Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid
Abstract
1. Introduction
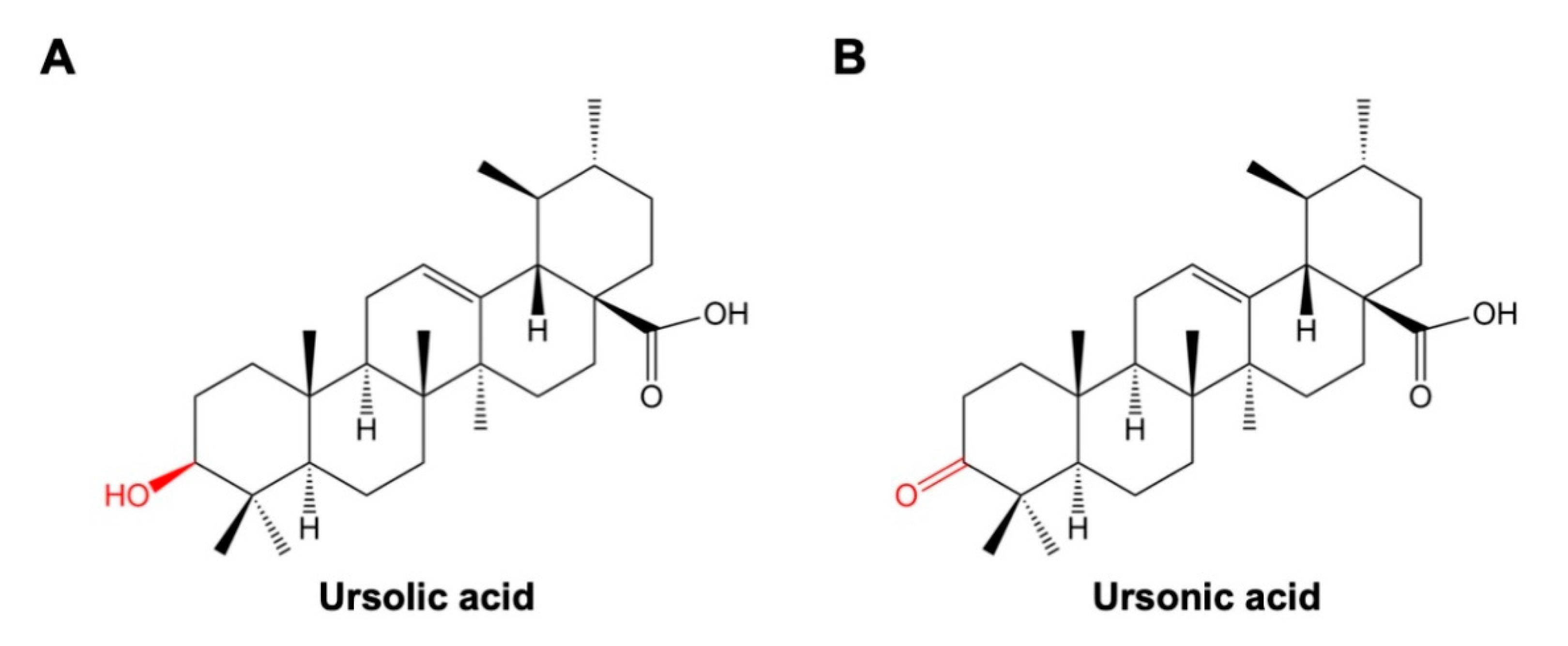
2. Chemical Properties
3. Synthesis of Ursonic Acid
4. Plant Sources of Ursonic Acid
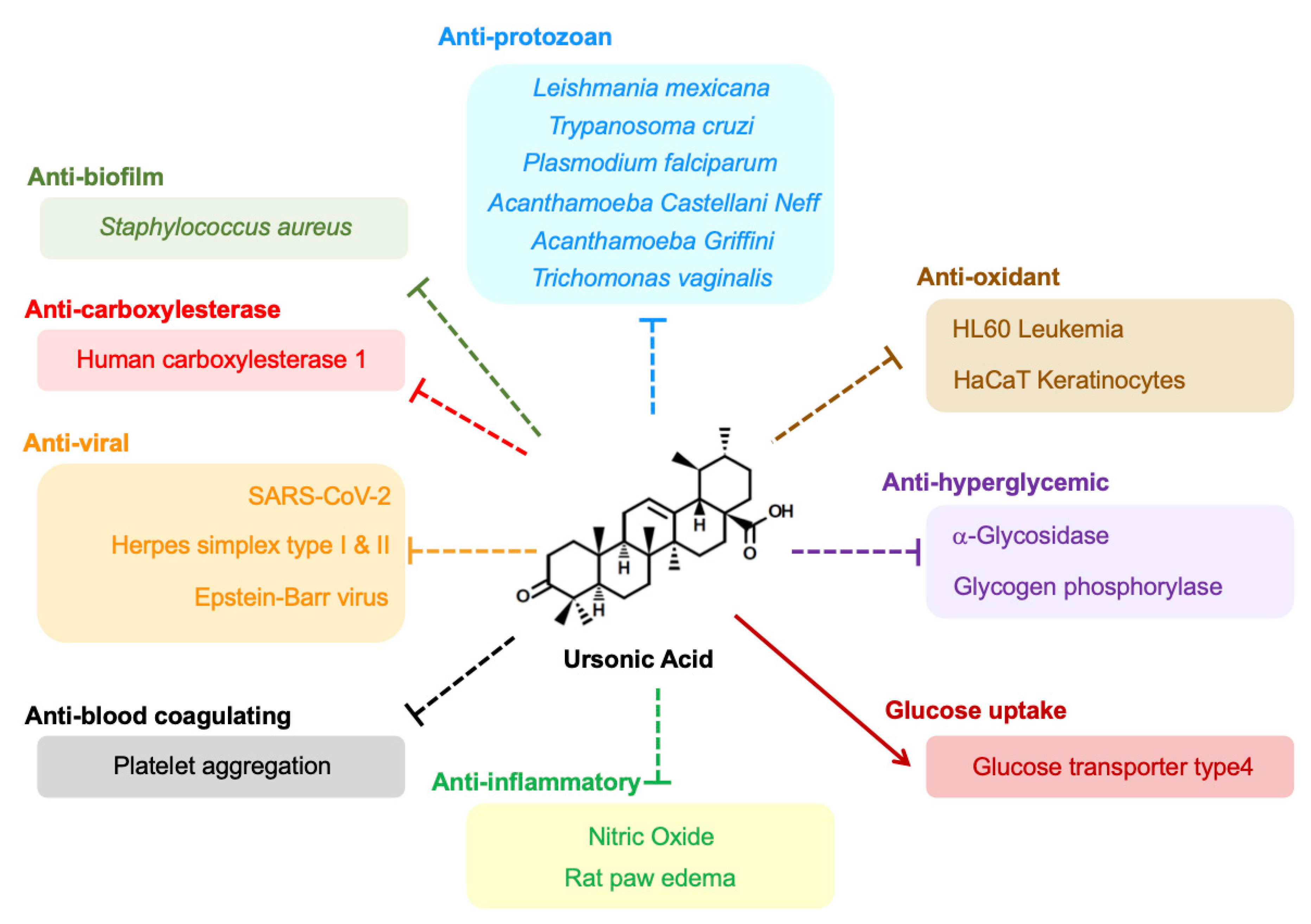
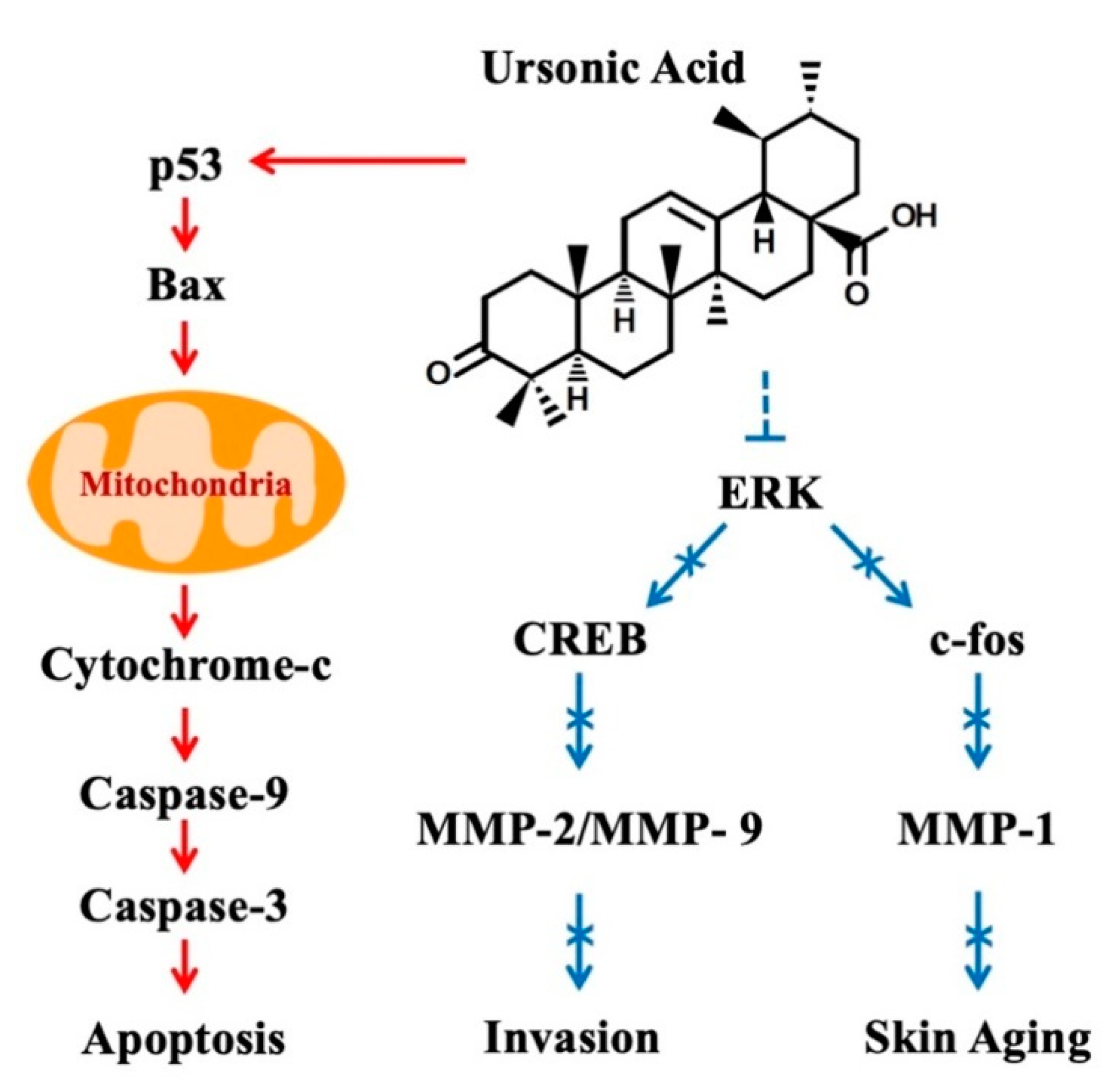
5. Anticancer Effects
6. Antiprotozoan Effects
7. Antihyperglycemic Effects
8. Anti-Inflammatory Effects
9. Antiviral Effects
10. Antioxidant Effects
11. Other Expected Therapeutic Potential
12. Modulation of Cell Signaling Pathways by Ursonic Acid
13. Modifications of Ursonic Acid
14. Future Perspectives
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UNA | Ursonic acid |
| ULA | Ursolic acid |
| NF-κB | Nuclear factor-κB |
| IPP | Isopentenyl diphosphate |
| DMAPP | Dimethylallyl diphosphate |
| NSCLC | Nonsmall cell lung cancer |
| MMP | Matrix metalloproteinase |
| NO | Nitric oxide |
| LPS | Lipopolysaccharide |
| EBV | Epstein–Barr virus |
| HIV | Human immunodeficiency virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 2019 |
| hCE1 | Human carboxylesterase 1 |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| CREB | cAMP response element-binding protein |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor-α |
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharm. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007, 66, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.M.; Arriaga, A.M.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Jang, S.M.; Yee, S.T.; Choi, J.; Choi, M.S.; Do, G.M.; Jeon, S.M.; Yeo, J.; Kim, M.J.; Seo, K.I.; Lee, M.K. Ursolic acid enhances the cellular immune system and pancreatic beta-cell function in streptozotocin-induced diabetic mice fed a high-fat diet. Int. Immunopharmacol. 2009, 9, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef]
- Choi, W.H.; Lee, I.A. Evaluation of Anti-Toxoplasma gondii Effect of Ursolic Acid as a Novel Toxoplasmosis Inhibitor. Pharmaceuticals 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-P.; Kong, T.; Zhang, L.; Tong, S.; Tian, Z.-Y.; Duan, Y.-H.; Zhang, X.-H. Solubilities of ursolic acid and oleanolic acid in four solvents from (283.2 to 329.7) K. J. Chem. Eng. Data 2011, 56, 2723–2725. [Google Scholar] [CrossRef]
