Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Palmitate and DCI Preparation
2.3. MTT and Crystal Violet Assays
2.4. α-TC1-6 Treatment with DCI
2.5. Glucagon Secretion
2.6. Cell Lysis, Immunoprecipitation, and Western Blot Analysis
2.7. Statistical Analysis
3. Results
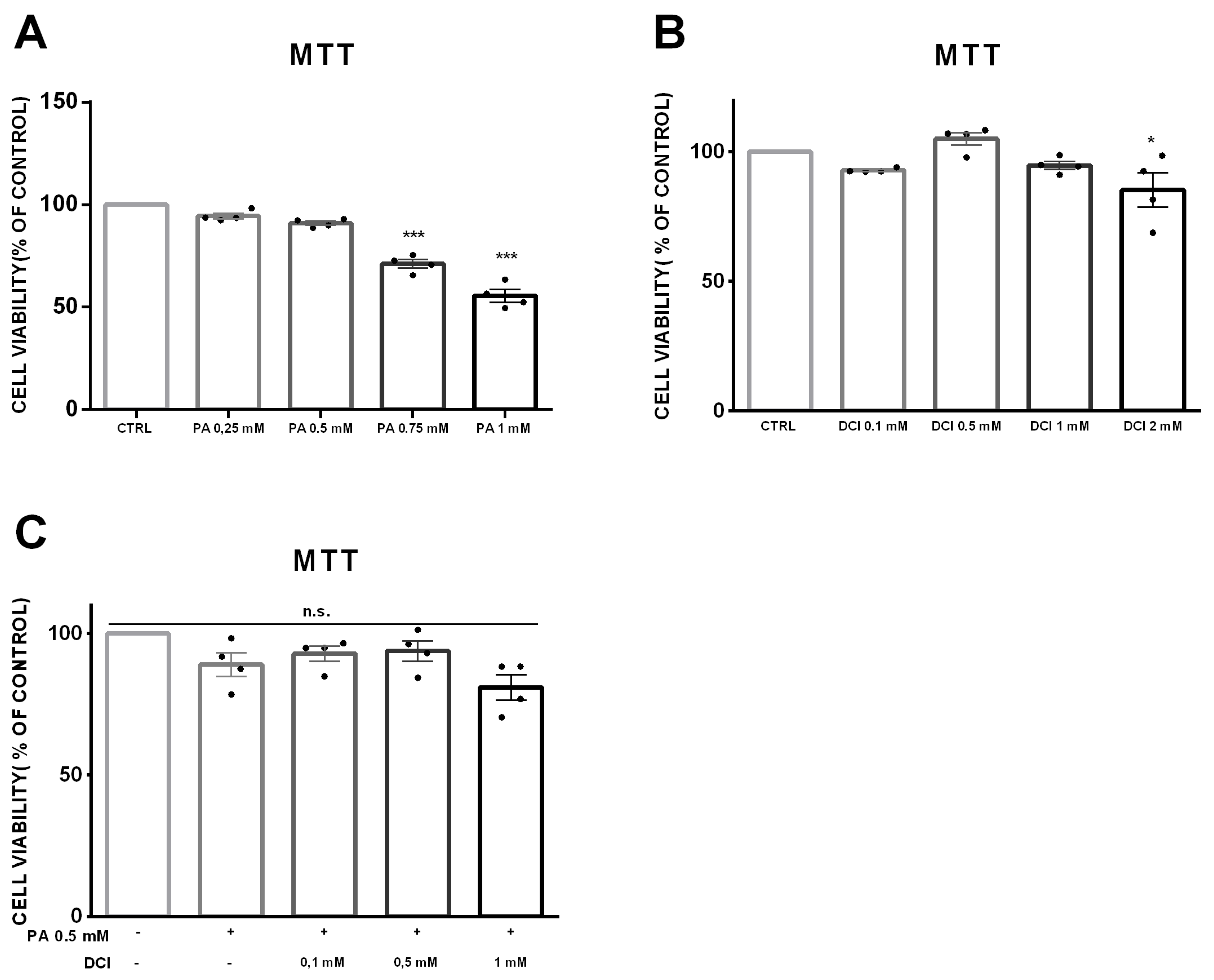
3.1. Cell Viability of α-TC1-6 Cells Treated with DCI
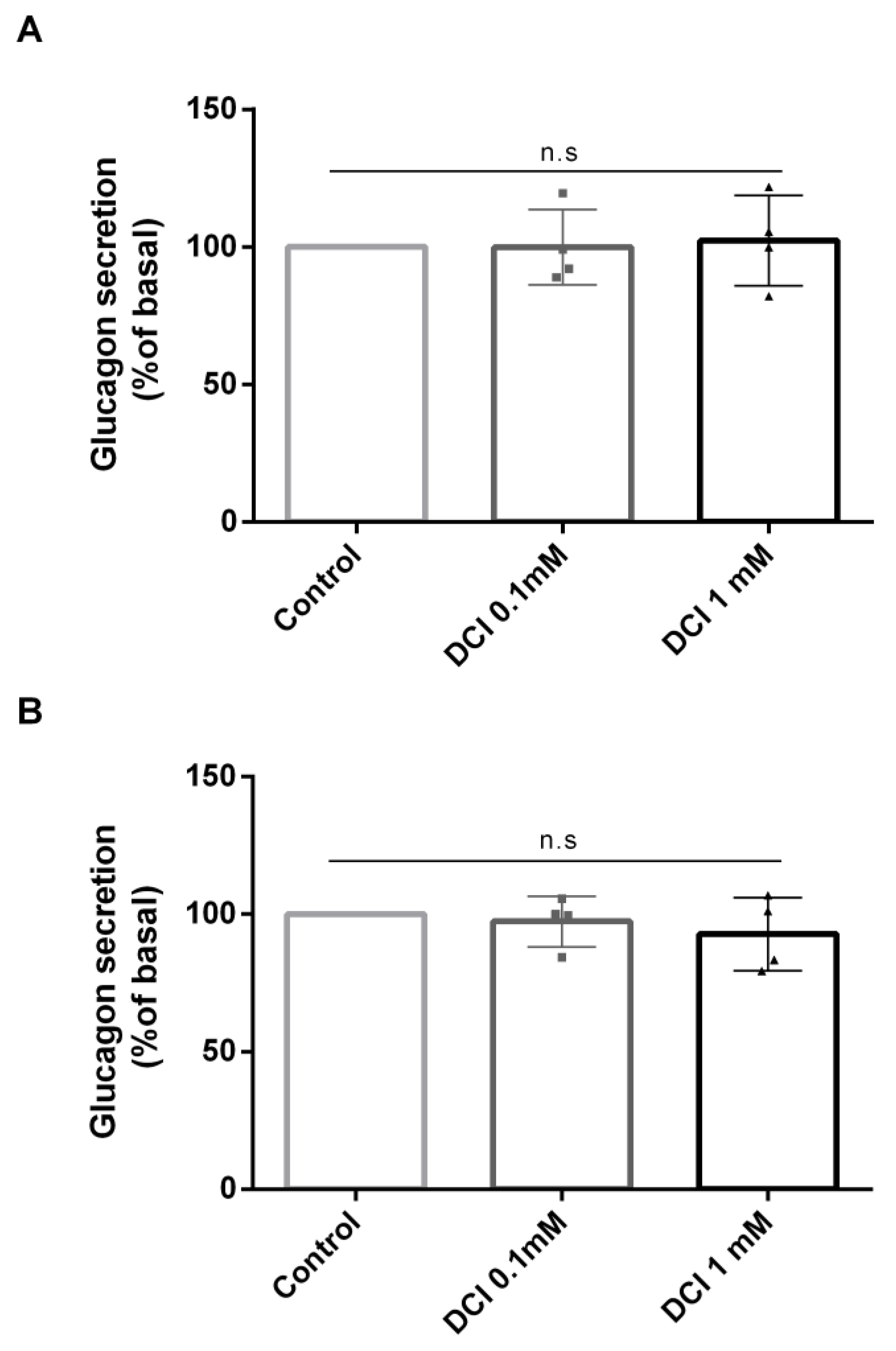
3.2. Effects of DCI Treatment on Glucagon Secretion
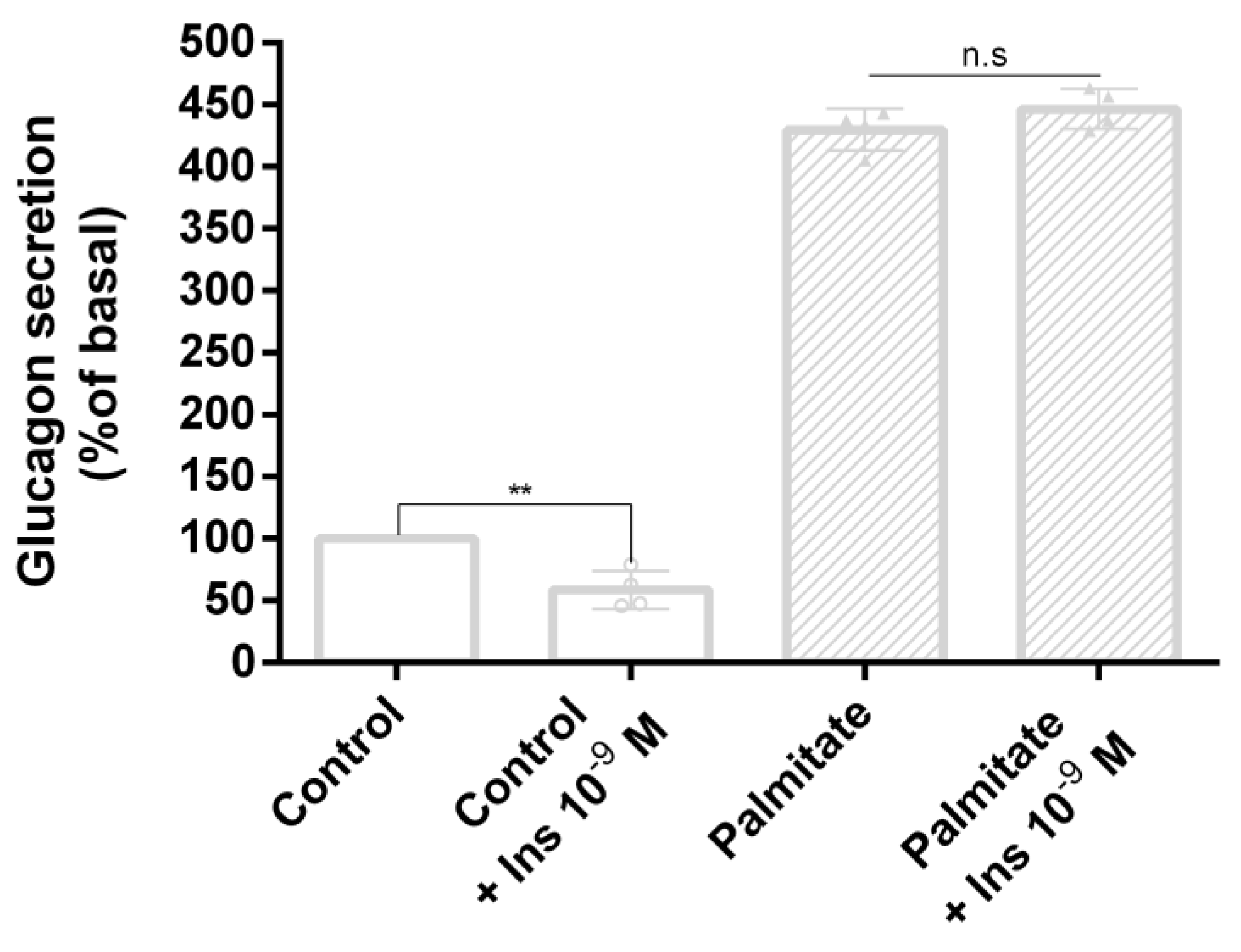
3.3. Palmitate Induces Insulin Resistance and Impaired Glucagon Secretion
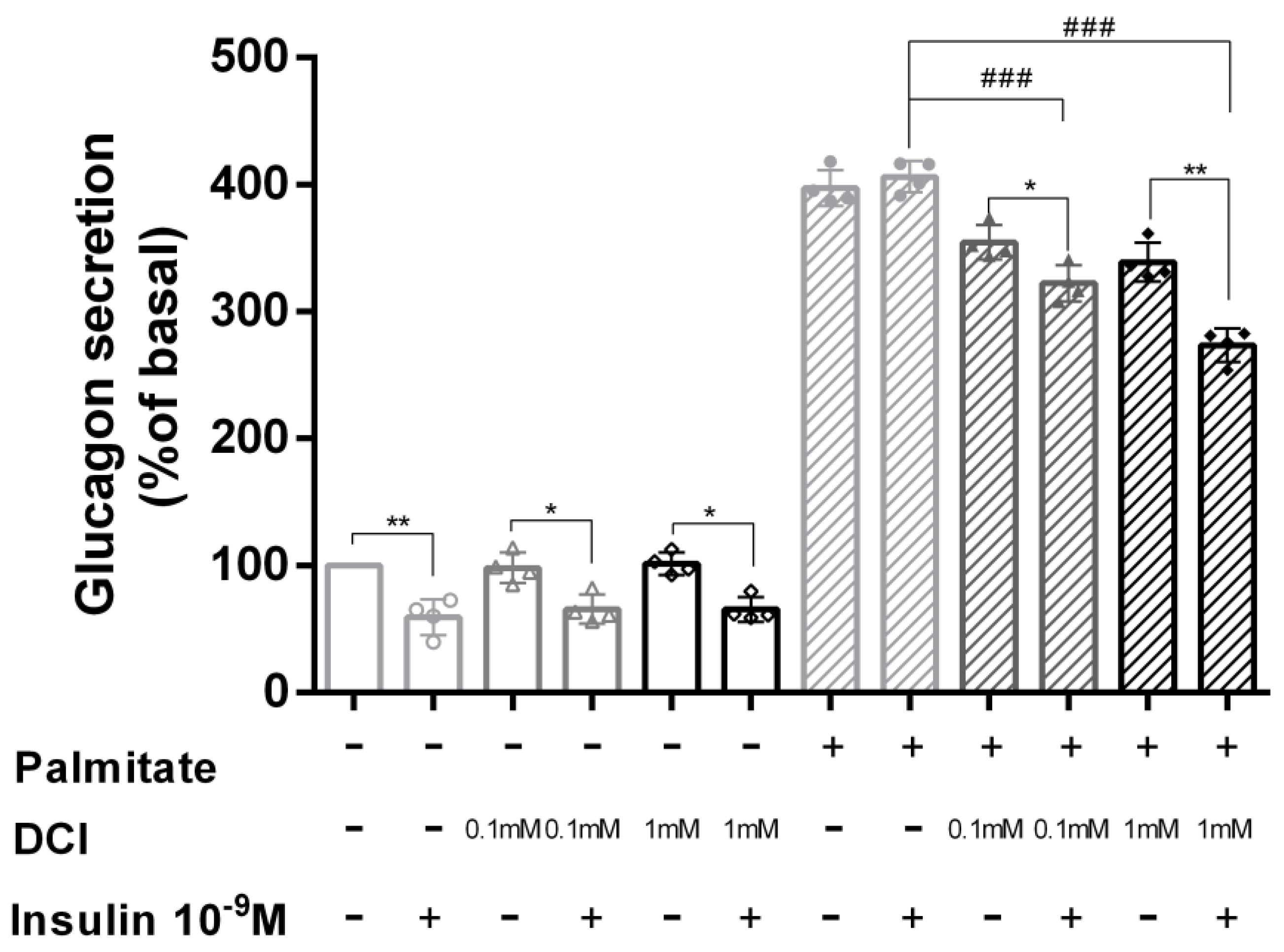
3.4. DCI Treatment Prevented Palmitate Effects on Glucagon Secretion
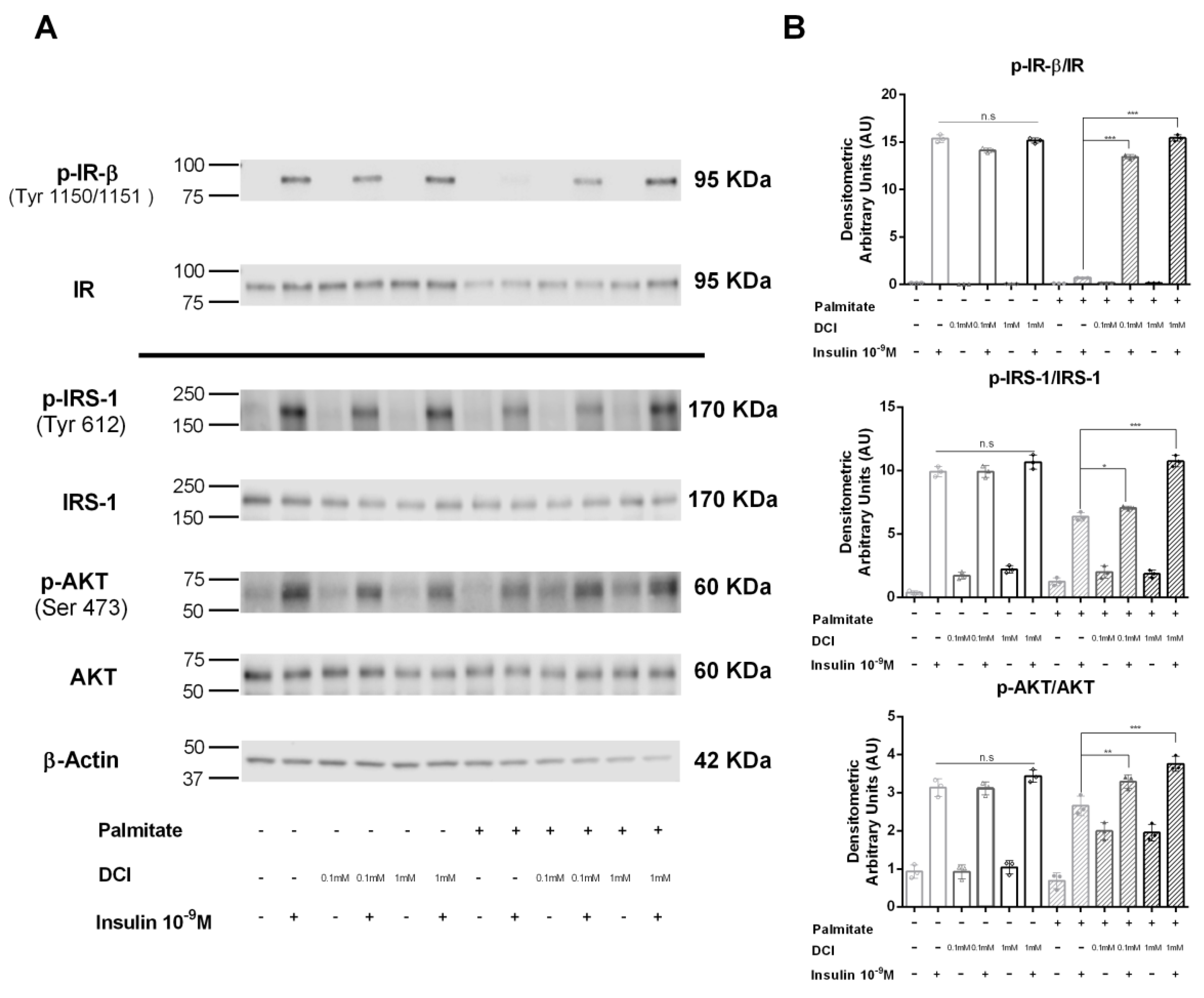
3.5. Influence of DCI on Insulin Signaling in α-TC1-6 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Serine-threonine protein kinase |
| CV | Crystal violet |
| DCI | D-chiro-inositol |
| GLP-1 | Glucagon-like peptide-1 |
| IR | Insulin receptor |
| IRS | Insulin receptor substrate |
| KRB | Krebs–Ringer buffer |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PCOS | Polycystic ovary syndrome |
| T2D | Type 2 diabetes |
References
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.H.; Orci, L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975, 1, 14–16. [Google Scholar] [CrossRef]
- Cryer, P.E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 2013, 369, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Honzawa, N.; Fujimoto, K.; Kitamura, T. Cell Autonomous Dysfunction and Insulin Resistance in Pancreatic alpha Cells. Int. Mol. Sci. 2019, 20, 3699. [Google Scholar] [CrossRef] [Green Version]
