Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression
Abstract
1. Gastrointestinal Cancer and Phytochemicals
1.1. Gastrointestinal Cancer
1.2. Colorectal Cancer
1.3. Esophageal Cancer
1.4. Diet and Microbial Metabolites
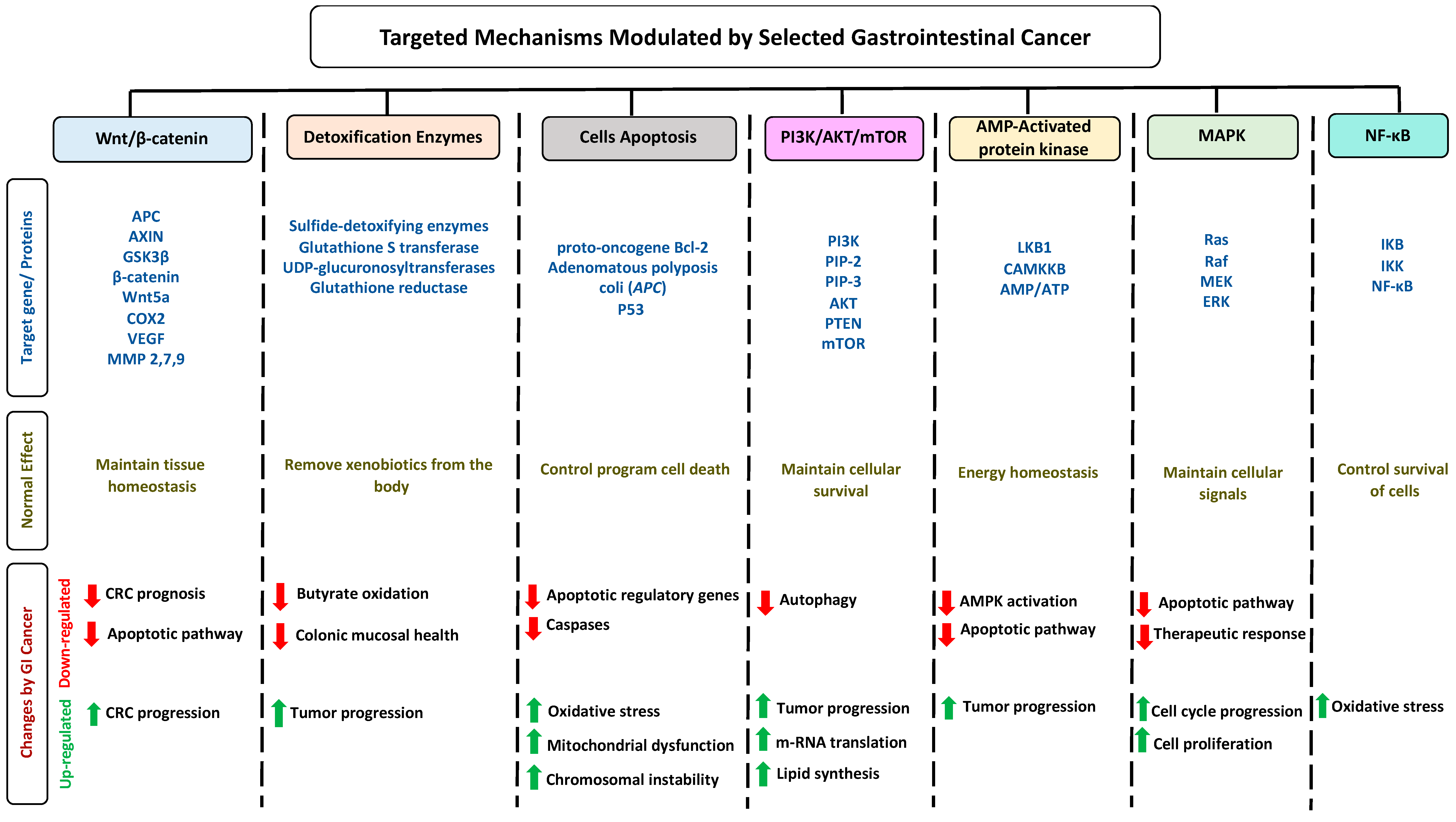
1.5. Impact of Gastrointestinal Cancer on Selected Pathways
1.6. Phytochemicals
1.7. Metabolism of Phytochemicals
1.8. Search Strategy and Selection Criteria
2. Anti-Cancerous Effects of Selected Phytochemicals
2.1. Carotenoids
2.1.1. Lutein
2.1.2. Lycopene
2.1.3. β-Carotene
2.2. Proanthocyanidins
2.2.1. Quercetin
2.2.2. Ellagic Acid
2.3. Organosulfur Compounds
2.3.1. Allicin
2.3.2. Allyl Propyl Disulfide
2.3.3. Asparagusic Acid
2.3.4. Sulforaphane
2.4. Other Phytochemicals
2.4.1. Pectin
2.4.2. Curcumin
2.4.3. p-Coumaric Acid
2.4.4. Ferulic Acid
| Phytochemical Subclass | Phytochemical and Structure | Dietary Source | Conversion Reaction | Metabolites Produced | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | |||||||
| Carotenoids | Lutein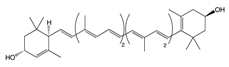 | Egg yolk, kale, spinach, parsley, and peas | Oxidation | 3′-dehydro-lutein |
|
|
| [74,78] |
Lycopene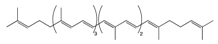 | Tomato, guava, papaya, grapefruit, and watermelon | Auto-oxidation Radical-mediated oxidation | Apo-10′-lycopenoids |
|
|
| [86,89] | |
β-Carotene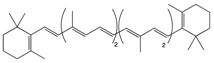 | Carrot | Oxidation | Falcarindiol 6-methoxymellein |
|
|
| [97,98,99] | |
| Pro-anthocy-anidins | Quercetin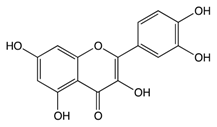 | Cranberry | Sulfation Conjugation | 3-(4hydroxyphenyl) -propionic acid hippuric acid catechol-O-sulfate |
|
|
| [108,109] |
Ellagic Acid | Bilberry | Glucuronidation O-methylation | Peonidin-3-galactoside |
|
|
| [120,121] | |
| Organosulfur Compounds | Allicin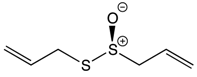 | Garlic | Oxidation Hydrolysis | Allyl methyl sulfide (AMS) Allyl methyl sulfoxide (AMSO) Allyl methyl sulfone (AMSO2) |
|
|
| [135,136] |
Allyl propyl disulfide | Onion | Reduction | Quercetin 3,4‘-diglucoside Quercetin 4‘-glucoside |
|
| [146,147] | ||
Asparagusic acid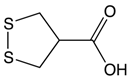 | Asparagus | Sulfation | Asparagus polysaccharide dimethyl sulfide |
|
| [152,153] | ||
Sulforaphane | Broccoli, cabbage, Brussels sprout, and cauliflower | Hydrolysis | Thiocyanates Isothiocyanates Epithionitrile nitrile |
|
| [164,165] | ||
