HIV-Infected Patients: Cross Site-Specific Hydrolysis of H2a and H2b Histones and Myelin Basic Protein with Antibodies against These Three Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Donors, and Patients
2.2. Antibody Purification
2.3. Affinity Chromatography of IgGs on MBP- and Histone-Sepharose
2.4. ELISA of Anti-MBP and Anti-Histones Autoantibodies
2.5. Proteolytic Activity Assay
2.6. Kinetic Analysis
2.7. MALDI-TOF Analysis of Ab-Dependent Proteins Hydrolysis
2.8. Analysis of Sequence Homology
2.9. Statistical Analysis
3. Results
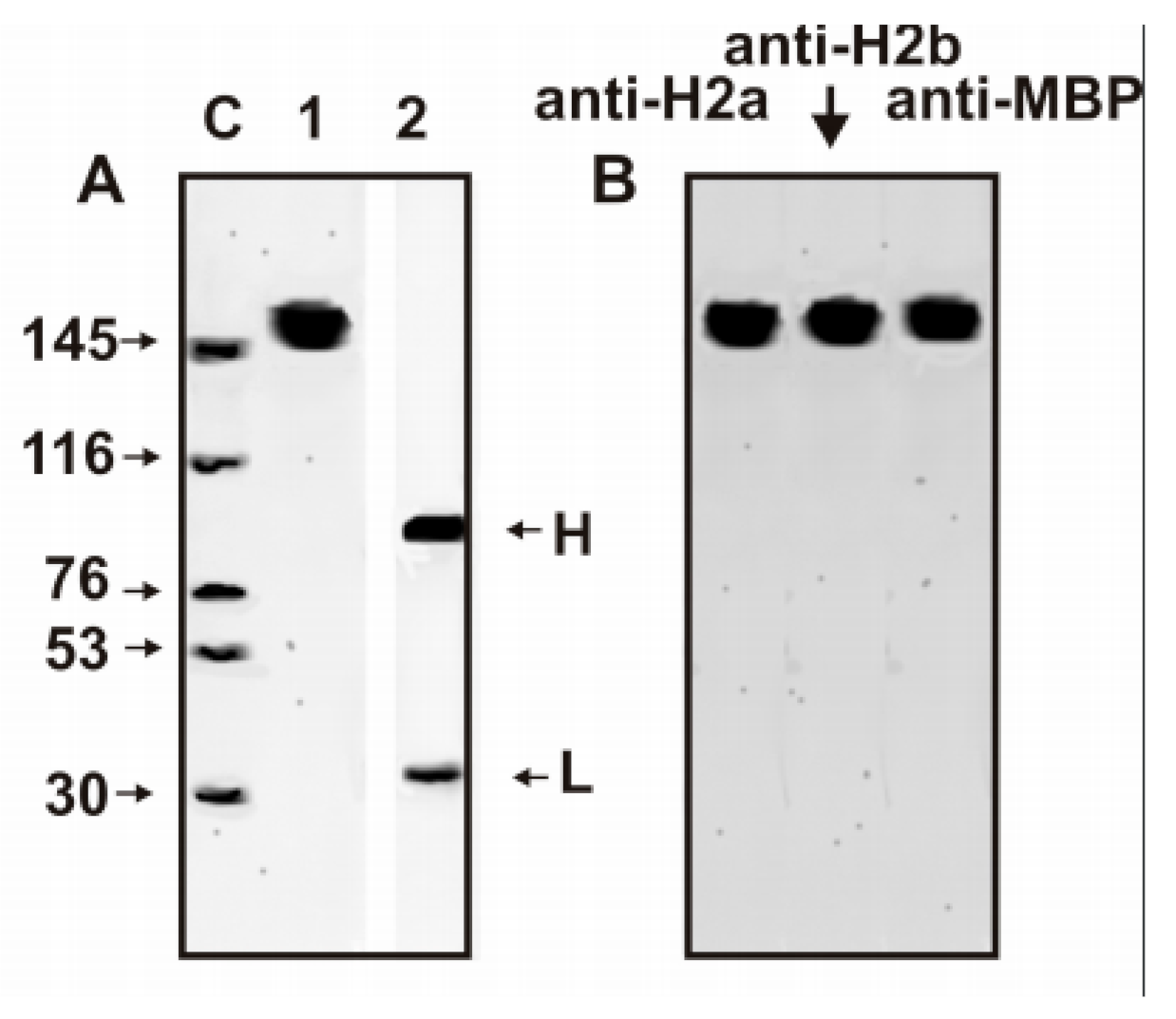
3.1. Purification of Antibodies
3.2. ELISA of Anti-MBP and Anti-Histones Autoantibodies
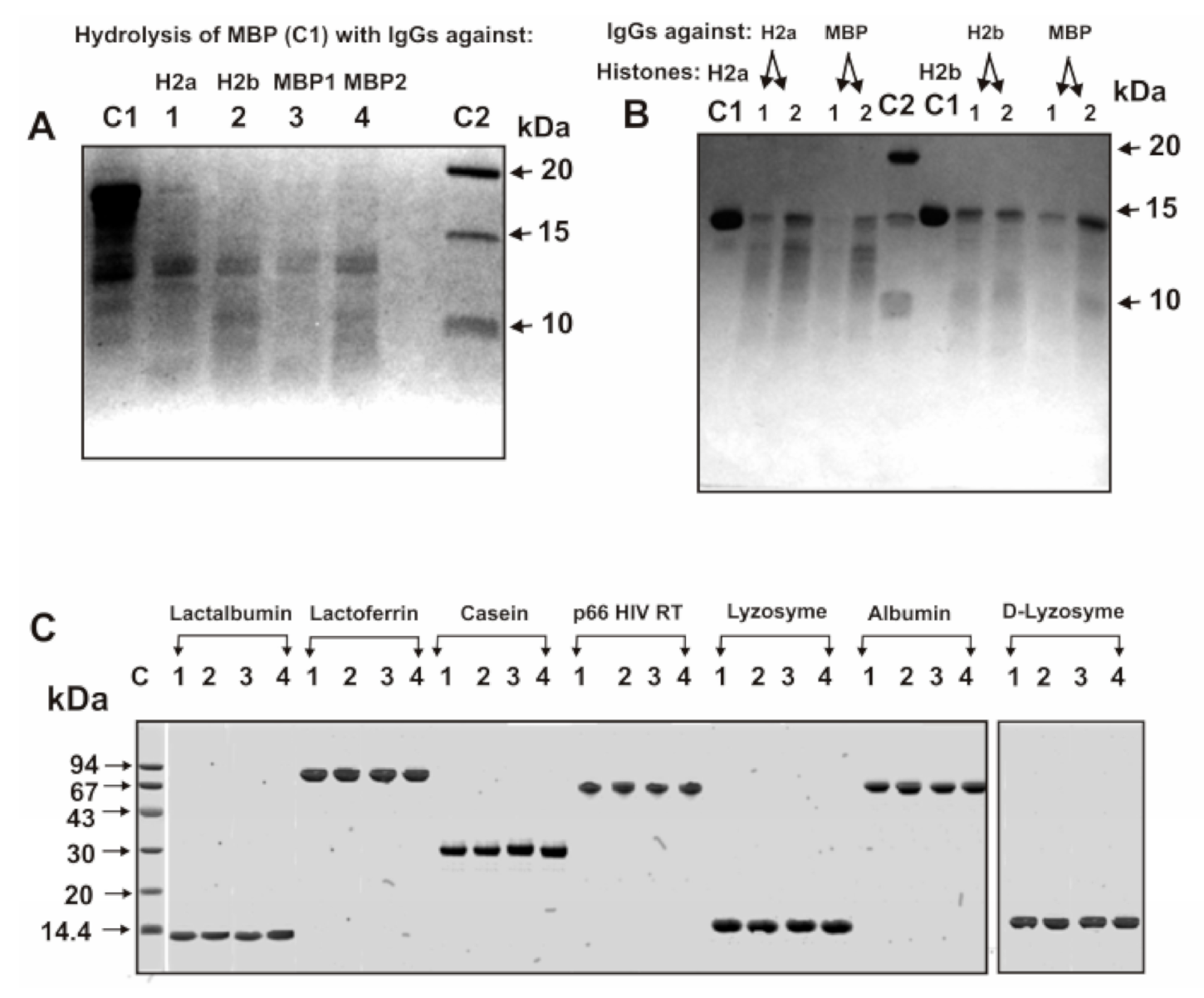
3.3. SDS-PAGE Analysis of Catalytic Cross-Reactivity
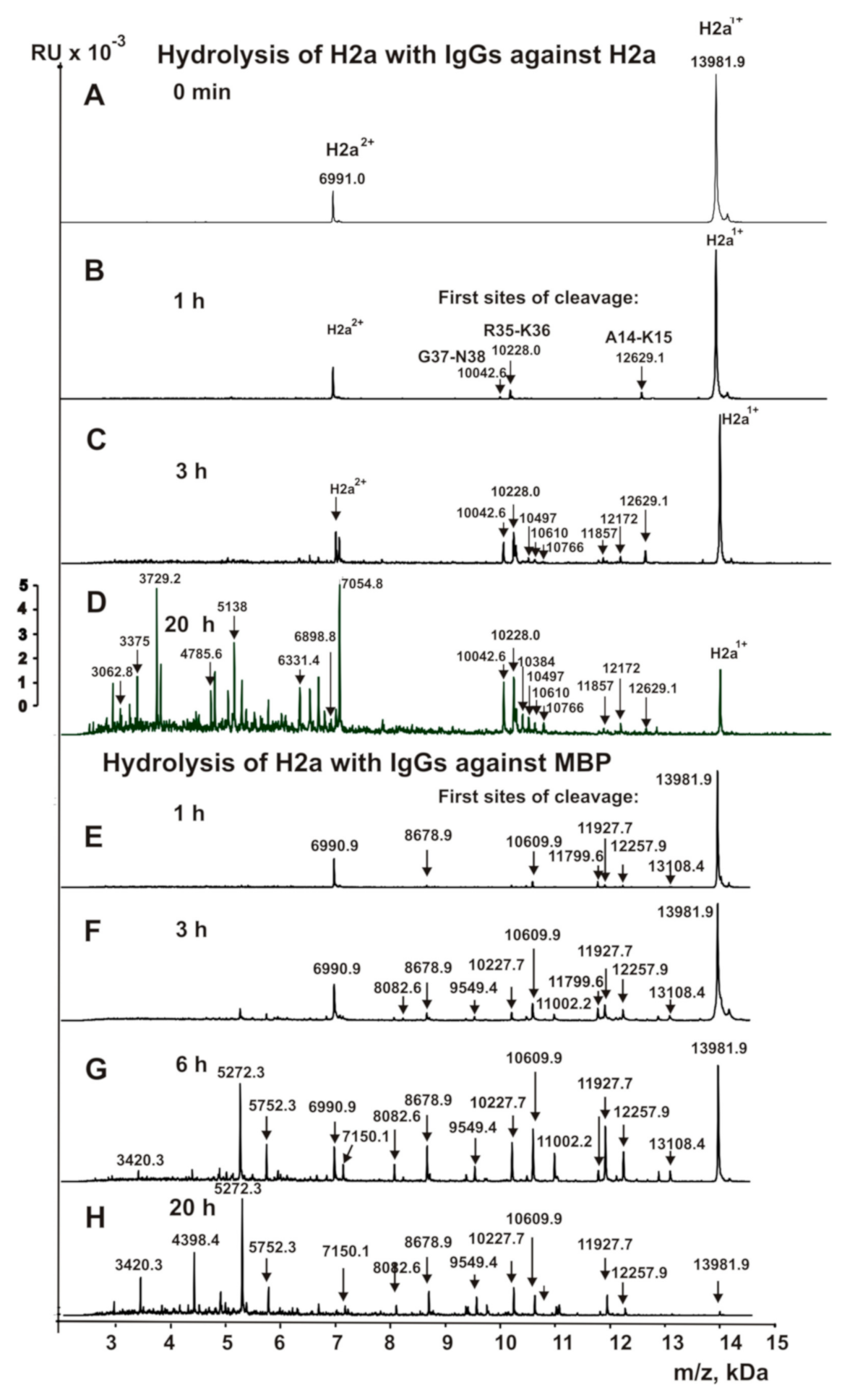
3.4. Analysis of Site-Specificity of H2a Histone Hydrolysis
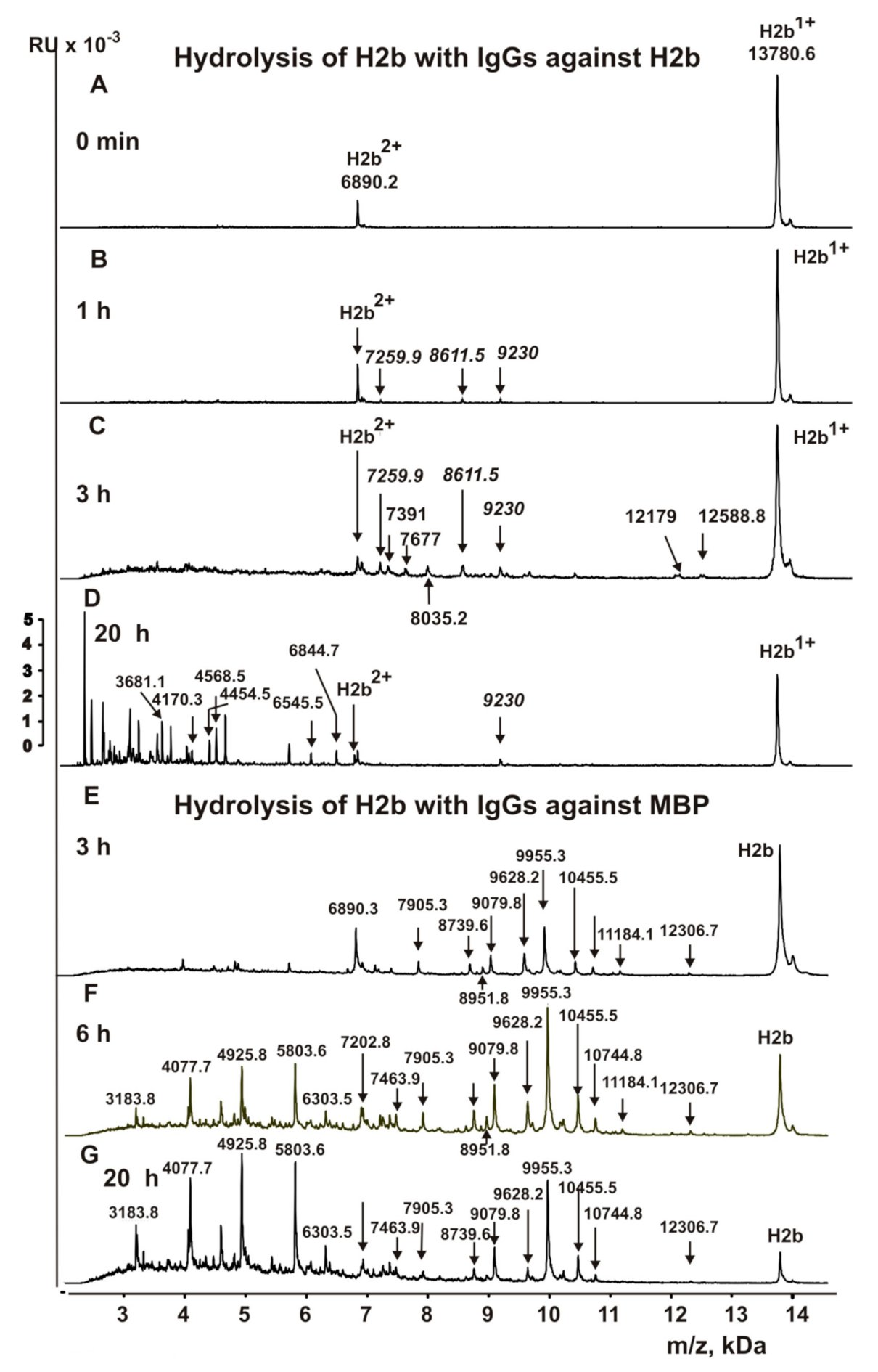
3.5. Analysis of Site-Specificity of H2b Histone Hydrolysis with Abzymes
3.6. Analysis of Possible Homology of H2a and H2b Histones with MBP
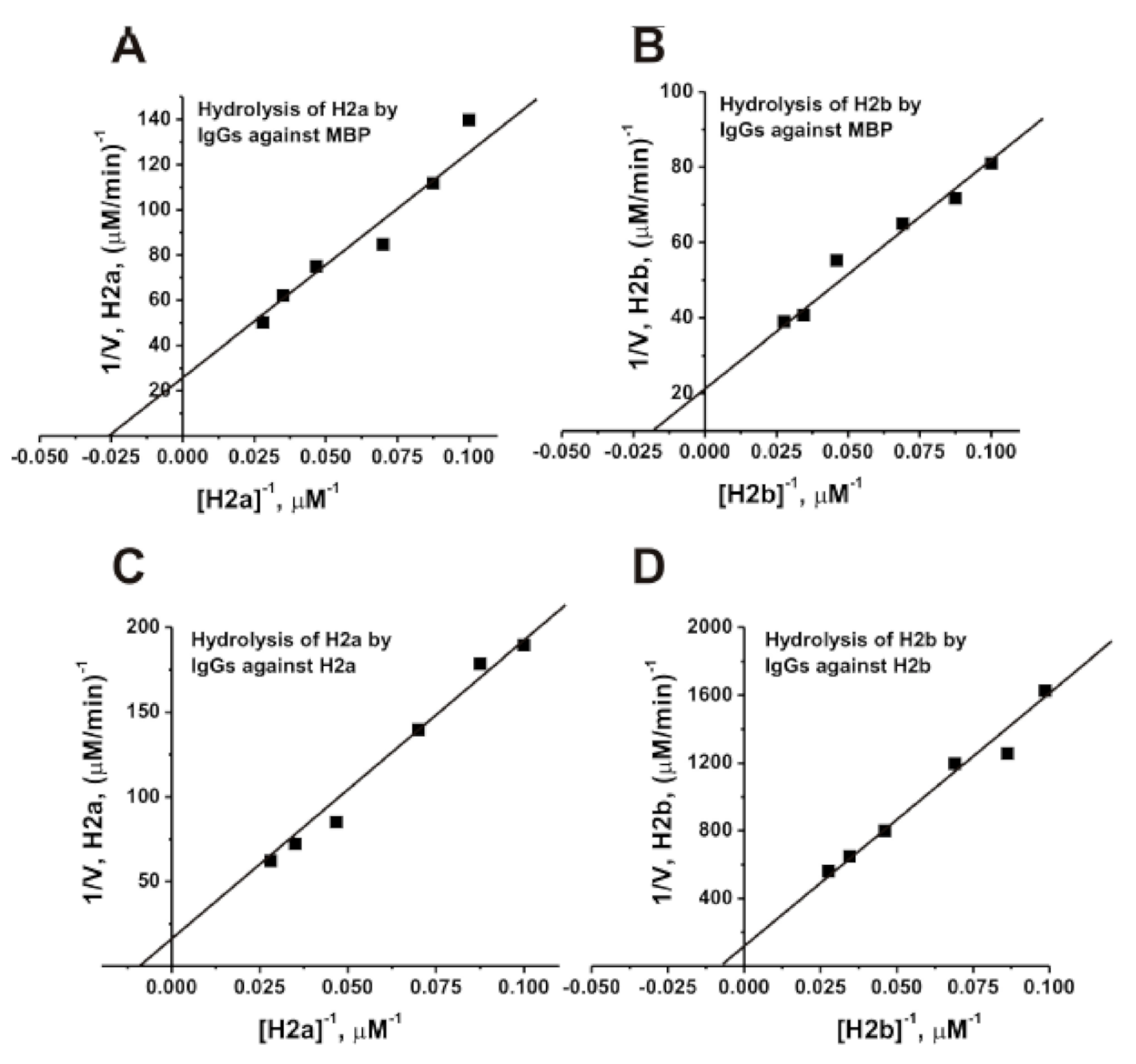
3.7. Affinity of IgGs for Histones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | amino acid |
| Ab | antibody |
| Abz | abzyme |
| AI | autoimmune |
| AIDS | acquired immune deficiency syndrome |
| GL | generalized lymphoadenopathy |
| AGDs | antigenic determinants |
| HIV-1 | human immunodeficiency virus type 1 |
| OP | oligopeptide |
| MBP | myelin basic protein |
| MALDI-TOF | matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MS | multiple sclerosis |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SLE | systemic lupus erythematosus |
References
- Lerner, R.A.; Tramontano, A. Antibodies as enzymes. Trends Bioch. Sci. 1987, 12, 427–438. [Google Scholar] [CrossRef]