- Leipold, D.; Wünsch, G.; Schmidt, M.; Bart, H.-J.; Bley, T.; Neuhaus, H.E.; Bergmann, H.; Richling, E.; Muffler, K.; Ulber, R. Biosynthesis of ursolic acid derivatives by microbial metabolism of ursolic acid with Nocardia sp. strains—Proposal of new biosynthetic pathways. Process. Biochem. 2010, 45, 1043–1051. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Qian, D.; Tang, Y.; Wu, D.; Su, S.; Wang, H.; Zhao, Y. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem. 2015, 167, 468–474. [Google Scholar] [CrossRef]
- Mazumder, K.; Tanaka, K.; Fukase, K. Cytotoxic activity of ursolic acid derivatives obtained by isolation and oxidative derivatization. Molecules 2013, 18, 8929–8944. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharm. 2018, 22, 235–248. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Chang, J.Y.; Kuo, C.C.; Chang, C.Y.; Kuo, Y.H. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochemistry 2005, 66, 495–501. [Google Scholar] [CrossRef]
- Baglin, I.; Mitaine-Offer, A.C.; Nour, M.; Tan, K.; Cave, C.; Lacaille-Dubois, M.A. A review of natural and modified betulinic, ursolic and echinocystic acid derivatives as potential antitumor and anti-HIV agents. Mini Rev. Med. Chem. 2003, 3, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, S.H.; Ma, B.L.; Wang, W.W.; Yu, B.Y.; Zhang, J. New derivatives of ursolic acid through the biotransformation by Bacillus megaterium CGMCC 1.1741 as inhibitors on nitric oxide production. Bioorg. Med. Chem. Lett. 2017, 27, 2575–2578. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.K.; Chacko, A.R.; Gandhimathi, A.; Ghosh, P.; Harini, K.; Joseph, A.P.; Joshi, A.G.; Karpe, S.D.; Kaushik, S.; Kuravadi, N.; et al. Genome sequencing of herb Tulsi (Ocimum tenuiflorum) unravels key genes behind its strong medicinal properties. BMC Plant Biol. 2015, 15, 212. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khalifa, S.I.; Khafagi, I.; Youssef, D.T.; Khan, S.; Mesbah, M.; Khan, I. Microbial metabolism of biologically active secondary metabolites from Nerium oleander L. Chem. Pharm. Bull. 2008, 56, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kitamura, K.; Irie, K.; Naruse, S.; Matsuura, T.; Uemae, T.; Taira, S.; Ohigashi, H.; Murakami, S.; Takahashi, M.; et al. Triterpenoids Isolated from Ziziphus jujuba Enhance Glucose Uptake Activity in Skeletal Muscle Cells. J. Nutr. Sci. Vitam. 2017, 63, 193–199. [Google Scholar] [CrossRef]