| Other Phytochemicals | Pectin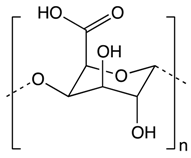 | Apples, plums, oranges, and gooseberries | Colonic fermentation | Butyrate |
|
|
| [169,170] |
Curcumin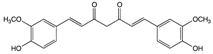 | Ginger | Hydrolysis | Curcumin glucuronide Curcumin sulfate |
|
| [183,184] | ||
p-Couramic acid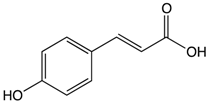 | Navy beans | Hydrolysis | N-methylpipecolate 2-aminoadipate Piperidine Vanillate |
|
| [189,190] | ||
Ferulic acid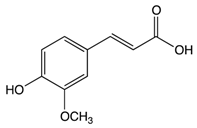 | Rice bran | Colonic fermentation | Tryptophan α-ketoglutarate γ-tocopherol/β-tocopherol γ-tocotrienol |
|
|
| [201,202] | |
3. Conclusions
3.1. Challenges with Studying Phytochemicals
3.1.1. Estimated Consumption Level of Phytochemicals
3.1.2. Could Phytochemicals be Carcinogenic
3.1.3. Could Phytochemical Combinations Have Synergistic Effects
3.1.4. Phytochemicals in Cancer Drug Delivery
3.2. Final Thoughts
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GI | gastrointestinal |
| CRC | colorectal cancer |
| APC | adenomatous polyposis coli |
| SMAD | small mothers against decapentaplegic |
| IL | interleukins |
| SCFA | short chain fatty acid |
| Wnt | wingless-related integration site |
| TNF | tumor necrosis factor |
| AMPK | adenosine monophosphate activated protein kinase |
| PI3K | Phosphoinositide 3-kinases |
| STZ | streptozotocin |
| NF-κB | nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Bcl-2 | B-cell lymphoma 2 |
| NO | nitric oxide |
| SULT | sulfotransferases |
| ACF | aberrant crypt foci |
| UGT | UDP-glucuronosyltransferase |
| GPx | glutathione peroxidase |
| GST | glutathione S-transferase |
| GR | glutathione reductase |
| BAX | Bcl-2-associated X protein |
| JNK | c-Jun N-terminal kinases |
| GSH | reduced glutathione |
| COX-2 | cyclooxygenase 2 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PACs | predominant A- type procyanidins |
| EGFR | epithelial growth factor receptor |
| TBARS | thiobarbituric acid reactive substances |
| ROS | reactive oxygen species |
| SAMC | S-allylmercaptocysteine |
| ACSOs | alk(en)yl cysteine sulphoxides |
| MseC | Se-methyl-L-selenocysteine |
| MDSCs | myeloid-derived suppressor cells |
| MMP | matrix metallopeptidase |
| ICAM | intercellular adhesion molecules |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Gastric cancer treatment PDQ. Available online: http://www.cancer.gov/cancertopics/pdq/treatment/gastric/HealthProfessional/page1 (accessed on 8 July 2010).
