Quantitative and Qualitative Identification of Bioactive Compounds in Edible Flowers of Black and Bristly Locust and Their Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples
2.1.1. Chemicals
2.1.2. Origins of Flowers
2.1.3. Plant Material Preparation
2.2. Chemical Analysis
2.2.1. Evaluation of Dry Matter
2.2.2. Polyphenols Analysis
Sample Preparation
HPLC Set and Analysis Parameters Description
Compounds Identification and Calculation
2.2.3. Antioxidant Activity
2.3. Statistical Evaluation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kropf, U.; Korošec, M.; Bertoncelj, J.; Ogrinc, N.; Nečemer, M.; Kump, P.; Golob, T. Determination of the geographical origin of Slovenian black locust, lime and chestnut honey. Food Chem. 2010, 121, 839–846. [Google Scholar] [CrossRef]
- Dobre, I.; Georgescu, L.A.; Alexe, P.; Escuredo, O.; Seijo, M.C. Rheological behavior of different honey types from Romania. Food Res. Int. 2012, 49, 126–132. [Google Scholar] [CrossRef]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT-Food Sci. Tech. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Gortzi, O.; Metaxa, X.; Mantanis, G.; Lalas, S. Effect of artificial ageing using different wood chips on the antioxidant activity, resveratrol and catechin concentration, sensory properties and colour of two Greek red wines. Food Chem. 2013, 141, 2887–2895. [Google Scholar] [CrossRef]
- Rédei, K. Management of black Locust (Robinia pseudoacacia L.) stands in Hungary. J. For. Res. 2002, 13, 260–264. [Google Scholar] [CrossRef]
- Park, C.-H.; Park, S.-Y.; Lee, S.Y.; Kim J., K.; Park, S.U. Analysis of metabolites in white flowers of Magnolia Denudata Desr. and violet flowers of Magnolia Liliiflora Desr. Molecules 2018, 23, 1558. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, camellia, centaurea and pansies: Nutritional, fatty acids, free sugars, vitamin E, carotenoids and organic acids characterization. Food Res. Int. 2020, 123, 2132. [Google Scholar] [CrossRef]