- Schultz, P.G.; Lerner, R.A. From molecular diversity to catalysis: Lessons from the immune system. Science 1995, 269, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Keinan, E. (Ed.) Catalytic Antibodies; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2005; 586p. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural catalytic antibodies–abzymes. In Catalytic Antibodies; Keinan, E., Ed.; VCH-Wiley Press: Weinheim, Germany, 2005; pp. 505–569. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In Autoimmune Diseases: Symptoms, Diagnosis and Treatment; Brenner, K.J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 1–107. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century; Kasenga, F.H., Ed.; InTech: Rijeka, Croatia, 2011; pp. 151–192. [Google Scholar]
- Nevinsky, G.A. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In Multiple Sclerosis; Conzalez-Quevedo, A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 100–147. [Google Scholar]
- Nevinsky, G.A. Catalytic antibodies in norm and systemic lupus erythematosus. In Lupus; Khan, W.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 41–101. [Google Scholar]
- Kalaga, R.; Li, L.; O’Dell, J.R.; Paul, S. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheumatoid arthritis. J. Immunol. 1995, 155, 2695–2702. [Google Scholar] [PubMed]
- Paul, S.; Volle, D.J.; Beach, C.M.; Johnson, D.R.; Powell, M.J.; Massey, R.J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science 1989, 244, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Desmazes, S.; Moreau, A.; Sooryanarayana; Bonnemain, C.; Stieltjes, N.; Pashov, A.; Sultan, Y.; Hoebeke, J.; Kazatchkine, M.D.; Kaveri, S.V. Catalytic activity of antibodies against factor VIII in patients with hemophilia A. Nat. Med. 1999, 5, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, P.; Dannenbring, R.; Matsuura, K.; Tramontano, A.; Gololobov, G.; Paul, S. Monoclonal antibody light chain with prothrombinase activity. Biochemistry 2000, 39, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J. Cell Mol. Med. 2004, 8, 359–368. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Nevinsky, G.A.; Favorova, O.O. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol. Lett. 2006, 103, 75–81. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Buneva, V.N.; Doronin, B.M.; Tyshkevich, O.B.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by IgM and IgA antibodies from the sera of patients with multiple sclerosis. Med. Sci. Monit. 2005, 11, BR266–BR272. [Google Scholar]
- Savel’ev, A.N.; Eneyskaya, E.V.; Shabalin, K.A.; Filatov, M.V.; Neustroev, K.N. Antibodies with amylolytic activity. Protein Pept. Lett. 1999, 6, 179–181. [Google Scholar]
- Paul, S.; Planque, S.A.; Nishiyama, Y.; Hanson, C.V.; Massey, R.J. Nature and nurture of catalytic antibodies. Adv. Exp. Med. Biol. 2012, 750, 56–75. [Google Scholar] [PubMed]
- Planque, S.A.; Nishiyama, Y.; Hara, M.; Sonoda, S.; Murphy, S.K.; Watanabe, K.; Mitsuda, Y.; Brown, E.L.; Massey, R.J.; Primmer, S.R.; et al. Physiological IgM class catalytic antibodies selective for transthyretin amyloid. J. Biol. Chem. 2014, 289, 13243–13258. [Google Scholar] [CrossRef] [PubMed]
- Bezuglova, A.V.; Konenkova, L.P.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity and metal-dependence of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. J. Mol. Recognit. 2011, 24, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Bezuglova, A.M.; Dmitrenok, P.S.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. Multiple sites of the cleavage of 17- and 19-mer encephalytogenic oligopeptides corresponding to human myelin basic protein (MBP) by specific anti-MBP antibodies from patients with systemic lupus erythematosus. Peptides 2012, 37, 69–78. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Dmitrenok, P.S.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. Multiple sites of the cleavage of 21- and 25-mer encephalytogenic oligopeptides corresponding to human myelin basic protein (MBP) by specific anti-MBP antibodies from patients with systemic systemic lupus erythematosus. PLoS ONE 2013, 8, e51600. [Google Scholar] [CrossRef][Green Version]