- Jin, X.Y.; Chen, H.; Li, D.D.; Li, A.L.; Wang, W.Y.; Gu, W. Design, synthesis, and anticancer evaluation of novel quinoline derivatives of ursolic acid with hydrazide, oxadiazole, and thiadiazole moieties as potent MEK inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 955–972. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-kappaB involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef] [PubMed]
- CHEN, B.-l.; FENG, K.; ZHENG, Z.-h.; CHEN, Y.-y. Determination of Oleanic Acid and Ursonic Acid in Crude Hawthorn Berry by HPLC. Chin. J. Exp. Tradit. Med. Formulae 2010, 5, 73. [Google Scholar]
- Kukina, T.; Frolova, T.; Sal’nikova, O. Lipophilic constituents from Malus baccata. Chem. Nat. Compd. 2014, 50, 1096–1098. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.; Su, S.; Shang, E.; Ni, S.; Qian, D. High-performance liquid chromatography--two wavelength detection of triterpenoid acids from the fruits of Ziziphus jujuba containing various cultivars in different regions and classification using chemometric analysis. J. Pharm. Biomed. Anal. 2009, 49, 1296–1302. [Google Scholar] [CrossRef]
- Liu, S.H.; Cheng, Y.C. Old formula, new Rx: The journey of PHY906 as cancer adjuvant therapy. J. Ethnopharmacol. 2012, 140, 614–623. [Google Scholar] [CrossRef]
- Mahjoub, F.; Akhavan Rezayat, K.; Yousefi, M.; Mohebbi, M.; Salari, R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J. Med. Life 2018, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Syamasundar, K.; Mallavarapu, G.R.; Krishna, E.M. Triterpenoids of the resin of Bursera delpechiana. Phytochemistry 1991, 30, 362–363. [Google Scholar] [CrossRef]
- Poehland, B.L.; Carte, B.K.; Francis, T.A.; Hyland, L.J.; Allaudeen, H.S.; Troupe, N. In vitro antiviral activity of dammar resin triterpenoids. J. Nat. Prod. 1987, 50, 706–713. [Google Scholar] [CrossRef]
- Yang, S.J.; Zhao, Q.; Xiang, H.M.; Liu, M.J.; Zhang, Q.Y.; Xue, W.; Song, B.A.; Yang, S. Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.A.; Farnaz, S.; Simjee, R.U.; Malik, A. Triterpenes and B-sitosterol from piper betle: Isolation, antiplatelet and anti-inflammatory effects. Biochem. Soc. Trans. 1993, 21, 462S. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Raza, S.M.; Siddiqui, B.S.; Siddiqui, S. Triterpenoids from the aerial parts of Lantana camara. J. Nat. Prod. 1995, 58, 1570–1574. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A.; Morton, T.C.; Suares, H.; Willing, R.I. Triterpenes of Lantana tiliaefolia. 24-Hydroxy-3-oxours-12-en-28-oic acid, a new triterpene. Aust. J. Chem. 1983, 36, 2537–2547. [Google Scholar] [CrossRef]
