MMP-8, TRAP-5, and OPG Levels in GCF Diagnostic Potential to Discriminate between Healthy Patients’, Mild and Severe Periodontitis Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Assessments
2.2. GCF Samples
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2000 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl 1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl 1), S74–S84. [Google Scholar] [CrossRef]
- Goodson, J.M.; Haffajee, A.D.; Socransky, S.S.; Kent, R.; Teles, R.; Hasturk, H.; Bogren, A.; Van Dyke, T.; Wennstrom, J.; Lindhe, J. Control of periodontal infections: A randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. J. Clin. Periodontol. 2012, 39, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl 2), 6–16. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. The complete periodontal examination. Periodontology 2000 2004, 34, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl 20), S149–S161. [Google Scholar] [CrossRef]
- Leppilahti, J.M.; Hernandez-Rios, P.A.; Gamonal, J.A.; Tervahartiala, T.; Brignardello-Petersen, R.; Mantyla, P.; Sorsa, T.; Hernandez, M. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J. Clin. Periodontol. 2014, 41, 348–356. [Google Scholar] [CrossRef]
- Baeza, M.; Garrido, M.; Hernandez-Rios, P.; Dezerega, A.; Garcia-Sesnich, J.; Strauss, F.; Aitken, J.P.; Lesaffre, E.; Vanbelle, S.; Gamonal, J.; et al. Diagnostic accuracy for apical and chronic periodontitis biomarkers in gingival crevicular fluid: An exploratory study. J. Clin. Periodontol. 2016, 43, 34–45. [Google Scholar] [CrossRef]
- Emingil, G.; Han, B.; Gurkan, A.; Berdeli, A.; Tervahartiala, T.; Salo, T.; Pussinen, P.J.; Kose, T.; Atilla, G.; Sorsa, T. Matrix metalloproteinase (MMP)-8 and tissue inhibitor of MMP-1 (TIMP-1) gene polymorphisms in generalized aggressive periodontitis: Gingival crevicular fluid MMP-8 and TIMP-1 levels and outcome of periodontal therapy. J. Periodontol. 2014, 85, 1070–1080. [Google Scholar] [CrossRef]