- Kawamori, D.; Kurpad, A.J.; Hu, J.; Liew, C.W.; Shih, J.L.; Ford, E.L.; Herrera, P.L.; Polonsky, K.S.; McGuinness, O.P.; Kulkarni, R.N. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009, 9, 350–361. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Berglund, E.D.; Yu, X.; Wang, M.Y.; Evans, M.R.; Scherer, P.E.; Holland, W.L.; Charron, M.J.; Roth, M.G.; Unger, R.H. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13217–13222. [Google Scholar] [CrossRef] [Green Version]
- Katsura, T.; Kawamori, D.; Aida, E.; Matsuoka, T.A.; Shimomura, I. Glucotoxicity induces abnormal glucagon secretion through impaired insulin signaling in InR1G cells. PLoS ONE 2017, 12, e0176271. [Google Scholar] [CrossRef] [Green Version]
- Piro, S.; Maniscalchi, E.T.; Monello, A.; Pandini, G.; Mascali, L.G.; Rabuazzo, A.M.; Purrello, F. Palmitate affects insulin receptor phosphorylation and intracellular insulin signal in a pancreatic alpha-cell line. Endocrinology 2010, 151, 4197–4206. [Google Scholar] [CrossRef] [Green Version]
- Ahren, B. Glucagon--Early breakthroughs and recent discoveries. Peptides 2015, 67, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wendt, A.; Eliasson, L. Pancreatic alpha-cells—The unsung heroes in islet function. Semin. Cell Dev. Biol. 2020, 103, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Broichhagen, J.; Yushchenko, D.A.; Trauner, D.; Schultz, C.; Hodson, D.J. Optical tools for understanding the complexity of beta-cell signalling and insulin release. Nat. Rev. Endocrinol. 2018, 14, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Belete, T.M. A Recent Achievement In the Discovery and Development of Novel Targets for the Treatment of Type-2 Diabetes Mellitus. J. Exp. Pharmacol. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Eddouks, M.; Bidi, A.; El Bouhali, B.; Hajji, L.; Zeggwagh, N.A. Antidiabetic plants improving insulin sensitivity. J. Pharm. Pharmacol. 2014, 66, 1197–1214. [Google Scholar] [CrossRef]
- Li, J.; Bai, L.; Wei, F.; Zhao, J.; Wang, D.; Xiao, Y.; Yan, W.; Wei, J. Therapeutic Mechanisms of Herbal Medicines Against Insulin Resistance: A Review. Front. Pharmacol. 2019, 10, 661. [Google Scholar] [CrossRef] [Green Version]