- Zheng, J.; Meenu, M.; Xu, B. A systematic investigation on free phenolic acids and flavonoids profiles of commonly consumed edible flowers in China. J. Pharm. Biomed. 2019, 172, 268–277. [Google Scholar] [CrossRef]
- Tanikawa, N.; Honma, K.; Tatsuzawa, F. Flavonoids of the rose-pink, blue, and white flowers of Nigella damascena L. (Ranunculaceae). Sci. Hort. 2019, 257, 108609. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Pereira, E.L.; Ramalhosa, E.; Saraiva, J.A. Effect of high hydrostatic pressure on the quality of four edible flowers: Viola× wittrockiana, Centaurea cyanus, Borago officinalis and Camellia japonica. Int. J. Food Sci. Techn. 2017, 52, 2455–2462. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Effects of different drying methods on the bioactive compounds and antioxidant properties of edible Centaurea (Centaurea cyanus) petals. Brazilian J. Food Techn. 2018, 21, 017211. [Google Scholar] [CrossRef]
- Christopher, D.A.; Mitchell, R.J.; Karron, J.D. Pollination intensity and paternity in flowering plants. Ann. Bot. 2019, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Olszewska, M.A.; Owczarek, A.; Wolbiś, M. Preliminary study on the composition of volatile fraction of fresh flowers and leaves of Robinia pseudoacacia L., growing in Poland. Acta Pol. Pharm. 2015, 72, 1217–1222. [Google Scholar]
- Călina, D.; Olah, N.K.; Pătru, E.; Docea, A.; Popescu, H.; Bubulica, M.V. Chromatographic analysis of the flavonoids from Robinia pseudoacacia species. Curr. Health Sci. J. 2013, 39, 232–236. [Google Scholar]
- Savic Gajic, I.; Savic, I.; Boskov, I.; Žerajić, S.; Markovic, I.; Gajic, D. Optimization of ultrasound-assisted extraction of phenolic compounds from black locust (Robiniae Pseudoacaciae) flowers and comparison with conventional methods. Antioxidants 2019, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Gardeazabal, I.; Romanos-Nanclares, A.; Martínez-González, M.; Sánchez-Bayona, R.; Vitelli-Storelli, F.; Gaforio, J.; Aramendía-Beitia, J.M.; Toledo, E. Total polyphenol intake and breast cancer risk in the Seguimiento Universidad de Navarra (SUN) cohort. Br. J. Nutr. 2019, 122, 542–551. [Google Scholar] [CrossRef]