- Fannang, S.V.; Kuete, V.; Mbazoa, C.D.; Momo, J.I.; Van-Dufat, H.T.; Tillequin, F.; Seguin, E.; Chosson, E.; Wandji, J. A new acylated triterpene with antimicrobial activity from the leaves of Rauvolfia vomitoria. Chem. Nat. Compd. 2011, 47, 404. [Google Scholar] [CrossRef]
- Thanh Tam, N.; Thien, D.D.; Sung, T.V.; Thi Hoang Anh, N.; Thuy, T.T.; Trung, K.H.; Xuan, T.D.; Khanh, T.D. Evaluation of ursolic acid as the main component isolated from Catharanthus roseus against hyperglycemia. Int. Lett. Nat. Sci. 2016, 50, 7–17. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kiuchi, F.; Ito, M.; Honda, G.; Takeda, Y.; Khodzhimatov, O.K.; Ashurmetov, O.A. Trypanocidal constituents of Dracocephalum komarovi. Tetrahedron 2006, 62, 4355–4359. [Google Scholar] [CrossRef]
- Heron, M.; Anderson, R.N. Changes in the Leading Cause of Death: Recent Patterns in Heart Disease and Cancer Mortality. NCHS Data Brief 2016, 254, 1–8. [Google Scholar]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35 (Suppl.), S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Zhang, W.; Men, X.; Lei, P. Review on anti-tumor effect of triterpene acid compounds. J. Cancer Res. 2014, 10 (Suppl. 1), 14–19. [Google Scholar]
- Ryu, S.Y.; Choi, S.U.; Lee, S.H.; Lee, C.O.; No, Z.; Ahn, J.W. Antitumor triterpenes from medicinal plants. Arch. Pharmacal Res. 1994, 17, 375. [Google Scholar] [CrossRef]
- Alves Monteath, S.A.F.; Maciel, M.A.M.; Vega, R.G.; de Mello, H.; de Araujo Martins, C.; Esteves-Souza, A.; Gattass, C.R.; Echevarria, A. Ultrasound-assisted Extraction of Ursolic Acid from the Flowers of Ixora coccinia Linn (Rubiaceae) and Antiproliferative Activity of Ursolic Acid and Synthesized Derivatives. Pharm. Mag. 2017, 13, 265–269. [Google Scholar]
- Kim, S.-H. Antitumor Activity and Inhibitory of Topoisomerase-I by ursonic acid. Korean J. Orient. Med. Pathol. 1997, 11, 12–15. [Google Scholar]
- Min, B.S.; Kim, Y.H.; Lee, S.M.; Jung, H.J.; Lee, J.S.; Na, M.K.; Lee, C.O.; Lee, J.P.; Bae, K. Cytotoxic triterpenes from Crataegus pinnatifida. Arch. Pharm. Res. 2000, 23, 155–158. [Google Scholar] [CrossRef]
- Hevener, K.; Verstak, T.A.; Lutat, K.E.; Riggsbee, D.L.; Mooney, J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B 2018, 8, 844–861. [Google Scholar] [CrossRef]