- Derakhshan, M.H.; Yazdanbod, A.; Sadjadi, A.R.; Shokoohi, B.; McColl, K.E.; Malekzadeh, R. High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut 2004, 53, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Zali, H.; Rezaei-Tavirani, M.; Azodi, M. Gastric cancer: Prevention, risk factors and treatment. Gastroenterol. Hepatol. Bed Bench. 2011, 4, 175–185. [Google Scholar] [PubMed]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef]
- Holian, O.; Wahid, S.; Atten, M.J.; Attar, B.M. Inhibition of gastric cancer cell proliferation by resveratrol: Role of nitric oxide. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G809–G816. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.M.; Wong, B.C.; Fan, X.M.; Zhang, H.B.; Lin, M.C.; Kung, H.F.; Lam, S.K. Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis 2011, 22, 1393–1397. [Google Scholar] [CrossRef]
- Hundahl, S.A.; Phillips, J.L.; Menck, H.R. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000, 88, 921–932. [Google Scholar] [CrossRef]
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. North. Am. 2013, 211–217. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Zali, H.; Rezaei-Tavirani, M.; Kariminia, A.; Yousefi, R.; Shokrgozar, M.A. Evaluation of growth inhibitory and apoptosis inducing activity of human calprotectin on the human gastric cell line (AGS). Iran. Biomed. J. 2008, 12, 7–14. [Google Scholar]
- Li, Y.H.; Niu, Y.B.; Sun, Y.; Zhang, F.; Liu, C.X.; Fan, L.; Mei, Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262–9272. [Google Scholar] [CrossRef] [PubMed]
- Perdue, D.G.; Haverkamp, D.; Perkins, C.; Daley, C.M.; Provost, E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990–2009. Am. J. Public Health 2014, 104, S404–S414. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Marchionni, L.; Nowak, M.A.; Parmigiani, G.; Vogelstein, B. Only three driver gene mutations are required for the development of lung and colorectal cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 118–123. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Chen, G.Y. Flavonoids and Colorectal Cancer Prevention. Antioxid 2018, 7, 187. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef]
- Marmol, I.; Sanchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers. 2015, 1, 15065. [Google Scholar] [CrossRef]
- Lambert, R.; Hainaut, P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pr. Res. Clin. Gastroenterol. 2007, 21, 921–945. [Google Scholar] [CrossRef] [PubMed]
- Herszenyi, L.; Tulassay, Z. Epidemiology of gastrointestinal and liver tumors. Eur. Rev. Med. Pharm. Sci. 2010, 14, 249–258. [Google Scholar]
- Hongo, M.; Nagasaki, Y.; Shoji, T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J. Gastroenterol. Hepatol. 2009, 24, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Corley, D.A. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am. J. Gastroenterol. 2004, 99, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Haidry, R.J.; Butt, M.A.; Dunn, J.M.; Gupta, A.; Lipman, G.; Smart, H.L.; Registry, U.R. Improvement over time in outcomes for patients undergoing endoscopic therapy for Barrett’s oesophagus-related neoplasia: 6-year experience from the first 500 patients treated in the UK patient registry. Gut 2015, 64, 1192–1199. [Google Scholar] [CrossRef]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and future perspectives in virus discovery. Curr. Opin Virol. 2012, 2, 63–77. [Google Scholar] [CrossRef]
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Knight, R. The intestinal metabolome: An intersection between microbiota and host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M.Jr.; Coburn, L.A. The Role of the Microbiome in Gastrointestinal Cancer. Gastroenterol. Clin. North. Am. 2016, 45, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Chirivella, I. Oncological emergencies. Ann. Oncol. 2004, 15, iv299–iv306. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, M.D.; Bitencourt, A.G.; Marchiori, E.; Chojniak, R.; Gross, J.L.; Kundra, V. Imaging acute complications in cancer patients: What should be evaluated in the emergency setting? Cancer Imaging 2014, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Schatoff, E.M.; Leach, B.I.; Dow, L.E. Wnt Signaling and Colorectal Cancer. Curr. Colorectal. Cancer Rep. 2017, 13, 101–110. [Google Scholar] [CrossRef]
- Ramasamy, S.; Singh, S.; Taniere, P.; Langman, M.J.; Eggo, M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G288–G296. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr. Colorectal. Cancer Rep. 2013, 9, 331–340. [Google Scholar] [CrossRef]
- Tapia, O.; Riquelme, I.; Leal, P.; Sandoval, A.; Aedo, S.; Weber, H.; Roa, J.C. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014, 465, 25–33. [Google Scholar] [CrossRef]
- Walker, J.; Jijon, H.B.; Diaz, H.; Salehi, P.; Churchill, T.; Madsen, K.L. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: A possible role for AMPK. Biochem. J. 2005, 385, 485–491. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, M.J. AMP-activated protein kinase: A therapeutic target in intestinal diseases. Open Biol. 2017, 7, 170104. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Naumann, M. NF-kappaB Signaling in Gastric Cancer. Toxins Basel 2017, 9, 119. [Google Scholar] [CrossRef]
- Yang, M.; Huang, C.Z. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J. Gastroenterol. 2015, 21, 11673–11679. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Lee, H.S.; Jung, J.; Cho, S.J.; Chung, H.Y.; Kim, W.H.; Nam, S.Y. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin. Cancer Res. 2005, 11, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.; Samowitz, W.S.; Wolff, R.K.; Stevens, J.R.; Herrick, J.S. The NF-kappaB signalling pathway in colorectal cancer: Associations between dysregulated gene and miRNA expression. J. Cancer Res. Clin. Oncol. 2018, 144, 269–283. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Gingras, D.; Beliveau, R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011, 4, 133–139. [Google Scholar] [CrossRef]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Komplementmed 2016, 23, 69–74. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015, 2015, 15. [Google Scholar] [CrossRef]