- Yamagata, K. Polyphenols regulate endothelial functions and reduce the risk of cardiovascular disease. Curr. Pharm. Des. 2019, 5, 2443–2458. [Google Scholar] [CrossRef]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.-Y.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. HPLC Fingerprint combined with quantitation of phenolic compounds and chemometrics as an efficient strategy for quality consistency evaluation of Sambucus nigra berries. Nat. Prod. Comm. 2016, 10, 1449–1454. [Google Scholar] [CrossRef]
- Polish Norm PN-R-04013. The Estimation of Dry Matter in Fruits and Vegetables; Polish Standard Committee: Warszawa, Poland, 1988; pp. 1–5. [Google Scholar]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef] [PubMed]
- Średnicka-Tober, D.; Ponder, A.; Hallmann, E.; Głowacka, A.; Rozpara, E. The profile and content of polyphenols and carotenoids in local and commercial sweet cherry fruits (Prunus avium L.) and their antioxidant activity in vitro. Antioxidants 2019, 8, 534. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Pachlewska, A. Biological value of various edible flower species. Acta Sci. Pol. Hort. Cult. 2016, 15, 109–119. [Google Scholar]
- Barragán-Fonseca, K.Y.; Loon, J.J.A.; Dicke, M.; Lucas-Barbosa, D. Use of visual and olfactory cues of flowers of two brassicaceous species by insect pollinators. Ecol. Entomol. 2020, 45, 45–55. [Google Scholar] [CrossRef]
- Ricker, J.G.; Lubell, J.D.; Brand, M.H. Comparing insect pollinator visitation for six native shrub species and their cultivars. Hort. Sci. 2019, 4, 2086–2090. [Google Scholar] [CrossRef]
- Ojeda, F.; Midgley, J.; Pauw, A.; Lavola, A.; Casimiro-Soriguer, R.; Hattas, D.; Segarra-Moragues, J.G.; Julkunen-Tiitto, R. Flower colour divergence is associated with post-fire regeneration dimorphism in the fynbos heath Erica coccinea subsp. coccinea (Ericaceae). Evol. Ecol. 2019, 33, 345–367. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound identification of selected rose species and cultivars: An insight to petal and leaf phenolic profiles. J. Am. Soc. Hort. Sci. 2014, 39, 157–166. [Google Scholar] [CrossRef]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of phenolic acids in honeys of different floral origin by solid-pase extraction and high-performance liquid chromatography. Phytochem. Ann. 2006, 18, 24–32. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Pauter, P.; Smarzyński, K.; Różańska, M.B.; Jeżowski, P.; Dwiecki, K.; Mildner-Szkudlarz, S. Thermal processing of pasta enriched with black locust flowers affect quality, phenolics, and antioxidant activity. J. Food Proc. Pres. 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Gayibova, S.; Ivanišová, E.; Árvay, J.; Hŕstková, M.; Slávik, M.; Petrová, J.; Hleba, L.; Tóth, T.; Kačániová, M.; Aripov, T. In vitro screening of antioxidant and antimicrobial activities of medicinal plants growing in Slovakia. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1281–1289. [Google Scholar]
- Jin-Ping, F.; Hai-Xiao, Z.; Mei-Mei, M.; Dai-di, C. Components of flower pigments in petals of lily. J. North. Agric. Univ. 2016, 23, 10–22. [Google Scholar]
- Park, Y.; Park, S.-Y.; Valan Arasu, M.; Al-Dhabi, N.; Ahn, H.; Kim, J.; Park, S. Accumulation of carotenoids and metabolic profiling in different cultivars of tagetes flowers. Molecules 2017, 22, 313. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Bhattarai, K. Gerbera. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; pp. 407–438. [Google Scholar]
- Mato, M.; Onozaki, T.; Ozeki, Y.; Higeta, D.; Itoh, Y.; Hisamatsu, T.; Yoshida, H.; Shibata, M. Flavonoid biosynthesis in pink-flowered cultivars derived from “William Sim” carnation (Dianthus caryophyllus). Engei. Gakkai. Zasshi. 2001, 70, 315–319. [Google Scholar] [CrossRef][Green Version]
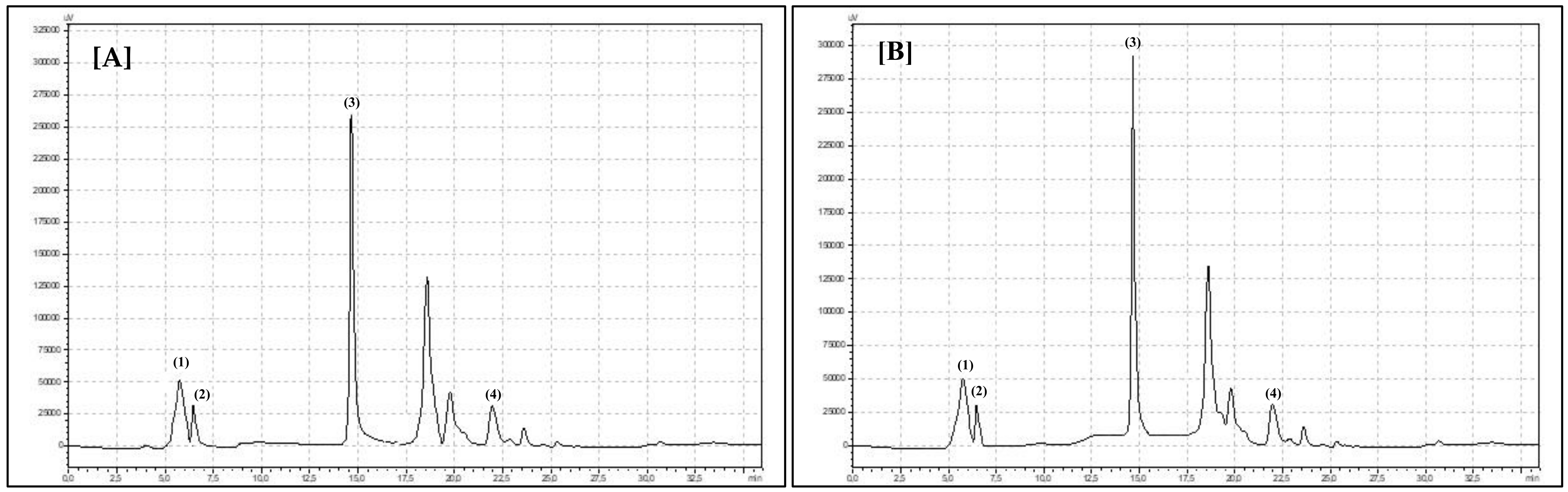

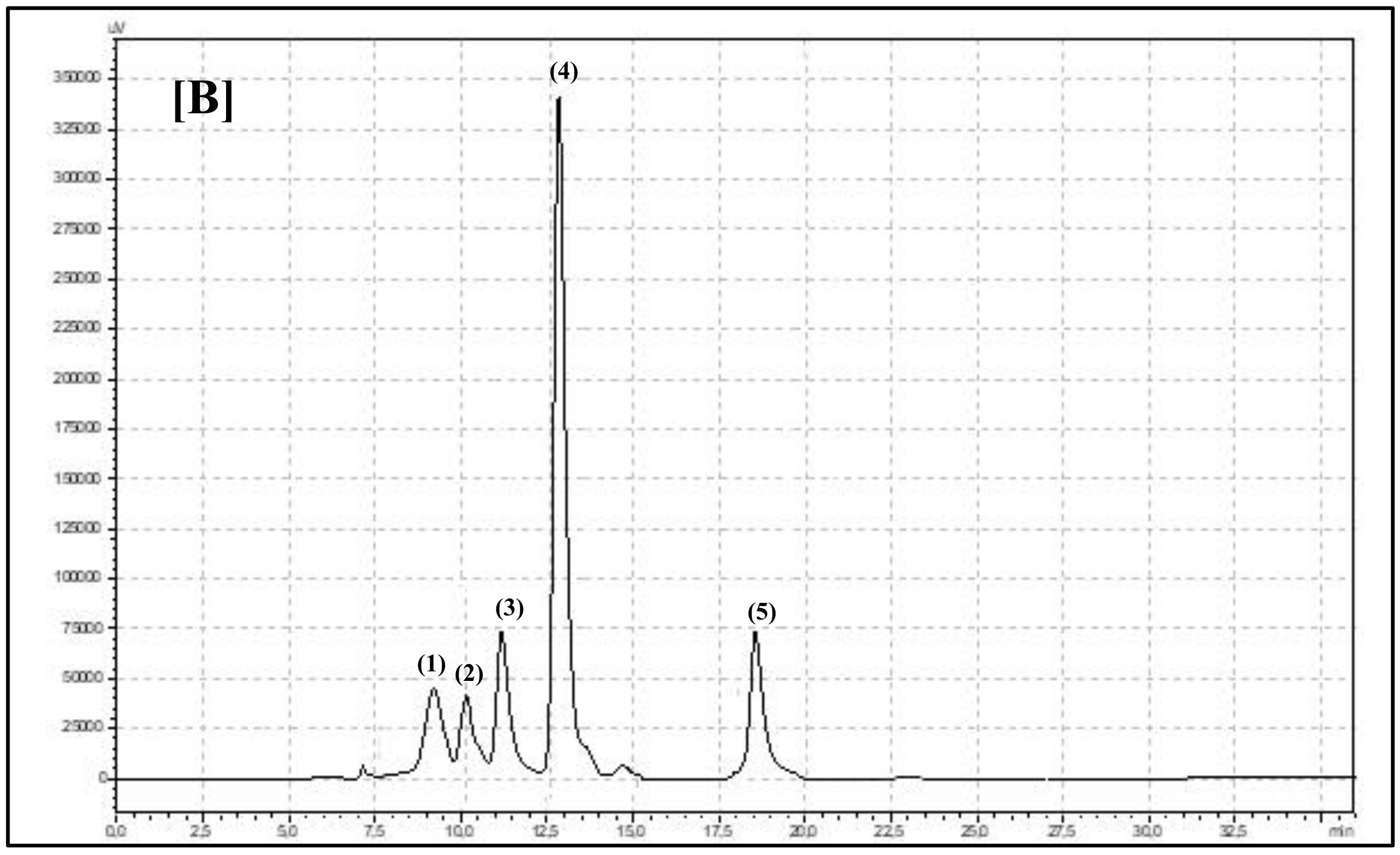

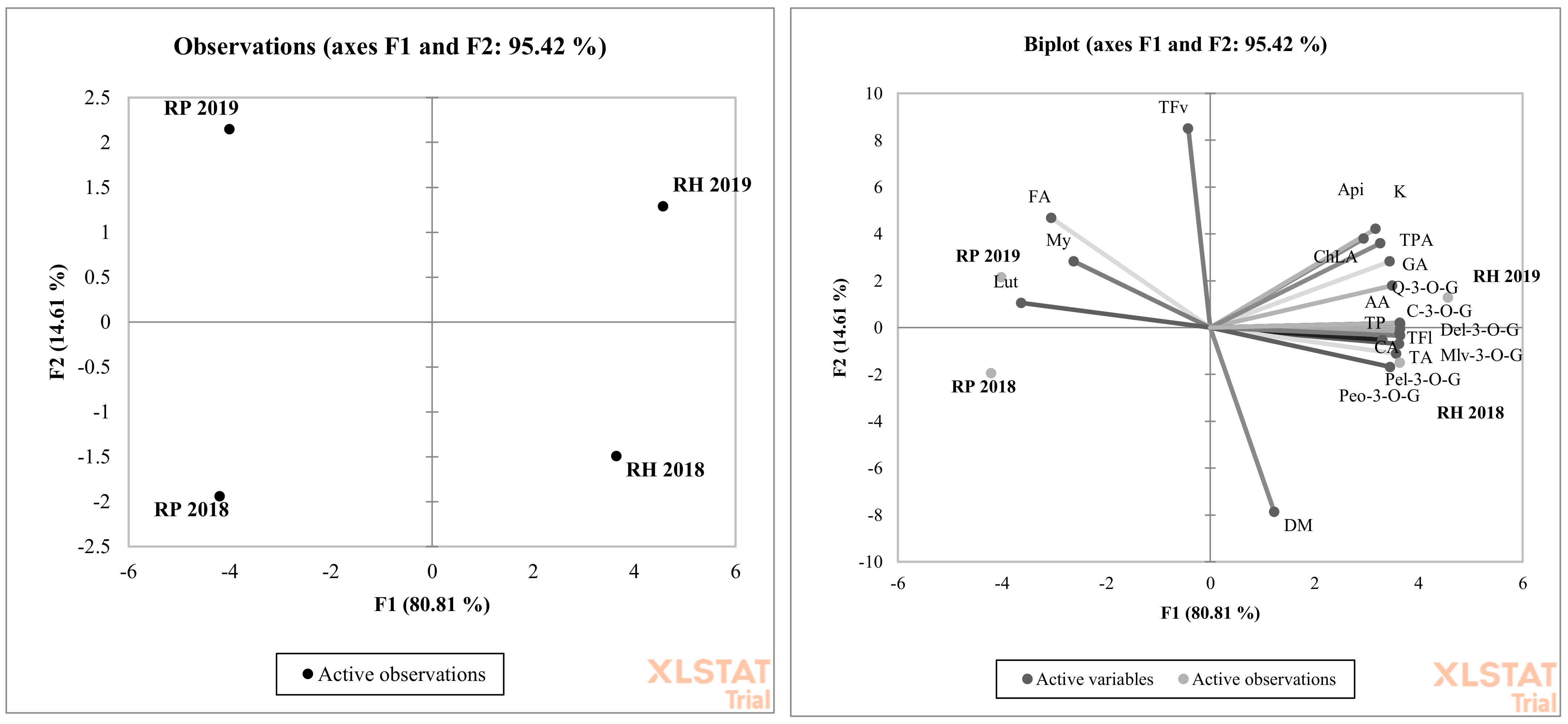