- Parshukova, D.; Smirnova, L.P.; Ermakov, E.A.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Autoimmunity and immune system dysregulation in schizophrenia: IgGs from sera of patients hydrolyze myelin basic protein. J. Mol. Recognit. 2019, 32, e2759. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Fan, X.-G.; Tang, D. Release and activity of histone in diseases. Cell Death Dis. 2014, 5, e1370. [Google Scholar] [CrossRef]
- Fournel, S.; Muller, S. Antinucleosome antibodies and T-cell response in systemic lupus erythematosus. Ann. Med. Interne 2002, 153, 513–519. [Google Scholar]
- Fauci, A.S.; Braunwald, E.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 17th ed.; McGraw-Hill Professional: New York, NY, USA, 2008. [Google Scholar]
- Ternynck, P.; Falanga, B.; Unterkircher, C.; Gregoire, J.; Da Silva, L.P.; Avrameas, S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudi. Int. Immunol. 1991, 3, 29–37. [Google Scholar] [CrossRef]
- Hentati, B.; Sato, M.N.; Payelle, B.; Avrameas, S.; Ternynck, T. Beneficial effect of polyclonal immunoglobulins from malaria-infected BALB/c mice on the lupus-like syndrome of (NZB x NZW)F1 mice. Eur. J. Immunol. 1994, 24, 8–15. [Google Scholar] [CrossRef]
- Matsiota-Bernard, P.; Mahana, W.; Avrameas, S.; Nauciel, C. Specific and natural antibody production during Salmonella typhimurium infection in genetically susceptible and resistant mice. Immunology 1993, 79, 375–380. [Google Scholar] [PubMed]