- Probst, Y.C.; Guan, V.X.; Kent, K. Dietary phytochemical intake from foods and health outcomes: A systematic review protocol and preliminary scoping. BMJ Open 2017, 7, e013337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharm. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; Ramírez de Molina, A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef]
- Johnson, I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007, 66, 207–215. [Google Scholar] [CrossRef]
- Cinzia, F.; Francesco, F.; Manuela, B. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 16. [Google Scholar]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Piver, B.; Fer, M.; Vitrac, X.; Merillon, J.M.; Dreano, Y.; Berthou, F. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem. Pharmacol. 2004, 68, 773–782. [Google Scholar] [CrossRef]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Chang, J.L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Burchell, B. Genetic variation of human UDP-glucuronosyltransferase: Implications in disease and drug glucuronidation. Am. J. Pharm 2003, 3, 37–52. [Google Scholar] [CrossRef]
- Slattery, M.L.; Benson, J.; Curtin, K.; Ma, K.N.; Schaeffer, D.; Potter, J.D. Carotenoids and colon cancer. Am. J. Clin. Nutr. 2000, 71, 575–582. [Google Scholar] [CrossRef]
- Palozza, P.; Calviello, G.; Serini, S.; Maggiano, N.; Lanza, P.; Ranelletti, F.O.; Bartoli, G.M. Beta-carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free Radic. Biol. Med. 2001, 30, 1000–1100. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Malila, N.; Virtamo, J.; Virtanen, M.; Pietinen, P.; Albanes, D.; Teppo, L. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. Eur. J. Clin. Nutr. 2002, 56, 615–621. [Google Scholar] [CrossRef]
- Smith-Warner, S.A.; Elmer, P.J.; Tharp, T.M.; Fosdick, L.; Randall, B.; Gross, M.; Potter, J.D. Increasing vegetable and fruit intake: Randomized intervention and monitoring in an at-risk population. Cancer Epidemiol. Biomark. Prev. 2000, 9, 307–317. [Google Scholar]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Ohnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, K.O.; Nolan, J.; Stack, J.; Moran, R.; Feeney, J.; Kenny, R.A.; Peto, T.; Dooley, C.; O’Halloran, A.M.; Cronin, H. Prevalence of age-related macular degeneration in the Republic of Ireland. Br. J. Ophthalmol. 2015, 99, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W.J.r. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Oh, J.; Chang, H.J.; Sohn, D.; Kwon, O.; Shin, A.; Kim, J. Dietary Lutein Plus Zeaxanthin Intake and DICER1 rs3742330 A > G Polymorphism Relative to Colorectal Cancer Risk. Sci. Rep. 2019, 9, 3406. [Google Scholar] [CrossRef]
- Collins, A.R.; Harrington, V. Antioxidants; not the only reason to eat fruit and vegetables. Phytochem. Rev. 2003, 1, 167–174. [Google Scholar] [CrossRef]
- Femia, A.P.; Tarquini, E.; Salvadori, M.; Ferri, S.; Giannini, A. K-ras mutations and mucin profile in preneoplastic lesions and colon tumors induced in rats by 1,2-dimethylhydrazine. Int. J. Cancer 2008, 1, 117–123. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.U.; Younes, I.H.; Karchesy, J.J.; el-Sabban, M.E. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1,2-dimethylhydrazine in mice. Nutr. Cancer 2001, 39, 108–116. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; González-Jasso, E.; Ferriz-Martínez, R.; Villalón-Corona, B.; Salgado, L.; Ramos-Gómez, M. Dietary Supplementation of Lutein Reduces Colon Carcinogenesis in DMH-Treated Rats by Modulating K-ras, PKB, and β-catenin Proteins. Nutr. Cancer 2010, 63, 39–45. [Google Scholar] [CrossRef]
- Satia-Abouta, J.; Galanko, J.A.; Martin, C.F.; Potter, J.D.; Ammerman, A.; Sandler, R.S. Associations of micronutrients with colon cancer risk in African Americans and whites: Results from the North Carolina Colon Cancer Study. Cancer Epidemiol. Biomark. Prev. 2003, 12, 747–754. [Google Scholar]
- Santocono, M.; Zurria, M.; Berrettini, M.; Fedeli, D.; Falcioni, G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J. Photochem. Photobiol. B 2006, 85, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Complement. Alternat. Med. 2013, 2013, 705121. [Google Scholar] [CrossRef] [PubMed]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, A.C.; da Silva, T.P.; de Araujo, G.R. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. Biol. Interact. 2016, 263, 7–17. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Truscott, T.G.; Witt, C. A dramatic effect of oxygen on protection of human cells against γ-radiation by lycopene. FEBS Lett. 2016, 590, 1086–1093. [Google Scholar] [CrossRef]
- Slattery, M.L.; Lundgreen, A.; Welbourn, B.; Wollf, R.K.; Corcoran, C. Oxidative balance and colon and rectal cancer: Interaction of lifestyle factors and genes. Mutat. Res. 2012, 734, 30–40. [Google Scholar] [CrossRef]
- Youn, S.W. Systemic inflammatory response as a prognostic factor in patients with cancer. J. Korean Orient Oncol. 2012, 17, 1–7. [Google Scholar]
- Lin, M.C.; Wang, F.Y.; Kuo, Y.H.; Tang, F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318. [Google Scholar] [CrossRef]
- Palozza, P.; Colangelo, M.; Simone, R. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Velmurugan, B.; Nagini, S. Lycopene, an antioxidant carotenoid modulates glutathione-dependent hepatic biotransformation enzymes during experimental gastric carcinogenesis. Nutr. Res. 2001, 8, 1117–1124. [Google Scholar]
- Kalt, W. Effects of production and processing factor on major fruit and vegetable antioxidants. J. Food Sci. 2005, 70, 11–19. [Google Scholar] [CrossRef]
- Wally, O.S.; Punja, Z.K. Carrot (Daucus carota L.). Methods Mol. Biol. 2015, 1224, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Nielsen, D.S.; Kot, W.; Krych, L.; Christensen, L.P.; Baatrup, G. Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer. BMC Res. Note 2018, 11, 411. [Google Scholar] [CrossRef]