- Leppilahti, J.M.; Sorsa, T.; Kallio, M.A.; Tervahartiala, T.; Emingil, G.; Han, B.; Mantyla, P. The utility of gingival crevicular fluid matrix metalloproteinase-8 response patterns in prediction of site-level clinical treatment outcome. J. Periodontol. 2015, 86, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.S.; Morelli, T.; Oh, M.; Braun, T.M.; Ramseier, C.A.; Sugai, J.V.; Giannobile, W.V. Crevicular fluid biomarkers and periodontal disease progression. J. Clin. Periodontol. 2014, 41, 113–120. [Google Scholar] [CrossRef]
- Buduneli, N.; Kinane, D.F. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J. Clin. Periodontol. 2011, 38 (Suppl 11), 85–105. [Google Scholar] [CrossRef]
- Dereka, X.E.; Markopoulou, C.E.; Fanourakis, G.; Tseleni-Balafouta, S.; Vrotsos, I.A. RANKL and OPG mRNA level after non-surgical periodontal treatment. Inflammation 2010, 33, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Analysis of gingival crevice fluid and risk of progression of periodontitis. Periodontology 2000 2004, 34, 109–119. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Kononen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology 2000 2016, 70, 142–163. [Google Scholar] [CrossRef]
- Baltacioglu, E.; Kehribar, M.A.; Yuva, P.; Alver, A.; Atagun, O.S.; Karabulut, E.; Akalin, F.A. Total oxidant status and bone resorption biomarkers in serum and gingival crevicular fluid of patients with periodontitis. J. Periodontol. 2014, 85, 317–326. [Google Scholar] [CrossRef]
- Salinas-Munoz, M.; Garrido-Flores, M.; Baeza, M.; Huaman-Chipana, P.; Garcia-Sesnich, J.; Bologna, R.; Vernal, R.; Hernandez, M. Bone resorptive activity in symptomatic and asymptomatic apical lesions of endodontic origin. Clin. Oral. Investig. 2017, 21, 2613–2618. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Wuyts, A.; Husson, S.J.; Proost, P.; Van Damme, J.; Opdenakker, G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 2003, 270, 3739–3749. [Google Scholar] [CrossRef]
- Hernandez, M.; Martinez, B.; Tejerina, J.M.; Valenzuela, M.A.; Gamonal, J. MMP-13 and TIMP-1 determinations in progressive chronic periodontitis. J. Clin. Periodontol. 2007, 34, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl 20), S68–S77. [Google Scholar] [CrossRef]
