Abstract
The effects of cation substitution are the main emphasis of this investigation into the structural, mechanical, electronic, and optical properties of double perovskites K2AgSbBr6 and K2NaScBr6. Outwardly favorable tolerance and octahedral factors and negative formation energy confirmed structural stability and thermodynamic feasibility. Mechanical analysis showed that K2AgSbBr6 possesses greater volumetric stability and rigidity, while K2NaScBr6 exhibits greater ductility and isotropic characteristics. The electronic properties determined based on density functional theory (DFT) calculations indicate that K2AgSbBr6 has an indirect bandgap of 0.857 eV, making it suitable for applications using visible light, and K2NaScBr6 has a direct bandgap of 3.107 eV, making it ideal for UV-specific technologies. Optical analyses demonstrate complementary characteristics, particularly in terms of the dielectric function, absorption, reflectivity, energy loss function, refractive index, extinction coefficient, and optical conductivity. K2AgSbBr6 exhibits strong visible light absorptivity.
1. Introduction
The recently emerged organic–inorganic hybrid halide perovskites (), more specifically , have attracted immense attention in optoelectronics and photonics due to their unique properties such as an optimal optical bandgap, high photoluminescence quantum yield, large absorption coefficient, long carrier diffusion length, good carrier mobility, defect tolerance, and low-cost synthesis options [1,2]. The strides we have made since their emergence have been extraordinary. Photovoltaic devices using lead halide perovskite materials improved power conversion efficiency from to . These improvements have excited scientists everywhere, and materials with these properties seem to be promising candidates for alternatives to silicon-based solar cells [3].
In-depth experimental and theoretical studies have highlighted certain exceptional structural and electronic properties that are behind the remarkable photovoltaic characteristics of . First and foremost, the high symmetry of the perovskite structure serves as a basis for its functionalities, and its defect-tolerant behavior maximizes efficiency. Moreover, the - and - orbitals have strong interactions at the edge of the valence band, leading to an antibonding configuration, which, overall, contributes to the remarkable photovoltaic properties of [4]. In quantum physics, the intrinsic thermodynamic instability of may result from the weak chemical bonding of the organic cation and its intrinsic instability [5]. Organic constituents and the toxic properties of lead (), both relevant properties of the halide-based organic–inorganic hybrid perovskites that play a pivotal role in stability issues, present important challenges for chemical stability. While having remarkable properties such as low-cost solution processing, high defect tolerance, and tunability in light emission across visible wavelengths, -based perovskites are limited in some critical aspects [6,7]. These involve susceptibility to extended exposure to light, sensitivity to humidity, thermal instability under environmental conditions, and the toxicological effects of the ions that can bioaccumulate within ecosystems. Moreover, the issues presented by the properties can potentially be amplified by restrictions from the Restriction of Hazardous Substances () directive, greatly limiting their use [8,9,10].
Tackling stability issues has become a part of the research agenda, building on partial success in replacing the organic cation with an inorganic one, potassium. With this step, researchers want to examine the potential of alternative metal halide perovskites, which deliver optoelectronic performance similar to that of the original structure, low toxicity, and enhanced structural stability. Recent studies have uncovered a new type of lead-free halide double perovskite () that has generated a lot of attention. This nanomaterial has shown impressive properties, including excellent environmental stressor resistance, intrinsic thermodynamic stability, and low effective mass for charge carriers; these double perovskites appear to be excellent, non-toxic candidates for usage as photovoltaic absorbers as a substitute for in halide perovskite solar cells [11,12]. are also being investigated for assorted optoelectronic applications, including sensing, thermally stable scintillators for nuclear monitoring, broad-spectrum white phosphors, and detection devices [13,14].
Although compounds have been synthesized and studied since the 1970s, earlier methods were limited. Clearly, the synthesis of Pb-free , which can be written with the chemical formula , where (one of each) and replace two toxic Pb2+ ions, is now worthy of considerable attention. This research aims to develop non-toxic perovskite structures, paving the way for eco-friendly and non-toxic material solutions. are broadly categorized into two types based on the presence or absence of lone-pair electrons in the B′ cations. Type are characterized by cations that lack lone-pair s-electrons (), while Type involve cations with lone-pair -electrons (). These lead-free also exhibit significant potential for ecological sustainability [3].
Recent reviews have provided an extensive assessment of research on a series of and , which have many uses [15,16]. Further, these materials exhibit optoelectronic properties similar to those of lead-based organic–inorganic , thus increasing their utility for various applications [17,18,19,20,21].
In a previous work, we examined the structural and optoelectronic properties of [22], and in this research we will provide a comparative study between and the most recently proposed double perovskite . We propose as a new candidate for UV transparent and lead-free optoelectronics. This general idea was previously considered in terms of but never with an in-depth examination of replacing the B′B″ site with Na+/Sc3+. We will primarily examine what the effects of cation substitution at the sites () are on the structural, optoelectronic, and mechanical properties of these compounds. We center on the first-principles density functional theory () methodology to fully understand the structural characteristics, electronic conductivity, optical properties, elastic constants, and thermodynamic stability of these double perovskite candidates.
This thorough theoretical study utilizes based on to expose the essential relationships between the electronic properties and optical properties, as well as additional mechanical parameters like elastic moduli, Poisson’s ratio, the anisotropy required to characterize these materials, hardness, mean sound velocity, and the Debye temperature of and .