- Shebaby, W.N.; Bodman-Smith, K.B.; Mansour, A.; Mroueh, M.; Taleb, R.I.; El-Sibai, M.; Daher, C.F. Daucus carota Pentane-Based Fractions Suppress Proliferation and Induce Apoptosis in Human Colon Adenocarcinoma HT-29 Cells by Inhibiting the MAPK and PI3K Pathways. J. Med. Food. 2005, 18, 745–752. [Google Scholar] [CrossRef]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef]
- Pan, M.H.; Ho, C.T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008, 37, 2558–2574. [Google Scholar] [CrossRef]
- Huang, X.E.; Hirose, K.; Wakai, K.; Matsuo, K.; Ito, H.; Xiang, J. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pac. J. Cancer Prev. 2004, 5, 419–427. [Google Scholar]
- De la Iglesia, R.; Milagro, F.I.; Campion, J.; Boque, N.; Martinez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Blade, C.; Aragones, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvado, M.J.; Suarez, M. Proanthocyanidins in health and disease. Biofactors 2016, 42, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- Casanova-Marti, A.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Cancer Chemopreventive Potential of Procyanidin. Toxicol. Res. 2017, 33, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberries: Ripe for more cancer research? J. Sci. Food Agric. 2011, 91, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef]
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Cai, X.; Neto, C.; Tata, A.; Han, Y.; Xiao, H. Chemopreventive Effects of Whole Cranberry (Vaccinium macrocarpon) on Colitis-Associated Colon Tumorigenesis. Mol. Nutr. Food Res. 2018, 62, e1800942. [Google Scholar] [CrossRef]
- Boateng, J.; Verghese, M.; Shackelford, L.; Walker, L.T.; Khatiwada, J.; Ogutu, S.; Chawan, C.B. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol. 2007, 45, 725–732. [Google Scholar] [CrossRef]
- Xiao, S.D.; Shi, T. Is cranberry juice effective in the treatment and prevention of Helicobacter pyloriinfection of mice? Chin. J. Dig. Dis. 2003, 4, 136–139. [Google Scholar] [CrossRef]
- Jin, D.; Liu, T.; Dong, W.; Zhang, Y.; Wang, S.; Xie, R.; Cao, H. Dietary feeding of freeze-dried whole cranberry inhibits intestinal tumor development in Apc(min/+) mice. Oncotarget 2017, 8, 97787–97800. [Google Scholar] [CrossRef] [PubMed]
- Koosha, S.; Alshawsh, M.A.; Looi, C.Y.; Seyedan, A.; Mohamed, Z. An Association Map on the Effect of Flavonoids on the Signaling Pathways in Colorectal Cancer. Int. J. Med. Sci. 2016, 13, 374–385. [Google Scholar] [CrossRef]
- Sun, Q.; Yue, Y.; Shen, P.; Yang, J.J.; Park, Y. Cranberry Product Decreases Fat Accumulation in Caenorhabditis elegans. J. Med. Food. 2016, 19, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Upton, R. Bilberry Fruit Vaccinium myrtillus L. Standards of Analysis, Quality Control, and Therapeutics; AHP: Santa Cruz, CA, USA, 2001. [Google Scholar]
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, Sissi, Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Mazza, G.; Kay, C.D.; Correll, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef] [PubMed]
- Valentova, K.; Ulrichova, J.; Cvak, L.; Simanek, V. Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem. 2006, 101, 912–917. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 760689. [Google Scholar] [CrossRef]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef]
- Mutanen, M.; Pajari, A.M.; Paivarinta, E.; Misikangas, M.; Rajakangas, J.; Marttinen, M.; Oikarinen, S. Berries as preventive dietary constituents-a mechanistic approach with ApcMin+ mouse. Asia Pac. J. Clin. Nutr. 2008, 17, 123–125. [Google Scholar]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Endlicher, E. Anthocyanins Prevent Colorectal Cancer Development in a Mouse Model. Digestion 2017, 95, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Gescher, A.J. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. Phila 2009, 2, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Esselen, M.; Fritz, J.; Hutter, M.; Teller, N.; Baechler, S.; Boettler, U.; Marko, D. Anthocyanin-rich extracts suppress the DNA-damaging effects of topoisomerase poisons in human colon cancer cells. Mol. Nutr. Food Res. 2011, 55, S143–S153. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Cunningham, D. Adjuvant therapy in colon cancer—what, when and how? Ann. Oncol. 2006, 17, 1347–1359. [Google Scholar] [CrossRef]
- Xiao, D.; Pinto, J.T.; Gundersen, G.G.; Weinstein, I.B. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol. Cancer Ther. 2005, 4, 1388–1398. [Google Scholar] [CrossRef]
- Moriarty, R.M.; Naithani, R.; Surve, B. Organosulfur compounds in cancer chemoprevention. Mini. Rev. Med. Chem. 2007, 7, 827–838. [Google Scholar] [CrossRef]
- Nagini, S. Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise? Anticancer Agents Med. Chem. 2008, 8, 313–321. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R.; Pinto, J.T. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J. Nutr. 2016, 136, S864–S869. [Google Scholar] [CrossRef]
- Hu, J.Y.; Hu, Y.W.; Zhou, J.J.; Zhang, M.W.; Li, D.; Zheng, S. Consumption of garlic and risk of colorectal cancer: An updated meta-analysis of prospective studies. World J. Gastroenterol. 2014, 20, 15413–15422. [Google Scholar] [CrossRef]
- Ross, S.A.; Finley, J.W.; Milner, J.A. Allyl sulfur compounds from garlic modulate aberrant crypt formation. J. Nutr. 2006, 136, S852–S854. [Google Scholar] [CrossRef]
- Powolny, A.A.; Singh, S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related allium vegetable-derived organosulfur compounds. Cancer Lett. 2008, 269, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Su, Q. Molecular mechanisms for the anticancer effects of diallyl disulfide. Food Chem. Toxicol. 2013, 57, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Nazeem, P.A.; Babu, T.D.; Abida, P.S.; Narayanankutty, A.; Valsalan, R.; Raghavamenon, A.C. EGFR gene regulation in colorectal cancer cells by garlic phytocompounds with special emphasis on S-Allyl-L-Cysteine Sulfoxide. Interdiscip Sci. 2008, 10, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.M.; Li, W.; Gray, Z.; Matter, M.S.; Colburn, N.H.; Young, M.R.; Kim, Y.S. Diallyl Disulfide (DADS), a Constituent of Garlic, Inactivates NF-kappaB and Prevents Colitis-Induced Colorectal Cancer by Inhibiting GSK-3beta. Cancer Prev. Res. Phila 2016, 9, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Rao, C.V. The role of inflammation in colon cancer. Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2014; Volume 816, pp. 25–52. [Google Scholar]