- Hernandez Rios, M.; Sorsa, T.; Obregon, F.; Tervahartiala, T.; Valenzuela, M.A.; Pozo, P.; Dutzan, N.; Lesaffre, E.; Molas, M.; Gamonal, J. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: Initial evidence for MMP-13/MMP-9 activation cascade. J. Clin. Periodontol. 2009, 36, 1011–1017. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Kononen, E.; Huumonen, S.; Tervahartiala, T.; Pussinen, P.J.; Suominen, A.L.; Sorsa, T. Salivary type I collagen degradation end-products and related matrix metalloproteinases in periodontitis. J. Clin. Periodontol. 2013, 40, 18–25. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Kononen, E.; Pradhan-Palikhe, P.; Tervahartiala, T.; Pussinen, P.J.; Suominen-Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomas, I. Accuracy of single molecular biomarkers in gingival crevicular fluid for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, A.M.; Meschiari, C.A.; Zuardi, L.R.; de Sousa, T.S.; Taba, M., Jr.; Teofilo, J.M.; Jacob-Ferreira, A.L.; Tanus-Santos, J.E.; Novaes, A.B., Jr.; Gerlach, R.F. Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J. Clin. Periodontol. 2010, 37, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Raisanen, I.T.; Parnanen, P.; Nwhator, S.O.; Gieselmann, D.R.; Sakellari, D. Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Kinney, J.S.; Herr, A.E.; Braun, T.; Sugai, J.V.; Shelburne, C.A.; Rayburn, L.A.; Tran, H.M.; Singh, A.K.; Giannobile, W.V. Identification of pathogen and host-response markers correlated with periodontal disease. J. Periodontol. 2009, 80, 436–446. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Bostanci, N.; Ilgenli, T.; Emingil, G.; Afacan, B.; Han, B.; Toz, H.; Berdeli, A.; Atilla, G.; McKay, I.J.; Hughes, F.J.; et al. Differential expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin mRNA in periodontal diseases. J. Periodontal. Res. 2007, 42, 287–293. [Google Scholar] [CrossRef]
- Bostanci, N.; Ilgenli, T.; Emingil, G.; Afacan, B.; Han, B.; Toz, H.; Atilla, G.; Hughes, F.J.; Belibasakis, G.N. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: Implications of their relative ratio. J. Clin. Periodontol. 2007, 34, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Otogoto, J.; Ota, N.; Togari, A. Differential expression of RANKL and osteoprotegerin in gingival crevicular fluid of patients with periodontitis. J. Dent. Res. 2004, 83, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Buduneli, E.; Kutukculer, N. Interleukin-17, RANKL, and osteoprotegerin levels in gingival crevicular fluid from smoking and non-smoking patients with chronic periodontitis during initial periodontal treatment. J. Periodontol. 