- Barzilai, O.; Ram, M.; Shoenfeld, Y. Viral infection can induce the production of autoantibodies. Curr. Opin. Rheumatol. 2007, 19, 636–643. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Shoenfeld, Y. HIV and autoimmunity. Autoimmun. Rev. 2002, 1, 329–337. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Kharitonova, M.A.; Baranovskii, A.G.; Siziakina, L.P.; Buneva, V.N.; Nevinsky, G.A. DNA-hydrolyzing IgG antibodies from the blood of patients with acquired immune deficiency syndrome. Mol. Biol. 2006, 40, 857–864. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Buneva, V.N.; Nevinsky, G.A. Autoantibodies in HIV-infected patients: Cross site-specific hydrolysis of H1 histone and myelin basic protein. Biofactors 2019, 45, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies from the sera of HIV-infected patients efficiently hydrolyze all human histones. J. Mol. Recognit. 2016, 29, 346–362. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H1 histone from the sera of HIV-infected patients recognize and catalyze site-specific degradation of this histone. J. Mol. Recognit. 2017, 30. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H2a and H2b histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. Mol. Biosyst. 2017, 13, 1090–1101. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrenok, P.S.; Zubkova, A.D.; Ivanisenko, N.V.; Odintsova, E.S.; Buneva, V.N.; Nevinsky, G.A. Antibodies against H3 and H4 histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. J. Mol. Recognit. 2018, 31, e2703. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing antibodies from sera of HIV-infected patients. Biochimie 2009, 91, 1081–1086. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing IgM antibodies from sera of HIV-infected patients. Int. Immunol. 2010, 22, 671–680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Odintsova, E.S.; Baranova, S.V.; Dmitrenok, P.S.; Rasskazov, V.A.; Calmels, C.; Parissi, V.; Andreola, M.L.; Buneva, V.N.; Zakharova, O.D.; Nevinsky, G.A. Antibodies to HIV integrase catalyze site-specific degradation of their antigen. Int. Immunol. 2011, 23, 601–612. [Google Scholar] [CrossRef][Green Version]