- Erstad, D.J.; Cusack, J.C. Targeting the NF-kappaB pathway in cancer therapy. Surg. Oncol. Clin. N. Am. 2013, 22, 705–746. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, G.; Zhu, X.; Cheng, L.; Sun, Y.; Zhao, Z. Combination of rapamycin and garlic-derived S-allylmercaptocysteine induces colon cancer cell apoptosis and suppresses tumor growth in xenograft nude mice through autophagy/p62/Nrf2 pathway. Oncol. Rep. 2017, 38, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Raghu, R.; Lu, K.H.; Sheen, L.Y. Recent Research Progress on Garlic (da suan) as a Potential Anticarcinogenic Agent Against Major Digestive Cancers. J. Tradit. Complement Med. 2012, 2, 192–201. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhuang, W.; Hu, W.; Liu, G.J.; Wu, T.X.; Wu, X.T. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011, 141, 80–89. [Google Scholar] [CrossRef]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions--a global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef]
- Suleria, H.A.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef]
- Izzo, A.A.; Capasso, R.; Capasso, F. Eating garlic and onion: A matter of life or death. Br. J. Cancer 2004, 91, 194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murayyan, A.I.; Manohar, C.M.; Hayward, G.; Neethirajan, S. Antiproliferative activity of Ontario grown onions against colorectal adenocarcinoma cells. Food Res. Int. 2017, 96, 12–18. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jin, H.; Gong, W.; Zhang, C.; Zhou, A. Effect of onion flavonoids on colorectal cancer with hyperlipidemia: An in vivo study. Onco Targets Ther. 2014, 7, 101–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tung, Y.C.; Tsai, M.L.; Kuo, F.L.; Lai, C.S.; Badmaev, V.; Ho, C.T.; Pan, M.H. Se-Methyl-L-selenocysteine Induces Apoptosis via Endoplasmic Reticulum Stress and the Death Receptor Pathway in Human Colon Adenocarcinoma COLO 205 Cells. J. Agric. Food Chem. 2015, 63, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Ibanez-Redin, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.; Oliveira, O.N. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1502–1508. [Google Scholar] [CrossRef]
- Negi, J.S.; Singh, P.; Joshi, G.P.; Rawat, M.S.; Bisht, V.K. Chemical constituents of Asparagus. Pharm. Rev. 2010, 4, 215–220. [Google Scholar] [CrossRef]
- Saxena, V.K.; Chaurasia, S. A new isoflavone from the roots of Asparagus racemosus. Fitoterapia 2001, 72, 307–309. [Google Scholar] [CrossRef]
- Hamdi, A.; Jaramillo-Carmona, S.; Srairi Beji, R.; Tej, R.; Zaoui, S.; Rodriguez-Arcos, R.; Guillen-Bejarano, R. The phytochemical and bioactivity profiles of wild Asparagus albus L. plant. Food Res. Int. 2017, 99, 720–729. [Google Scholar] [CrossRef]
- Jaramillo-Carmona, S.; Guillen-Bejarano, R.; Jimenez-Araujo, A.; Rodriguez-Arcos, R.; Lopez, S. In Vitro Toxicity of Asparagus Saponins in Distinct Multidrug-Resistant Colon Cancer Cells. Chem. Biodivers. 2018, 15, e1800282. [Google Scholar] [CrossRef]
- Zhang, W.; He, W.; Shi, X.; Li, X.; Wang, Y.; Hu, M.; Qin, Z. An Asparagus polysaccharide fraction inhibits MDSCs by inducing apoptosis through toll-like receptor 4. Phytother. Res. 2018, 32, 1297–1303. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhao, J.; Zhang, W.; Pang, X. Saponins extracted from by-product of Asparagus officinalis L. suppress tumour cell migration and invasion through targeting Rho GTPase signalling pathway. J. Sci. Food Agric. 2013, 93, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Bousserouel, S.; Le Grandois, J.; Gosse, F.; Werner, D.; Barth, S.W.; Marchioni, E.; Raul, F. Methanolic extract of white asparagus shoots activates TRAIL apoptotic death pathway in human cancer cells and inhibits colon carcinogenesis in a preclinical model. Int. J. Oncol. 2013, 43, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Eslick, G.D. Cruciferous vegetables and risk of colorectal neoplasms: A systematic review and meta-analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Bergner, A.; Gershenzon, J.; Wittstock, U. Glucosinolate hydrolysis in Lepidium sativum—Identification of the thiocyanate-forming protein. Plant Mol. Biol. 2007, 63, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, O.A.; Davies, A.; Deeken, R.; Thorpe, M.R.; Tomos, A.D.; Hedrich, R. Identification of a New Glucosinolate-Rich Cell Type in Arabidopsis Flower Stalk. Plant Physiol. 2000, 124, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef]
- Ramos-Gomez, M.; Kwak, M.K.; Dolan, P.M.; Itoh, K.; Yamamoto, M.; Talalay, P.; Kensler, T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3410–3415. [Google Scholar] [CrossRef]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef]
- Higgins, L.G.; Kelleher, M.O.; Eggleston, I.M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox–cycling agents. Toxicol. Appl. Pharmacol. 2009, 237, 267–280. [Google Scholar] [CrossRef]
- Keum, Y.S.; Yu, S.; Chang, P.P.; Yuan, X.; Kim, J.H.; Xu, C.; Han, J.; Agarwal, A.; Kong, A.N. Mechanism of Action of Sulforaphane: Inhibition of p38 Mitogen-Activated Protein Kinase Isoforms Contributing to the Induction of Antioxidant Response Element-Mediated Heme Oxygenase-1 in Human Hepatoma HepG2 Cells. Cancer Res. 2006, 66, 8804–8813. [Google Scholar] [CrossRef]
- Kim, J.K.; Gallaher, D.D.; Chen, C.; Gallaher, C.M.; Yao, D.; Trudo, S.P. Phenethyl isothiocyanate and indole-3-carbinol from cruciferous vegetables, but not furanocoumarins from apiaceous vegetables, reduced PhIP-induced DNA adducts in Wistar rats. Mol. Nutr. Food Res. 2016, 60, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lim, D.Y.; Park, J.H. Induction of G1 and G2/M cell cycle arrests by the dietary compound 3,30-diindolylmethane in HT-29 human colon cancer cells. BMC Gastroenterol. 2009, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, e1701000. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A Review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Tamburino, A.; Carnaroglio, D.; Cravotto, G.; Pagliaro, M. Controlling the Degree of Esterification of Citrus Pectin for Demanding Applications by Selection of the Source. ACS Omega 2017, 2, 7991–7995. [Google Scholar] [CrossRef]