2009, 80, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Biyikoglu, B.; Sherrabeh, S.; Lappin, D.F. Saliva concentrations of RANKL and osteoprotegerin in smoker versus non-smoker chronic periodontitis patients. J. Clin. Periodontol. 2008, 35, 846–852. [Google Scholar] [CrossRef]
- Pozo, P.; Valenzuela, M.A.; Melej, C.; Zaldivar, M.; Puente, J.; Martinez, B.; Gamonal, J. Longitudinal analysis of metalloproteinases, tissue inhibitors of metalloproteinases and clinical parameters in gingival crevicular fluid from periodontitis-affected patients. J. Periodontal. Res. 2005, 40, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ingman, T.; Tervahartiala, T.; Ding, Y.; Tschesche, H.; Haerian, A.; Kinane, D.F.; Konttinen, Y.T.; Sorsa, T. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J. Clin. Periodontol. 1996, 23, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjaderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral. Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef]
- De Morais, E.F.; Pinheiro, J.C.; Leite, R.B.; Santos, P.P.A.; Barboza, C.A.G.; Freitas, R.A. Matrix metalloproteinase-8 levels in periodontal disease patients: A systematic review. J. Periodontal. Res. 2018, 53, 156–163. [Google Scholar] [CrossRef]
- Lopez, R.; Smith, P.C.; Gostemeyer, G.; Schwendicke, F. Ageing, dental caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl 18), S145–S152. [Google Scholar] [CrossRef]
- Gamonal, J.; Mendoza, C.; Espinoza, I.; Munoz, A.; Urzua, I.; Aranda, W.; Carvajal, P.; Arteaga, O. Clinical attachment loss in Chilean adult population: First Chilean National Dental Examination Survey. J. Periodontol. 2010, 81, 1403–1410. [Google Scholar] [CrossRef]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018, 45 (Suppl 20), S130–S148. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Leppilahti, J.M.; Kallio, M.A.; Tervahartiala, T.; Sorsa, T.; Mantyla, P. Gingival crevicular fluid matrix metalloproteinase-8 levels predict treatment outcome among smokers with chronic periodontitis. J. Periodontol. 