- de Sousa, L.R.F.; Wu, H.M.; Nebo, L.; Fernandes, J.B.; da Silva, M.F.D.F.; Kiefer, W.; Schirmeister, T.; Vieira, P.C. Natural products as inhibitors of recombinant cathepsin L of Leishmania mexicana. Exp. Parasitol. 2015, 156, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple roles in cancer. Proteom. Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H. Apoptic and antimetastatic effects of ursolic acid isolated from Oldenlandia diffusae Herba. J. Haehwa Med. 1997, 5, 523–533. [Google Scholar]
- Thien, D.D.; Tam, N.T.; Thien, D.G.; Anh, N.T.H.; Van Sung, T. Synthesis and cytotoxic activity of ursolic acid derivatives. Z. Für Nat. B 2013, 68, 201–206. [Google Scholar] [CrossRef]
- Dillekas, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Bravo-Cordero, J.J.; Hodgson, L.; Condeelis, J. Directed cell invasion and migration during metastasis. Curr. Opin. Cell Biol. 2012, 24, 277–283. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Lee, S.Y. Ursonic acid exerts inhibitory effects on matrix metalloproteinases via ERK signaling pathway. Chem. Biol. Interact. 2020, 315, 108910. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lin, C.Y.; Tsai, C.W.; Yin, M.C. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol. In Vitro 2011, 25, 1274–1280. [Google Scholar] [CrossRef]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef]
- Monzote, L.; Siddiq, A. Drug development to protozoan diseases. Open Med. Chem. J. 2011, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D.; Wright, C.W. Antiprotozoal agents from plant sources. Planta Med. 1991, 57, S53–S59. [Google Scholar] [CrossRef]
- Wozniak, L.; Skapska, S.; Marszalek, K. Ursolic Acid--A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- da Silva Filho, A.A.; Resende, D.O.; Fukui, M.J.; Santos, F.F.; Pauletti, P.M.; Cunha, W.R.; Silva, M.L.; Gregorio, L.E.; Bastos, J.K.; Nanayakkara, N.P. In Vitro antileishmanial, antiplasmodial and cytotoxic activities of phenolics and triterpenoids from Baccharis dracunculifolia D. C. (Asteraceae). Fitoterapia 2009, 80, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; Rodriguez-Exposito, R.L.; Reyes-Batlle, M.; Rizo-Liendo, A.; Pinero, J.E.; Bazzocchi, I.L.; Lorenzo-Morales, J.; Jimenez, I.A. Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba Spp. Pathogens 2019, 8, 130. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Staudt, A.F.; Menezes, C.; de Azevedo, A.P.; Fialho, S.N.; Tasca, T.; Teles, C.B.G.; Gnoatto, S.B. Evaluation of triterpenes derivatives in the viability of Leishmania amazonensis and Trichomonas vaginalis. Braz. J. Pharm. Sci. 2019, 55, e17481. [Google Scholar] [CrossRef]