- Wikiera, A.; Irla, M.; Mika, M. Health-promoting properties of pectin. Postepy Hig. Med. Dosw. Online 2014, 68, 590–596. [Google Scholar] [CrossRef]
- Sriamornsak, P. Application of pectin in oral drug delivery. Expert Opin Drug. Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef]
- Verma, A.K.; Sachin, K. Novel hydrophilic drug polymer nano-conjugatesof cisplatin showing long blood retention profile—Its release kinetics, cellularuptake and bio-distribution. Curr. Drug. Deliv. 2008, 5, 120–126. [Google Scholar] [CrossRef]
- Izadi, Z.; Divsalar, A.; Saboury, A.A.; Sawyer, L. beta-lactoglobulin-pectin Nanoparticle-based Oral Drug Delivery System for Potential Treatment of Colon Cancer. Chem. Biol. Drug. Des. 2016, 88, 209–216. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Lu, S.M.; Ling, Z.Q. Chemoprevention of Low-Molecular-Weight Citrus Pectin (LCP) in Gastrointestinal Cancer Cells. Int. J. Biol. Sci. 2016, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastatic properties: One bullet multiple targets. Carbohydr. Res. 2009, 14, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Miller, M.C.; Zheng, Y.; Zhang, Z.; Xue, H.; Zhao, D. Macromolecular assemblies of complex polysaccharides with galectin-3 and their synergistic effects on function. Biochem. J. 2017, 474, 3849–3868. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan I containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression. Carbohydr. Polym. 2015, 132, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Mellem, J.; Naicker, T.; Reddy, L. Chemoprevention of Azoxymethane-induced Colonic Carcinogenesis in Balb/c mice Using a Modified Pectin Alginate Probiotic. Anticancer Res. 2015, 35, 4765–4775. [Google Scholar] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Mazzolani, F.; Togni, S. Oral administration of a curcumin-phospholipid delivery system for the treatment of central serous chorioretinopathy: A 12-month follow-up study. Clin. Ophthalmol. 2013, 7, 939–945. [Google Scholar] [CrossRef]
- Jalili-Nik, M.; Soltani, A.; Moussavi, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; Hassanian, S.M.; Avan, A. Current status and future prospective of Curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J. Cell. Physiol. 2018, 233, 6337–6345. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis., N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- Bahrami, A.; Majeed, M.; Sahebkar, A. Curcumin: A potent agent to reverse epithelial-to-mesenchymal transition. Cell. Oncol. Dordr 2019, 42, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of Curcumin on the Diversity of Gut Microbiota in Ovariectomized Rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): An overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2018, 58, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Hangen, L.; Bennink, M.R. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Borresen, E.C.; Brown, D.G.; Harbison, G.; Taylor, L.; Fairbanks, A.; O’Malia, J.; Ryan, E.P. A Randomized Controlled Trial to Increase Navy Bean or Rice Bran Consumption in Colorectal Cancer Survivors. Nutr. Cancer 2016, 68, 1269–1280. [Google Scholar] [CrossRef]
- Baxter, B.A.; Oppel, R.C.; Ryan, E.P. Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients 2018, 11, 28. [Google Scholar] [CrossRef]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Power, K.A. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef]
- Borresen, E.C.; Gundlach, K.A.; Wdowik, M.; Rao, S.; Brown, R.J.; Ryan, E.P. Feasibility of Increased Navy Bean Powder Consumption for Primary and Secondary Colorectal Cancer Prevention. Curr. Nutr. Food Sci. 2014, 10, 112–119. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.J.; Bohm, M.; Laufs, U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Jolfaie, N.R.; Rouhani, M.H.; Surkan, P.J.; Siassi, F.; Azadbakht, L. Rice Bran Oil Decreases Total and LDL Cholesterol in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Horm. Metab. Res. 2016, 48, 417–426. [Google Scholar] [CrossRef] [PubMed]
- So, W.K.; Law, B.M.; Law, P.T.; Chan, C.W.; Chair, S.Y. Current Hypothesis for the Relationship between Dietary Rice Bran Intake, the Intestinal Microbiota and Colorectal Cancer Prevention. NutrIents 2016, 8, 569. [Google Scholar] [CrossRef] [PubMed]
- Law, B.M.H.; Waye, M.M.Y.; So, W.K.W.; Chair, S.Y. Hypotheses on the Potential of Rice Bran Intake to Prevent Gastrointestinal Cancer through the Modulation of Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 1352. [Google Scholar] [CrossRef]
- Ryan, E.P.; Heuberger, A.L.; Weir, T.L.; Barnett, B.; Broeckling, C.D.; Prenni, J.E. Rice bran fermented with Saccharomyces boulardii generates novel metabolite profiles with bioactivity. J. Agric. Food Chem. 2011, 59, 1862–1870. [Google Scholar] [CrossRef]
- Fan, H.; Morioka, T.; Ito, E. Induction of apoptosis and growth inhibition of cultured human endometrial adenocarcinoma cells (Sawano) by an antitumor lipoprotein fraction of rice bran. Gynecol. Oncol. 2000, 76, 170–175. [Google Scholar] [CrossRef]
- Kong, C.K.; Lam, W.S.; Chiu, L.C.; Ooi, V.E.; Sun, S.S.; Wong, Y.S. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem. Pharmacol. 2009, 77, 1487–1496. [Google Scholar] [CrossRef]
- Norris, L.; Malkar, A.; Horner-Glister, E.; Hakimi, A.; Ng, L.L.; Gescher, A.J.; Jones, D.J. Search for novel circulating cancer chemopreventive biomarkers of dietary rice bran intervention in Apc(Min) mice model of colorectal carcinogenesis, using proteomic and metabolic profiling strategies. Mol. Nutr. Food Res. 2015, 59, 1827–1836. [Google Scholar] [CrossRef]