2014, 85, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Mantyla, T. Assessing absentmindedness: Prospective memory complaint and impairment in middle-aged adults. Mem. Cognit. 2003, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.H.; Fitzsimmons, T.R.; Bartold, P.M. Effect of smoking on concentrations of receptor activator of nuclear factor kappa B ligand and osteoprotegerin in human gingival crevicular fluid. J. Clin. Periodontol. 2009, 36, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Bunaes, D.F.; Mustafa, M.; Mohamed, H.G.; Lie, S.A.; Leknes, K.N. The effect of smoking on inflammatory and bone remodeling markers in gingival crevicular fluid and subgingival microbiota following periodontal therapy. J. Periodontal. Res. 2017, 52, 713–724. [Google Scholar] [CrossRef]
- Ozcaka, O.; Nalbantsoy, A.; Kose, T.; Buduneli, N. Plasma osteoprotegerin levels are decreased in smoker chronic periodontitis patients. Aust. Dent. J. 2010, 55, 405–410. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl 20), S162–S170. [Google Scholar] [CrossRef]
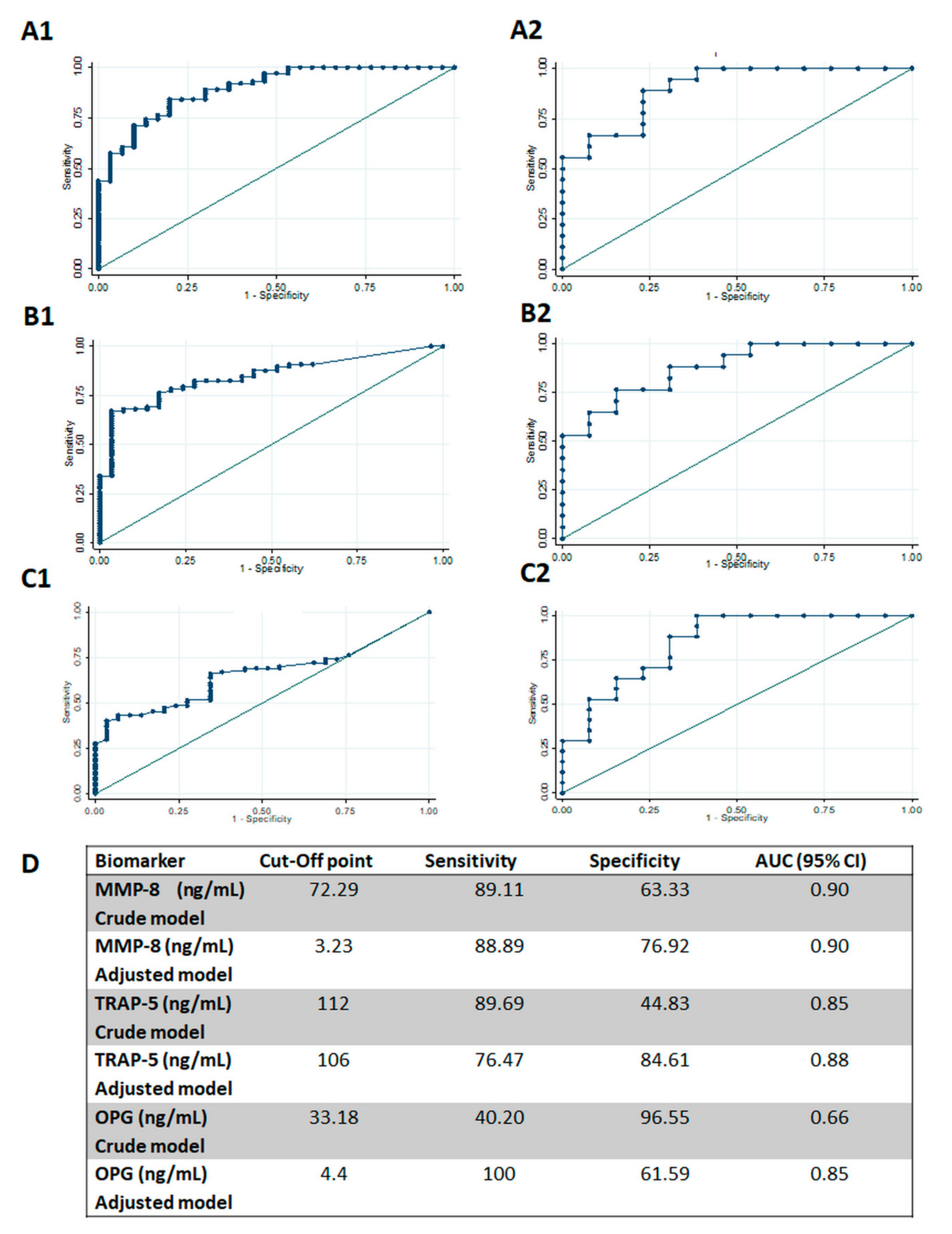
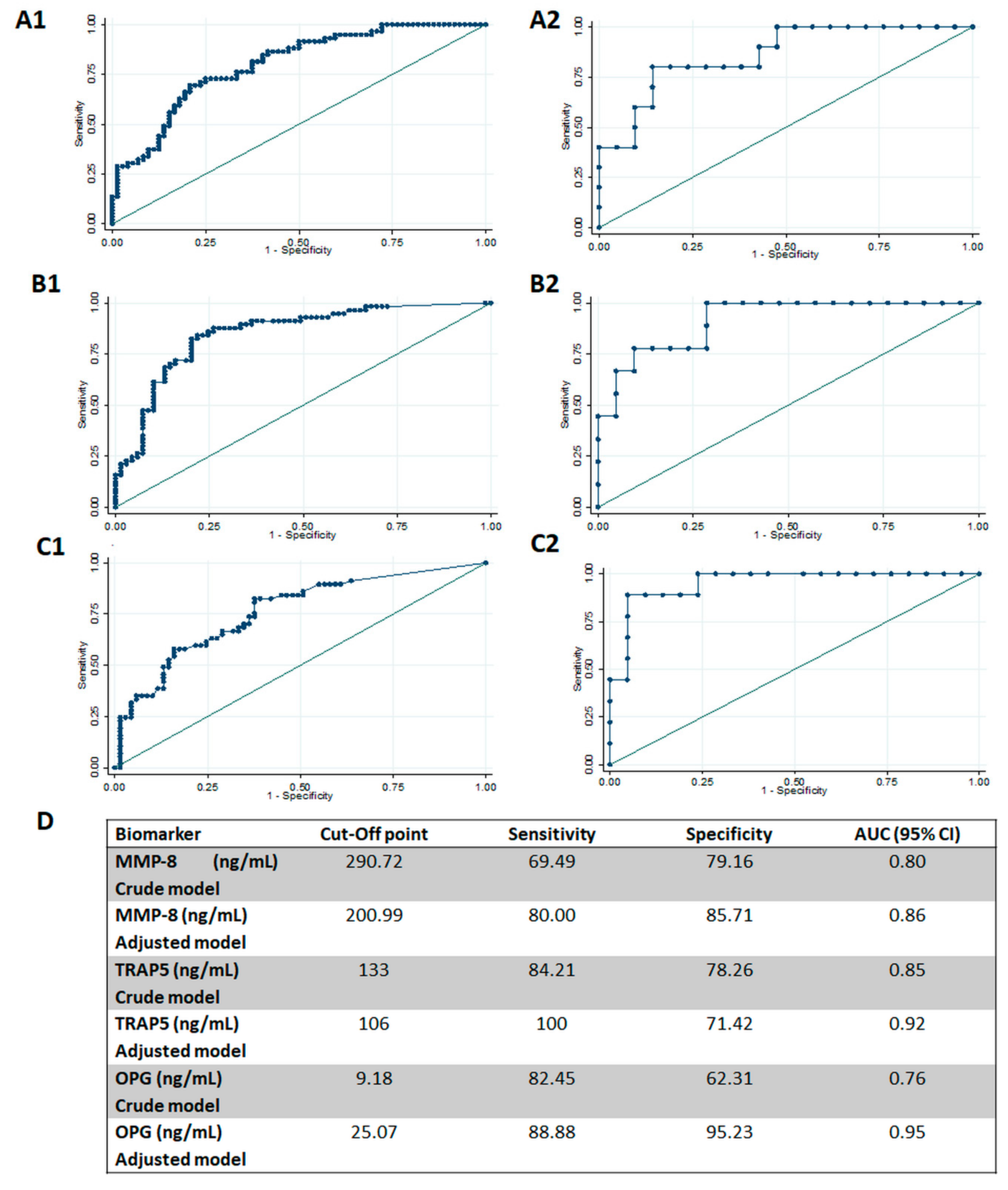
| Parameter | Healthy (n = 13) | Periodontitis (n = 18) | p |
|---|---|---|---|
| Age | 43.7 ± 14.0 | 54.1 ± 8.5 | 0.02 |
| Gender (females) | 7 | 12 | >0.05 |
| Smoking | 2 | 3 | >0.05 |
| Parameter | H (n = 30) | M (n = 42) | S (n = 59) | Overall p | H v/s M p | H v/s S p |
M v/s S p |
|---|---|---|---|---|---|---|---|
| PPD (mm) | 2.2 ± 0.40 | 2.83 ± 1.20 | 6.25 ± 1.80 | 0.000 | 0.187 | 0.000 | 0.000 |
| CAL (mm) | 1.63 ± 0.49 | 2.78 ± 1.25 | 7.42 ± 2.11) | 0.000 | 0.010 | 0.000 | 0.000 |
| BOP n (%) | 0 | 30 (71%) | 49 (83%) | 0.000 | 0.000 | 0.000 | 0.346 |
| MMP-8 (ng/mL) | 60.49 ± 95.67 | 270.82 ± 238.96 | 464.2 ± 281.31 | 0.000 | 0.001 | 0.000 | 0.000 |
| TRAP-5 (ng/mL) | 56.79 ± 65.73 | 177.89 ± 221.46 | 478.59 ± 422.73 | 0.000 | 0.345 | 0.000 | 0.000 |
| OPG (ng/mL) | 13.12 ± 11.49 | 23.24 ± 43.18 | 53.3 ± 57.88 | 0.000 | 1.000 | 0.001 | 0.006 |
| Healthy Sites (H v/s M and S) | Crude Model | Adjusted Model | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| MMP-8 | 1.011 (1.005–1.016) | 0.000 | 1.009 (1.001–1.018) | 0.021 |
| Intercept | 0.614 (0.316–1.192) | 0.150 | 0.015 (0.00–2.525) | 0.109 |