| Species and Experimental Year/Bioactive Compounds | 2018 | 2019 | Robinia pseudoacacia L. (White Folwers) | Robinia hispida (Pink Flowers) | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Robinia pseudoacacia L. (White Folwers) | Robinia hispida (Pink Flowers) | Robinia pseudoacacia L. (White Folwers) | Robinia hispida(Pink Flowers) | Year x Species | Species | |||
| dry matter | 15.88 ± 0.17 a | 17.71 ± 0.04 a | 12.17 ± 0.15 a | 13.72 ± 0.04 a | 14.03 ± 0.55 B | 15.72 ± 0.57 A | N.S. | <0.0001 |
| total polyphenols | 3.51 ± 0.01 b | 10.81 ± 0.13 a | 4.31 ± 0.06 b | 11.08 ± 0.07 a | 3.91 ± 0.12 B | 10.94 ± 0.08 A | 0.0017 | <0.0001 |
| total phenolic acids | 1.94 ± 0.01 c | 2.94 ± 0.03 b | 2.39 ± 0.07 b | 3.27 ± 0,02 a | 2.16 ± 0.07 B | 3.10 ± 0.05 A | 0.005 | <0.0001 |
| gallic acid | 0.37 ± 0.01 c | 0.66 ± 0.12 b | 0.41 ± 0.01 c | 0.85 ± 0.08 a | 0.39 ± 0.05 B | 0.75 ± 0.07 A | <0.0001 | <0.0001 |
| chlorogenic acid | 1.25 ± 0.01 c | 1.90 ± 0.02 a | 1.70 ± 0.01 b | 1.90 ± 0.07 a | 1.48 ± 0.05 B | 1.90 ± 0.06 A | <0.0001 | <0.0001 |
| caffeic acid | 0.25 ± 0.00 b | 0.34 ± 0.13 ab | 0.16 ± 0.00 c | 0.47 ± 0.05 a | 0.20 ± 0.00 B | 0.40 ± 0.09 A | <0.0001 | <0.0001 |
| ferulic acid | 0.07 ± 0.01 a | 0.04 ± 0.01 a | 0.11 ± 0.00 a | 0.05 ± 0.01 a | 0.09 ± 0.01 A | 0.04 ± 0.03 A | N.S. | N.S. |
| total flavonoids | 1.56 ± 0.01 c | 7.88 ± 0.02 a | 1.92 ± 0.01 b | 7.81 ± 0.01 a | 1.74 ± 0.07 B | 7.84 ± 0.01 A | 0.005 | <0.0001 |
| total flavonols | 1.56 ± 0.00 a | 1.54 ± 0.01 a | 1.92 ± 0.00 a | 1.83 ± 0.01 a | 1.74 ± 0.01 A | 1.68 ± 0.02 A | N.S. | N.S. |
| quercetin-3-O-glucoside | 0.06 ± 0.00 a | 0.37 ± 0.00 a | 0.08 ± 0.06 a | 0.40 ± 0.00 a | 0.07 ± 0.03 B | 0.38 ± 0.00 A | N.S. | <0.0001 |
| myricetin | 0.39 ± 0.00 a | 0.39 ± 0.02 a | 0.43 ± 0.00 a | 0.36 ± 0.07 b | 0.41 ± 0.00 A | 0.37 ± 0.03 B | 0.0002 | <0.0001 |
| luteolin | 0.77 ± 0.01 a | 0.04 ± 0.01 b | 0.84 ± 0.01 a | 0.07 ± 0.01 b | 0.80 ± 0.01 A | 0.05 ± 0.01 B | 0.006 | <0.0001 |
