A. Biosynthesis of silver nanoparticles by using Camellia japonica leaf extract for the electrocatalytic reduction of nitrobenzene and photocatalytic degradation of Eosin-Y. J. Photochem. Photobiol. B Biol. 2017, 170, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2020, 104725. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B Biol. 2019, 199, 111621. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. Photocatalytic degradation of Rhodamine-B dye by stable ZnO nanostructures with different calcination temperature induced defects. Appl. Surf. Sci. 2019, 465, 546–556. [Google Scholar] [CrossRef]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Addi, A.A.; Saleh, T.A. A comparative study of photocatalytic degradation of rhodamine B using natural-based zeolite composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J. Photocatalytic degradation of rhodamine B catalyzed by TiO2 films on a capillary column. RSC Adv. 2018, 8, 11921–11929. [Google Scholar] [CrossRef] [Green Version]
- Podasca, V.E.; Buruiana, T.; Buruiana, E.C. Photocatalytic degradation of Rhodamine B dye by polymeric films containing ZnO, Ag nanoparticles and polypyrrole. J. Photochem. Photobiol. A Chem. 2019, 371, 188–195. [Google Scholar] [CrossRef]


| Temperature ºC | % Degradation | Rate Constant (k) | R2 | pH | % Degradation | Rate Constant (k) | R2 |
|---|---|---|---|---|---|---|---|
| 30 | 93.36 | 0.02117 | 0.982 | 2 | 70.85 | 0.00972 | 0.968 |
| 40 | 95.25 | 0.02368 | 0.982 | 4 | 80.23 | 0.01259 | 0.978 |
| 50 | 97.12 | 0.02736 | 0.975 | 6 | 87.74 | 0.01663 | 0.981 |
| 60 | 98.55 | 0.03225 | 0.967 | 8 | 93.36 | 0.02117 | 0.982 |
| RB Dye. (M) | % Degradation | Rate Constant (k) | R2 | AgNPs (mg) | % Degradation | Rate Constant (k) | R2 |
|---|---|---|---|---|---|---|---|
| 1.0 × 10−5 | 93.36 | 0.02117 | 0.982 | 10 | 45.40 | 0.00201 | 0.989 |
| 2.0 × 10−5 | 79.59 | 0.01275 | 0.995 | 20 | 57.65 | 0.00692 | 0.989 |
| 3.0 × 10−5 | 67.85 | 0.00927 | 0.997 | 30 | 70.91 | 0.00987 | 0.997 |
| 4.0 × 10−5 | 54.55 | 0.00641 | 0.990 | 40 | 82.65 | 0.01386 | 0.993 |
| 5.0 × 10−5 | 39.79 | 0.00462 | 0.989 | 50 | 93.36 | 0.02117 | 0.982 |
| Catalyst | Light Source | Irradiation Time | Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|
| Au-ZnO | UV light | 180 min | 95% | [76] |
| ZnO | Sunlight | 200 min | 98% | [77] |
| ZnO | UV light | 70 min | 97.75% | [78] |
| Zeo-TiO2 | UV irradiation | 80 min | 100% | [79] |
| Zeo-ZnO | UV irradiation | 80 min | 81% | [79] |
| TiO2 film | UV irradiation | 30 min | 98.46% | [80] |
| ZnO/Ag | Visible light | 120 min | 99% | [81] |
| AgNPs | UV irradiation | 100 min | 90.41% | [74] |
| AgNPs | UV irradiation | 130 min | 93.39% | Present Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, A.A.; Malik, M.A. Phytomediated Photo-Induced Green Synthesis of Silver Nanoparticles Using Matricaria chamomilla L. and Its Catalytic Activity against Rhodamine B. Biomolecules 2020, 10, 1604. https://doi.org/10.3390/biom10121604
Alshehri AA, Malik MA. Phytomediated Photo-Induced Green Synthesis of Silver Nanoparticles Using Matricaria chamomilla L. and Its Catalytic Activity against Rhodamine B. Biomolecules. 2020; 10(12):1604. https://doi.org/10.3390/biom10121604
Chicago/Turabian StyleAlshehri, Abdulmohsen Ali, and Maqsood Ahmad Malik. 2020. "Phytomediated Photo-Induced Green Synthesis of Silver Nanoparticles Using Matricaria chamomilla L. and Its Catalytic Activity against Rhodamine B" Biomolecules 10, no. 12: 1604. https://doi.org/10.3390/biom10121604
APA StyleAlshehri, A. A., & Malik, M. A. (2020). Phytomediated Photo-Induced Green Synthesis of Silver Nanoparticles Using Matricaria chamomilla L. and Its Catalytic Activity against Rhodamine B. Biomolecules, 10(12), 1604. https://doi.org/10.3390/biom10121604