| TRAP5 | 1.014 (1.006–1.022) | 0.001 | 1.017 (0.996–1.039) | 0.096 |
| Intercept | 0.646 (0.316–1.322) | 0.232 | 0.013 (0.001–1.708) | 0.081 |
| OPG | 1.036 (1.009–1.064) | 0.009 | 1.072 (1.000–1.150) | 0.050 |
| Intercept | 1.561 (0.866–2.812) | 0.138 | 0.006 (0.000–0.976) | 0.049 |
| Severe Sites (H and M v/s S) | Crude Model | Adjusted Model | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| MMP-8 | 1.004 (1.002–1.005) | 0.000 | 1.005 (1.000–1.010) | 0.018 |
| Intercept | 0.229 (0.124–0.423) | 0.000 | 0.010 (0.000–6.103) | 0.160 |
| TRAP5 | 1.005 (1.002–1.007) | 0.000 | 1.009 (1.000–1.019) | 0.039 |
| Intercept | 0.236 (0.128–0.435) | 0.000 | 0.021 (0.000–18.763) | 0.266 |
| OPG | 1.023 (1.009–1.037) | 0.001 | 0.146 (1.020–1.287) | 0.021 |
| Intercept | 0.410 (0.245–0.687) | 0.001 | 0.000 (2.66e−09–8.165) | 0.113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, M.; Baeza, M.; Contreras, J.; Sorsa, T.; Tervahartiala, T.; Valdés, M.; Chaparro, A.; Hernández-Ríos, P. MMP-8, TRAP-5, and OPG Levels in GCF Diagnostic Potential to Discriminate between Healthy Patients’, Mild and Severe Periodontitis Sites. Biomolecules 2020, 10, 1500. https://doi.org/10.3390/biom10111500
Hernández M, Baeza M, Contreras J, Sorsa T, Tervahartiala T, Valdés M, Chaparro A, Hernández-Ríos P. MMP-8, TRAP-5, and OPG Levels in GCF Diagnostic Potential to Discriminate between Healthy Patients’, Mild and Severe Periodontitis Sites. Biomolecules. 2020; 10(11):1500. https://doi.org/10.3390/biom10111500
Chicago/Turabian StyleHernández, Marcela, Mauricio Baeza, Johanna Contreras, Timo Sorsa, Taina Tervahartiala, Macarena Valdés, Alejandra Chaparro, and Patricia Hernández-Ríos. 2020. "MMP-8, TRAP-5, and OPG Levels in GCF Diagnostic Potential to Discriminate between Healthy Patients’, Mild and Severe Periodontitis Sites" Biomolecules 10, no. 11: 1500. https://doi.org/10.3390/biom10111500
APA StyleHernández, M., Baeza, M., Contreras, J., Sorsa, T., Tervahartiala, T., Valdés, M., Chaparro, A., & Hernández-Ríos, P. (2020). MMP-8, TRAP-5, and OPG Levels in GCF Diagnostic Potential to Discriminate between Healthy Patients’, Mild and Severe Periodontitis Sites. Biomolecules, 10(11), 1500. https://doi.org/10.3390/biom10111500