- Dalla-Vechia, L.; Dassonville-Klimpt, A.; Grellier, P.; Sonnet, P.; Gosmann, G.; Gnoatto, S.C.B. The Beckmann Rearrangement Applied to Ursolic Acid with Antimalarial Activity in Medicinal Chemistry Studies. Lett. Org. Chem. 2012, 9, 92–95. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R.; et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef]
- Brealey, D.; Singer, M. Hyperglycemia in critical illness: A review. J. Diabetes Sci. Technol. 2009, 3, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The Role of Hyperglycemia in Endometrial Cancer Pathogenesis. Cancers 2020, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Thuong, P.T. Stimulation of glucose uptake by triterpenoids from Weigela subsessilis. Phytother. Res. 2010, 24, 49–53. [Google Scholar] [CrossRef]
- Han, J.H.; Zhou, W.; Li, W.; Tuan, P.Q.; Khoi, N.M.; Thuong, P.T.; Na, M.; Myung, C.S. Pentacyclic Triterpenoids from Astilbe rivularis that Enhance Glucose Uptake via the Activation of Akt and Erk1/2 in C2C12 Myotubes. J. Nat. Prod. 2015, 78, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.A.; Sun, H.B.; Liu, J.; Cheng, K.G.; Zhang, P.; Zhang, L.Y.; Hao, J.; Zhang, L.Y.; Ni, P.Z.; Zographos, S.E.; et al. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: Synthesis, structure-activity relationships, and X-ray crystallographic studies. J. Med. Chem 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
- Aiston, S.; Coghlan, M.P.; Agius, L. Inactivation of phosphorylase is a major component of the mechanism by which insulin stimulates hepatic glycogen synthesis. Eur. J. Biochem. 2003, 270, 2773–2781. [Google Scholar] [CrossRef]
- Wu, P.; Zheng, J.; Huang, T.; Li, D.; Hu, Q.; Cheng, A.; Jiang, Z.; Jiao, L.; Zhao, S.; Zhang, K. Synthesis and Evaluation of Novel Triterpene Analogues of Ursolic Acid as Potential Antidiabetic Agent. PLoS ONE 2015, 10, e0138767. [Google Scholar] [CrossRef]
- Lee, J.; Yee, S.T.; Kim, J.J.; Choi, M.S.; Kwon, E.Y.; Seo, K.I.; Lee, M.K. Ursolic acid ameliorates thymic atrophy and hyperglycemia in streptozotocin-nicotinamide-induced diabetic mice. Chem. Biol. Interact. 2010, 188, 635–642. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Si, L.; Meng, K.; Tian, Z.; Sun, J.; Li, H.; Zhang, Z.; Soloveva, V.; Li, H.; Fu, G.; Xia, Q.; et al. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes. Sci. Adv. 2018, 4, eaau8408. [Google Scholar] [CrossRef]