- Davinelli, S.; Nicolosi, D.; Di Cesare, C.; Scapagnini, G.; Di Marco, R. Targeting Metabolic Consequences of Insulin Resistance in Polycystic Ovary Syndrome by D-chiro-inositol and Emerging Nutraceuticals: A Focused Review. J. Clin. Med. 2020, 9. [Google Scholar]
- Bevilacqua, A.; Bizzarri, M. Inositols in Insulin Signaling and Glucose Metabolism. Int. J. Endocrinol. 2018, 2018, 1968450. [Google Scholar]
- Wojciechowska, A.; Osowski, A.; Jozwik, M.; Gorecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Inositols’ Importance in the Improvement of the Endocrine-Metabolic Profile in PCOS. Int. J. Mol. Sci. 2019, 20, 5787. [Google Scholar] [CrossRef] [Green Version]
- Antonowski, T.; Osowski, A.; Lahuta, L.; Gorecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes. Nutrients 2019, 11, 2314. [Google Scholar] [CrossRef] [Green Version]
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017, 11, CD003053. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Garzon, S.; Unfer, V. New clinical targets of d-chiro-inositol: Rationale and potential applications. Expert Opin. Drug Metab. Toxicol. 2020, 16, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Christiansen, M.; Horowitz, J.F.; Klein, S.; Hellerstein, M.K.; Ostlund, R.E., Jr. Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 2000, 23, 1000–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol. Med. 2010, 16, 543–552. [Google Scholar] [CrossRef]
- Pericuesta, E.; Laguna-Barraza, R.; Ramos-Ibeas, P.; Gutierrez-Arroyo, J.L.; Navarro, J.A.; Vera, K.; Sanjuan, C.; Baixeras, E.; de Fonseca, F.R.; Gutierrez-Adan, A. D-Chiro-Inositol Treatment Affects Oocyte and Embryo Quality and Improves Glucose Intolerance in Both Aged Mice and Mouse Models of Polycystic Ovarian Syndrome. Int. J. Mol. Sci. 2020, 21, 6049. [Google Scholar] [CrossRef]