- Odintsova, E.S.; Baranova, S.V.; Dmitrenok, P.S.; Calmels, C.; Parissi, V.; Nevinsky, G.A. Anti-integrase abzymes from the sera of HIV-infected patients specifically hydrolyze integrase but nonspecifically cleave short oligopeptides. J. Mol. Recognit. 2012, 25, 193–207. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Kharitonova, M.A.; Baranovskii, A.G.; Siziakina, L.P.; Buneva, V.N.; Nevinsky, G.A. Proteolytic activity of IgG antibodies from blood of acquired immunodeficiency syndrome patients. Biochemistry 2006, 71, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; Cusick, M.F.; Fujinami, R.S. Role of pathogens in multiple sclerosis. Int. Rev. Immunol. 2014, 33, 266–283. [Google Scholar] [CrossRef]
- Andersen, O.; Lygner, P.E.; Bergstrom, T. Viral infections trigger multiple sclerosis relapses: A prospective seroepidemiological study. J. Neurol. 1993, 240, 417–422. [Google Scholar] [CrossRef]
- Fersht, A. Enzyme Structure and Mechanism, 2nd ed.; W H Freeman Co.: New York, NY, USA, 1985. [Google Scholar]
- Zhou, Z.H.; Tzioufas, A.G.; Notkins, A.L. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J. Autoimmun. 2007, 29, 219–228. [Google Scholar] [CrossRef] [PubMed]
- James, L.C.; Roversi, P.; Tawfik, D.S. Antibody multispecificity mediated by conformational diversity. Science 2003, 299, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- James, L.C.; Tawfik, D.S. Conformational diversity and protein evolution—A 60-year-old hypothesis revisited. Trends Biochem. Sci. 2003, 28, 361–368. [Google Scholar] [CrossRef]
- James, L.C.; Tawfik, D.S. The specificity of cross-reactivity: Promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Sci. 2003, 12, 2183–2193. [Google Scholar] [CrossRef]