- Ikeda, T.; Yokomizo, K.; Okawa, M.; Tsuchihashi, R.; Kinjo, J.; Nohara, T.; Uyeda, M. Anti-herpes virus type 1 activity of oleanane-type triterpenoids. Biol. Pharm. Bull. 2005, 28, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Park, J.C. Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch. Pharm. Res. 2007, 30, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.Y.; Lee, C.-K.; Ahn, J.W.; Lee, S.H.; Zee, O.P. Antiviral activity of triterpenoid derivatives. Arch. Pharmacal Res. 1993, 16, 339–342. [Google Scholar] [CrossRef]
- Ohigashi, H.; Takamura, H.; Koshimizu, K.; Tokuda, H.; Ito, Y. Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation; ursolic acid and oleanolic acid from an anti-inflammatory Chinese medicinal plant, Glechoma hederaceae L. Cancer Lett. 1986, 30, 143–151. [Google Scholar] [CrossRef]
- Zuru, D.U. Theoretical model for the design and preparation of a CNT–ursonic acid drug matrix as HIV-gp120 entry inhibitor. Sci. Afr. 2019, 6, e00177. [Google Scholar] [CrossRef]
- Kumar, S.; Kashyap, P.; Chowdhury, S.; Kumar, S.; Panwar, A.; Kumar, A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine 2020, 153317. [Google Scholar] [CrossRef]
- Kim, Y.; Jedrzejczak, R.; Maltseva, N.I.; Wilamowski, M.; Endres, M.; Godzik, A.; Michalska, K.; Joachimiak, A. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. Protein Sci. 2020, 29, 1596–1605. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Zou, L.W.; Dou, T.Y.; Wang, P.; Lei, W.; Weng, Z.M.; Hou, J.; Wang, D.D.; Fan, Y.M.; Zhang, W.D.; Ge, G.B.; et al. Structure-Activity Relationships of Pentacyclic Triterpenoids as Potent and Selective Inhibitors against Human Carboxylesterase 1. Front. Pharmacol. 2017, 8, 435. [Google Scholar] [CrossRef]
- da Silva, G.N.S.; Primon-Barros, M.; Macedo, A.J.; Gnoatto, S.C.B. Triterpene Derivatives as Relevant Scaffold for New Antibiofilm Drugs. Biomolecules 2019, 9, 58. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, H.; Wan, H.; Zou, X.; Ma, X.; Gao, G. Harmine suppresses the proliferation and migration of human ovarian cancer cells through inhibiting ERK/CREB pathway. Oncol. Rep. 2017, 38, 2927–2934. [Google Scholar] [CrossRef]