- Fan, C.; Liang, W.; Wei, M.; Gou, X.; Han, S.; Bai, J. Effects of D-Chiro-Inositol on Glucose Metabolism in db/db Mice and the Associated Underlying Mechanisms. Front. Pharmacol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Yap, A.; Nishiumi, S.; Yoshida, K.; Ashida, H. Rat L6 myotubes as an in vitro model system to study GLUT4-dependent glucose uptake stimulated by inositol derivatives. Cytotechnology 2007, 55, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Owczarczyk-Saczonek, A.; Lahuta, L.B.; Ligor, M.; Placek, W.; Gorecki, R.J.; Buszewski, B. The Healing-Promoting Properties of Selected Cyclitols-A Review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef] [Green Version]
- Lazarenko, R.; Geisler, J.; Bayliss, D.; Larner, J.; Li, C. D-chiro-inositol glycan stimulates insulin secretion in pancreatic beta cells. Mol. Cell. Endocrinol. 2014, 387, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, S.; Scamporrino, A.; Petta, S.; Urbano, F.; Filippello, A.; Ragusa, M.; Di Martino, M.T.; Scionti, F.; Grimaudo, S.; Pipitone, R.M.; et al. Serum coding and non-coding RNAs as biomarkers of NAFLD and fibrosis severity. Liver Int. 2019, 39, 1742–1754. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Perez, M.E.; Ortega-Camarillo, C.; Del Carmen Escobar-Villanueva, M.; Blancas-Flores, G.; Alarcon-Aguilar, F.J. Cucurbita ficifolia Bouche increases insulin secretion in RINm5F cells through an influx of Ca(2+) from the endoplasmic reticulum. J. Ethnopharmacol. 2016, 188, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Vistica, D.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Chiba, K.; Kawakami, K.; Tohyama, K. Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol. In Vitro 1998, 12, 251–258. [Google Scholar] [CrossRef]
- Almutary, A.; Sanderson, B.J. The MTT and Crystal Violet Assays: Potential Confounders in Nanoparticle Toxicity Testing. Int. J. Toxicol. 2016, 35, 454–462. [Google Scholar] [CrossRef]