- Nevinsky, G.A. Important role of weak interactions in long DNA and RNA molecule recognition by enzymes. Mol. Biol. 1995, 29, 6–19. [Google Scholar]
- Nevinsky, G.A. Structural, thermodynamic, and kinetic basis of DNA and RNA-dependent enzymes functioning: Important role of weak nonspecific additive interactions between enzymes and long nucleic acids for their recognition and transformation. In Protein Structures: Kaleidoscope of Structural Properties and Functions; Uversky, V.N., Ed.; Research Signpost: Kerala, India, 2003; pp. 133–222. [Google Scholar]
- Nevinsky, G.A. Structural, thermodynamic, and kinetic basis for the activities of some nucleic acid repair enzymes. J. Mol. Recognit. 2011, 24, 656–677. [Google Scholar] [CrossRef] [PubMed]
- Andreev, S.L.; Buneva, V.N.; Nevinsky, G.A. How human IgGs against DNA recognize oligonucleotides and DNA. J. Mol. Recognit. 2016, 29, 596–610. [Google Scholar] [CrossRef]
- Belov, S.; Buneva, V.N.; Nevinsky, G.A. How human IgGs against myelin basic protein (MBP) recognize oligopeptides and MBP. J. Mol. Recognit. 2017, 30. [Google Scholar] [CrossRef] [PubMed]
- Kamholz, J.; de Ferra, F.; Pukkett, C.; Lazzarini, R. Indentification of three forms of human myelin basic protein by cDNA cloning, Proc. Natl. Acad. Sci. USA 1986, 83, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Deibler, G.E.; Boud, L.F.; Kies, M.W. Enzymatic and nonemzymatic degradation of myelin basic protein. Neurochem. Res. 1984, 9, 1371–1385. [Google Scholar] [CrossRef]
- O’Connor, K.C.; Bar-Or, A.; Hafler, D.A. The neuroimmunology of multiple sclerosis: Possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 2001, 21, 81–92. [Google Scholar] [CrossRef]
- Goldner, E.M.; Hsu, L.; Waraich, P.; Somers, J.M. Prevalence and incidence studies of schizophrenic disorders: A systematic review of the literature. Can. J. Psychiatry 2002, 47, 833–843. [Google Scholar] [CrossRef]