- Cheng, H.L.; Hsieh, M.J.; Yang, J.S.; Lin, C.W.; Lue, K.H.; Lu, K.H.; Yang, S.F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef]
- Tang, F.; Tang, S.; Guo, X.; Yang, C.; Jia, K. CT45A1 siRNA silencing suppresses the proliferation, metastasis and invasion of lung cancer cells by downregulating the ERK/CREB signaling pathway. Mol. Med. Rep. 2017, 16, 6708–6714. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci 2016, 17, 868. [Google Scholar] [CrossRef]
- Patil, K.R.; Mohapatra, P.; Patel, H.M.; Goyal, S.N.; Ojha, S.; Kundu, C.N.; Patil, C.R. Pentacyclic Triterpenoids Inhibit IKKbeta Mediated Activation of NF-kappaB Pathway: In Silico and In Vitro Evidences. PLoS ONE 2015, 10, e0125709. [Google Scholar] [CrossRef]
- NA, J.C.F.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; Andre, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar]
- Borkova, L.; Frydrych, I.; Jakubcova, N.; Adamek, R.; Liskova, B.; Gurska, S.; Medvedikova, M.; Hajduch, M.; Urban, M. Synthesis and biological evaluation of triterpenoid thiazoles derived from betulonic acid, dihydrobetulonic acid, and ursonic acid. Eur. J. Med. Chem. 2020, 185, 111806. [Google Scholar] [CrossRef]
- Huang, R.Z.; Hua, S.X.; Liao, Z.X.; Huang, X.C.; Wang, H.S. Side chain-functionalized aniline-derived ursolic acid derivatives as multidrug resistance reversers that block the nuclear factor-kappa B (NF-kappa B) pathway and cell proliferation. MedChemComm 2017, 8, 1421–1434. [Google Scholar] [CrossRef]
- Wu, P.P.; Zhang, B.J.; Cui, X.P.; Yang, Y.; Jiang, Z.Y.; Zhou, Z.H.; Zhong, Y.Y.; Mai, Y.Y.; Ouyang, Z.; Chen, H.S.; et al. Synthesis and biological evaluation of novel ursolic acid analogues as potential alpha-glucosidase inhibitors. Sci. Rep. 2017, 7, 45578. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.; Lee, S.Y. Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid. Biomolecules 2020, 10, 1505. https://doi.org/10.3390/biom10111505
Son J, Lee SY. Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid. Biomolecules. 2020; 10(11):1505. https://doi.org/10.3390/biom10111505
Chicago/Turabian StyleSon, Juhyeon, and Sang Yeol Lee. 2020. "Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid" Biomolecules 10, no. 11: 1505. https://doi.org/10.3390/biom10111505
APA StyleSon, J., & Lee, S. Y. (2020). Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid. Biomolecules, 10(11), 1505. https://doi.org/10.3390/biom10111505