- Urbano, F.; Di Pino, A.; Scicali, R.; Filippello, A.; Di Mauro, S.; Scamporrino, A.; Marchisello, S.; Rabuazzo, A.M.; Purrello, F.; Piro, S. Impaired glucagon suppression and reduced insulin sensitivity in subjects with prediabetes undergoing atorvastatin therapy. Eur. J. Endocrinol. 2019, 181, 579–590. [Google Scholar] [CrossRef]
- Filippello, A.; Urbano, F.; Di Mauro, S.; Scamporrino, A.; Di Pino, A.; Scicali, R.; Rabuazzo, A.M.; Purrello, F.; Piro, S. Chronic Exposure to Palmitate Impairs Insulin Signaling in an Intestinal L-cell Line: A Possible Shift from GLP-1 to Glucagon Production. Int. J. Mol. Sci. 2018, 19, 3791. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, S.; Ragusa, M.; Urbano, F.; Filippello, A.; Di Pino, A.; Scamporrino, A.; Pulvirenti, A.; Ferro, A.; Rabuazzo, A.M.; Purrello, M.; et al. Intracellular and extracellular miRNome deregulation in cellular models of NAFLD or NASH: Clinical implications. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1129–1139. [Google Scholar] [CrossRef]
- Hamaguchi, K.; Utsunomiya, N.; Takaki, R.; Yoshimatsu, H.; Sakata, T. Cellular interaction between mouse pancreatic alpha-cell and beta-cell lines: Possible contact-dependent inhibition of insulin secretion. Exp. Biol. Med. 2003, 228, 1227–1233. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wang, M.Y.; Yu, X.X.; Unger, R.H. Glucagon is the key factor in the development of diabetes. Diabetologia 2016, 59, 1372–1375. [Google Scholar] [CrossRef] [Green Version]
- Cryer, P.E. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 2012, 153, 1039–1048. [Google Scholar] [CrossRef]