| apigenin | 0.24 ± 0.01 b | 0.39 ± 0.00 a | 0.34 ± 0.01 ab | 0.46 ± 0.00 a | 0.29 ± 0.01 B | 0.42 ± 0.00 A | 0.012 | <0.0001 |
| kaempferol | 0.11 ± 0.00 c | 0.35 ± 0.01 b | 0.24 ± 0.01 c | 0.55 ± 0.00 a | 0.17 ± 0.02 B | 0.45 ± 0.01 A | <0.0001 | <0.0001 |
| total anthocyanins | N.D. | 6.34 ± 0.00 a | N.D. | 5.98 ± 0.00 b | N.D. | 6.16 ± 0.03 | 0.009 | <0.0001 |
| cyanidin-3-O-glucoside | N.D. | 1.52 ± 0.02 b | N.D. | 1.87 ± 0.01 a | N.D. | 1.70 ± 0.05 | <0.0001 | <0.0001 |
| pelargonidin-3-O-glucoside | N.D. | 4.41 ± 0.13 a | N.D. | 3.68 ± 0.06 b | N.D. | 4.05 ± 0.14 | <0.0001 | <0.0001 |
| delphinidin-3-O-glucoside | N.D. | 0.21 ± 0.01 a | N.D. | 0.25 ± 0.01 a | N.D. | 0.23 ± 0.01 | N.S. | <0.0001 |
| malvidin-3-O-glucoside | N.D. | 0.15 ± 0.00 a | N.D. | 0.16 ± 0.00 a | N.D. | 0.15 ± 0.00 | N.S. | <0.0001 |
| peonidin-3-O-glucoside | N.D. | 0.03 ± 0.00 a | N.D. | 0.02 ± 0.00 a | N.D. | 0.03 ± 0.00 | N.S. | <0.0001 |
| antioxidant activity | 1.43 ± 0.01 b | 4.40 ± 0.05 a | 1.75 ± 0.03 b | 4.51 ± 0.03 a | 1.59 ± 0.05 B | 4.45 ± 0.03 A | 0.0017 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallmann, E. Quantitative and Qualitative Identification of Bioactive Compounds in Edible Flowers of Black and Bristly Locust and Their Antioxidant Activity. Biomolecules 2020, 10, 1603. https://doi.org/10.3390/biom10121603
Hallmann E. Quantitative and Qualitative Identification of Bioactive Compounds in Edible Flowers of Black and Bristly Locust and Their Antioxidant Activity. Biomolecules. 2020; 10(12):1603. https://doi.org/10.3390/biom10121603
Chicago/Turabian StyleHallmann, Ewelina. 2020. "Quantitative and Qualitative Identification of Bioactive Compounds in Edible Flowers of Black and Bristly Locust and Their Antioxidant Activity" Biomolecules 10, no. 12: 1603. https://doi.org/10.3390/biom10121603
APA StyleHallmann, E. (2020). Quantitative and Qualitative Identification of Bioactive Compounds in Edible Flowers of Black and Bristly Locust and Their Antioxidant Activity. Biomolecules, 10(12), 1603. https://doi.org/10.3390/biom10121603