- Del Guerra, F.B.; Fonseca, J.L.; Figueiredo, V.M.; Ziff, E.B.; Konkiewitz, E.C. Human immunodeficiency virus-associated depression: Contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J. Neurovirol. 2013, 19, 314–327. [Google Scholar] [CrossRef]
- Corcoran, C.P. Neuropsychiatric changes in HIV/hepatitis C coinfected patients undergoing interferon therapy. J. Assoc. Nurses AIDS Care 2003, 14 (Suppl. 5), 80S–86S. [Google Scholar] [CrossRef]


| Substrate | Abs | KM, µM | kcat, min−1 |
|---|---|---|---|
| H2a | anti-MBP IgGs | 41.0 ± 7.0 | 0.15 ± 0.025 |
| H2b | anti-MBP IgGs | 59.0 ± 10.0 | 0.18 ± 0.03 |
| H2a | anti-H2a IgGs | 129.0 ± 19.0 | 0.33 ± 0.04 |
| H2b | anti-H2b IgGs | 160.0 ± 28.0 | 0.036 ± 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, S.V.; Dmitrienok, P.S.; Buneva, V.N.; Nevinsky, G.A. HIV-Infected Patients: Cross Site-Specific Hydrolysis of H2a and H2b Histones and Myelin Basic Protein with Antibodies against These Three Proteins. Biomolecules 2020, 10, 1501. https://doi.org/10.3390/biom10111501
Baranova SV, Dmitrienok PS, Buneva VN, Nevinsky GA. HIV-Infected Patients: Cross Site-Specific Hydrolysis of H2a and H2b Histones and Myelin Basic Protein with Antibodies against These Three Proteins. Biomolecules. 2020; 10(11):1501. https://doi.org/10.3390/biom10111501
Chicago/Turabian StyleBaranova, Svetlana V., Pavel S. Dmitrienok, Valentina N. Buneva, and Georgy A. Nevinsky. 2020. "HIV-Infected Patients: Cross Site-Specific Hydrolysis of H2a and H2b Histones and Myelin Basic Protein with Antibodies against These Three Proteins" Biomolecules 10, no. 11: 1501. https://doi.org/10.3390/biom10111501
APA StyleBaranova, S. V., Dmitrienok, P. S., Buneva, V. N., & Nevinsky, G. A. (2020). HIV-Infected Patients: Cross Site-Specific Hydrolysis of H2a and H2b Histones and Myelin Basic Protein with Antibodies against These Three Proteins. Biomolecules, 10(11), 1501. https://doi.org/10.3390/biom10111501