- Gromada, J.; Chabosseau, P.; Rutter, G.A. The alpha-cell in diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F. The role of glucagon on type 2 diabetes at a glance. Diabetol. Metab. Syndr. 2014, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.H.; Aguilar-Parada, E.; Muller, W.A.; Eisentraut, A.M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J. Clin. Investig. 1970, 49, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, H.; Tominaga, M.; Bolli, G.; Orci, L.; Unger, R.H. The alpha cell response to glucose change during perfusion of anti-insulin serum in pancreas isolated from normal rats. Diabetologia 1985, 28, 836–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.H.; Orci, L. Paracrinology of islets and the paracrinopathy of diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 16009–16012. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Wang, M.Y.; Du, X.Q.; Charron, M.J.; Unger, R.H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 2011, 60, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Unger, R.H.; Cherrington, A.D. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. J. Clin. Investig. 2012, 122, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Orci, L.; Unger, R.H. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet 1975, 2, 1243–1244. [Google Scholar] [CrossRef]
- Stefan, Y.; Orci, L.; Malaisse-Lagae, F.; Perrelet, A.; Patel, Y.; Unger, R.H. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes 1982, 31, 694–700. [Google Scholar] [CrossRef]
- Hare, K.J.; Vilsboll, T.; Asmar, M.; Deacon, C.F.; Knop, F.K.; Holst, J.J. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 2010, 59, 1765–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.M.; Dai, R.; Luo, Y.; Xiao, J.H. Glucose-lowering and hypolipidemic activities of polysaccharides from Cordyceps taii in streptozotocin-induced diabetic mice. BMC Complementary Altern. Med. 2019, 19, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cam, M.E.; Hazar-Yavuz, A.N.; Yildiz, S.; Ertas, B.; Ayaz Adakul, B.; Taskin, T.; Alan, S.; Kabasakal, L. The methanolic extract of Thymus praecox subsp. skorpilii var. skorpilii restores glucose homeostasis, ameliorates insulin resistance and improves pancreatic beta-cell function on streptozotocin/nicotinamide-induced type 2 diabetic rats. J. Ethnopharmacol. 2019, 231, 29–38. [Google Scholar] [CrossRef]