- Shafie, N.H.; Mohd Esa, N.; Ithnin, H.; Md Akim, A.; Saad, N.; Pandurangan, A.K. Preventive inositol hexaphosphate extracted from rice bran inhibits colorectal cancer through involvement of Wnt/beta-catenin and COX-2 pathways. BioMed Res. Int. 2013, 681027. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Ismail, S.; Esa, N.M.; Munusamy, M.A. Inositol-6 phosphate inhibits the mTOR pathway and induces autophagy-mediated death in HT-29 colon cancer cells. Arch. Med. Sci. 2018, 14, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, Y.; Cui, L.; Wen, Z.; & Lu, X. Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int. J. Clin. Exp. Pathol. 2015, 8, 1402–1410. [Google Scholar] [PubMed]
- Neto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar] [CrossRef]
- Aiyelaagbe, O.; Adeniyi, B.; Fatunsin, O.; Arimah, B. In vitro antimicrobial activity and phytochemical analysis of jatropha curcas roots. Int. J. Pharmacol. 2007, 3, 106–110. [Google Scholar]
- Broadhurst, C.L.; Polansky, M.M.; Anderson, R.A. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J. Agric. Food Chem. 2000, 48, 849–852. [Google Scholar] [CrossRef]
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Mäkelä, S.; Aittokallio, T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015, 32, 1249–1266. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Sergiu, C.; Braicu, C.; Berindan-Neagoe, I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef]
- Dreosti, I.E. Recommended dietary intake levels for phytochemicals: Feasible or fanciful? Asia Pac. J. Clin. Nutr. 2000, 9, S119–S122. [Google Scholar] [CrossRef]
- Most, M.M.; Tulley, R.; Morales, S.; Lefevre, M. Rice bran oil, not fiber, lowers cholesterol in humans. Am. J. Clin. Nutr. 2005, 81, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Lanzi, S.; D’Evoli, L.; Aguizzi, A.; Lombardi-Boccia, G. Intake of vitamin A and carotenoids from the Italian population–results of an Italian total diet study. Int. J. Vitam. Nutr. Res. 2006, 76, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.; Riso, P. What are typical lycopene intakes? J. Nutr. 2005, 135, 2042S–2045S. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Hathcock, J.N. Risk assessment for the carotenoids lutein and lycopene. Regul. Toxicol. Pharmacol. 2006, 45, 289–298. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Correa-Velez, I.; Clavarino, A.; Eastwood, H. Surviving, relieving, repairing, and boosting up: Reasons for using complementary/alternative medicine among patients with advanced cancer: A thematic analysis. J. Palliat. Med. 2005, 8, 953–961. [Google Scholar] [CrossRef]
- Fratamico, P.M.; Wasilenko, J.L.; Garman, B.; Demarco, D.R.; Varkey, S.; Jensen, M. Evaluation of a multiplex real-time PCR method for detecting shiga toxin-producing Escherichia coli in beef and comparison to the U.S. Department of Agriculture Food Safety and Inspection Service Microbiology laboratory guidebook method. J. Food Prot. 2014, 77, 180–188. [Google Scholar] [CrossRef]
- Gaige, S.; Djelloul, M.; Tardivel, C.; Airault, C.; Felix, B.; Jean, A. Modification of energy balance induced by the food contaminant T-2 toxin: A multimodal gut-to-brain connection. Brain Behav. Immun. 2014, 37, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.S.; Gubili, J.; Cassileth, B. Evidence-based botanical research: Applications and challenges. Hematol. Oncol. Clin. North Am. 2008, 22, 661–670. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. Toxic phytochemicals and their potential risks for human cancer. Cancer Prev. Res. Phila 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Yamamoto, M.; Calvo, A.; Cavada, G.; Baez, S.; Endoh, K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int. J. Cancer 2002, 102, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kisby, G.E.; Fry, R.C.; Lasarev, M.R.; Bammler, T.K.; Beyer, R.P.; Churchwell, M. The Cycad Genotoxin MAM Modulates Brain Cellular Pathways Involved in Neurodegenerative Disease and Cancer in a DNA Damage-Linked Manner. PLoS ONE 2011, 6, e20911. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.A.; Kuhnle, G.G.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T. Breast, colorectal, and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition-Norfolk in relation to phytoestrogen intake derived from an improved database. Am. J. Clin. Nutr. 2010, 91, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Masuda, E.K.; Kommers, G.D.; Martins, T.B.; Barros, C.S.; Piazer, J.V. Morphological factors as indicators of malignancy of squamous cell carcinomas in cattle exposed naturally to bracken fern (Pteridium aquilinum) . J. Comp. Pathol. 2010, 144, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Chung, Y.T.; Wang, P.F.; Chi, C.W.; Hsieh, L.L. Safrole-DNA adducts in human peripheral blood–an association with areca quid chewing and CYP2E1 polymorphisms. Mutat. Res. 2004, 559, 59–66. [Google Scholar] [CrossRef]
- Ho, J.W.; Cheung, M.W. Combination of phytochemicals as adjuvants for cancer therapy. Recent. Pat. Anticancer Drug. Discov. 2014, 9, 297–302. [Google Scholar] [CrossRef]
- Cho, K.J.; Wang, X.; Nie, S.M.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjugate. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Mansoori, G.A.; Mohazzabi, P.; McCormack, P. Nanotechnology in cancer prevention, detection and treatment: Bright future lies ahead. World Rev. Sci. Tech. Sust. Dev. 2007, 2, 226–257. [Google Scholar] [CrossRef]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing Delivery of Plant Based Anti-cancer Drugs. Front Pharmacol. 2017, 8, 1002. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. https://doi.org/10.3390/biom10010105
AL-Ishaq RK, Overy AJ, Büsselberg D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules. 2020; 10(1):105. https://doi.org/10.3390/biom10010105
Chicago/Turabian StyleAL-Ishaq, Raghad Khalid, Anthony J. Overy, and Dietrich Büsselberg. 2020. "Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression" Biomolecules 10, no. 1: 105. https://doi.org/10.3390/biom10010105
APA StyleAL-Ishaq, R. K., Overy, A. J., & Büsselberg, D. (2020). Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules, 10(1), 105. https://doi.org/10.3390/biom10010105