- Ramadan, B.K.; Schaalan, M.F.; Tolba, A.M. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naijil, G.; Anju, T.R.; Jayanarayanan, S.; Paulose, C.S. Curcumin pretreatment mediates antidiabetogenesis via functional regulation of adrenergic receptor subtypes in the pancreas of multiple low-dose streptozotocin-induced diabetic rats. Nutr. Res. 2015, 35, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Antu, K.A.; Riya, M.P.; Mishra, A.; Anilkumar, K.S.; Chandrakanth, C.K.; Tamrakar, A.K.; Srivastava, A.K.; Raghu, K.G. Antidiabetic property of Symplocos cochinchinensis is mediated by inhibition of alpha glucosidase and enhanced insulin sensitivity. PLoS ONE 2014, 9, e105829. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Oksbjerg, N.; Young, J.F.; Jeppesen, P.B. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes. Metab. 2014, 16, 602–612. [Google Scholar] [CrossRef]
- Lombardi, A.; Concepcion, E.; Hou, H.; Arib, H.; Mezei, M.; Osman, R.; Tomer, Y. Retro-inverso D-peptides as a novel targeted immunotherapy for Type 1 diabetes. J. Autoimmun. 2020. [Google Scholar] [CrossRef]
- Chen, X.; Hermansen, K.; Xiao, J.; Bystrup, S.K.; O’Driscoll, L.; Jeppesen, P.B. Isosteviol has beneficial effects on palmitate-induced alpha-cell dysfunction and gene expression. PLoS ONE 2012, 7, e34361. [Google Scholar]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Cruz, H.; Orostica, L.; Plaza-Parrochia, F.; Torres-Pinto, I.; Romero, C.; Vega, M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E237–E248. [Google Scholar] [CrossRef] [PubMed]
- Larner, J. D-chiro-inositol--its functional role in insulin action and its deficit in insulin resistance. Int. J. Exp. Diabetes Res. 2002, 3, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Zhang, M.N.; Wang, T.X.; Wu, T.C.; Ai, R.D.; Zhang, Z.S. Hypoglycemic effect of D-chiro-inositol in type 2 diabetes mellitus rats through the PI3K/Akt signaling pathway. Mol. Cell. Endocrinol. 2016, 433, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Han, L.; Xiao, Y.; Pan, C.; Li, Y.; Ge, X.; Zhang, Y.; Yan, S.; Wang, M. d- chiro-Inositol Ameliorates High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance via PKCepsilon-PI3K/AKT Pathway. J. Agric. Food Chem. 2019, 67, 5957–5967. [Google Scholar] [CrossRef] [PubMed]
- Formoso, G.; Baldassarre, M.P.A.; Ginestra, F.; Carlucci, M.A.; Bucci, I.; Consoli, A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab. Res. Rev. 2019, 35, e3154. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippello, A.; Scamporrino, A.; Di Mauro, S.; Malaguarnera, R.; Di Pino, A.; Scicali, R.; Purrello, F.; Piro, S. Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells. Biomolecules 2020, 10, 1404. https://doi.org/10.3390/biom10101404
Filippello A, Scamporrino A, Di Mauro S, Malaguarnera R, Di Pino A, Scicali R, Purrello F, Piro S. Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells. Biomolecules. 2020; 10(10):1404. https://doi.org/10.3390/biom10101404
Chicago/Turabian StyleFilippello, Agnese, Alessandra Scamporrino, Stefania Di Mauro, Roberta Malaguarnera, Antonino Di Pino, Roberto Scicali, Francesco Purrello, and Salvatore Piro. 2020. "Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells" Biomolecules 10, no. 10: 1404. https://doi.org/10.3390/biom10101404
APA StyleFilippello, A., Scamporrino, A., Di Mauro, S., Malaguarnera, R., Di Pino, A., Scicali, R., Purrello, F., & Piro, S. (2020). Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells. Biomolecules, 10(10), 1404. https://doi.org/10.3390/biom10101404