2. Results and Discussions
2.1. Structural Properties and Stability
The structural properties of K2AgSbBr6 and K2NaScBr6 were meticulously optimized in the cubic phase, adopting the space group (no. 225), using the functional. The computational method ramps up the dependability of our prediction on stability and lattice parameters, which facilitate an understanding of the structural considerations in these double perovskites [22,23].
Both K2AgSbBr6 and K2NaScBr6 crystallize in a face-centered cubic structure with the cations at B′ and B″ showing some order at their sites. The B′ and B″ sites are occupied by Ag and Sb in K2AgSbBr6 and by Na and Sc in K2NaScBr6, contributing to unique structural forms. Replacing AgSb with NaSc should dramatically change the lattice geometry, and while the geometry may only be subtly altered, it still changes the crystal structure. The optimized lattice parameters shown in Table 1 facilitate the structural comparisons associated with this cation substitution [24].

Table 1.
Selected bond distances of K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.
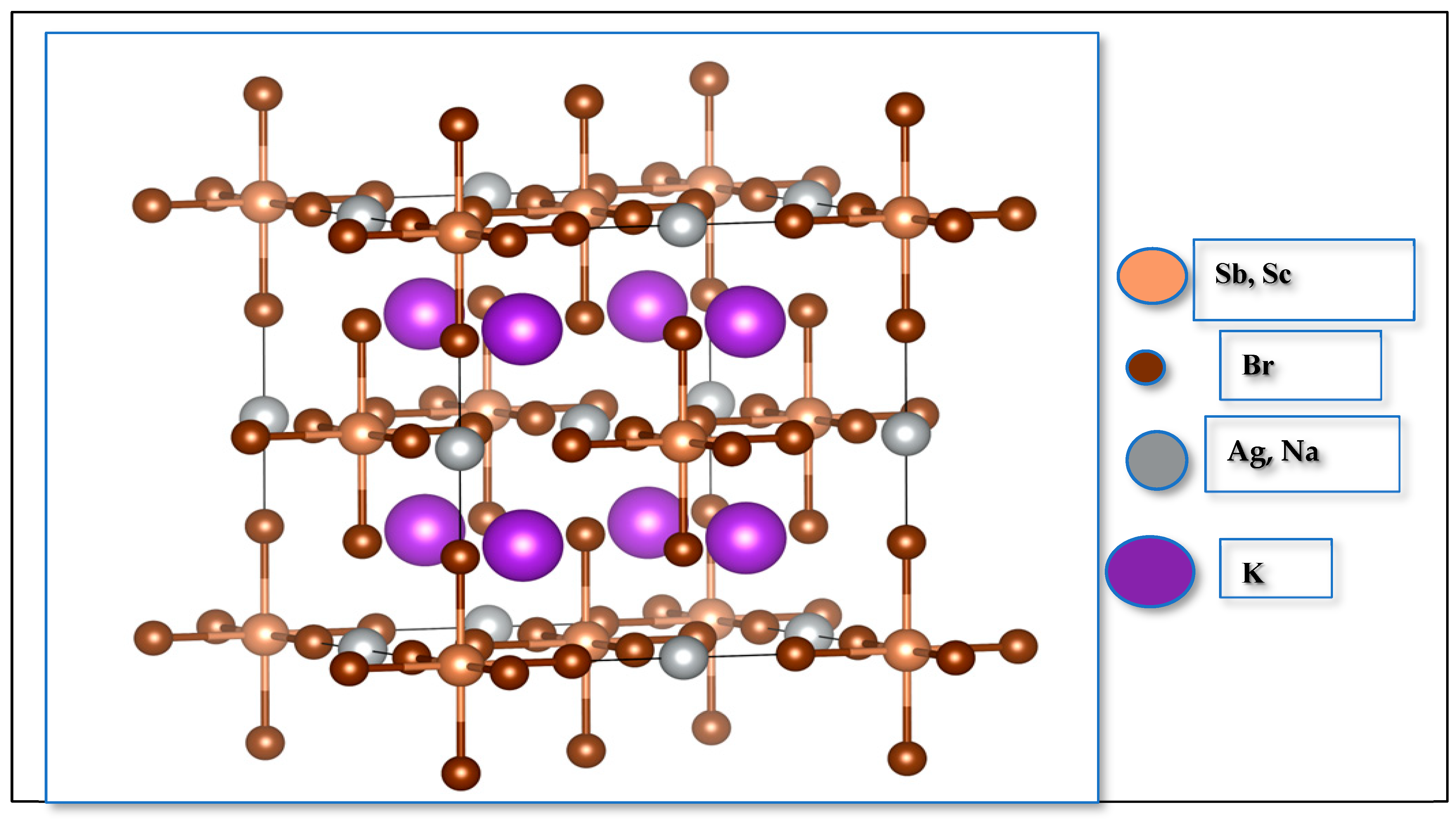
Figure 1 illustrates the structure of K2AgSbBr6 and K2NaScBr6 and their complex arrangements of alternating corner-sharing octahedra which have B′Br6−5 and B″Br6−3 units, where the and cations create local electronic and bonding environments. The octahedra connect to form a strong framework, with cations occupying the sites within cuboctahedral voids [22,23].
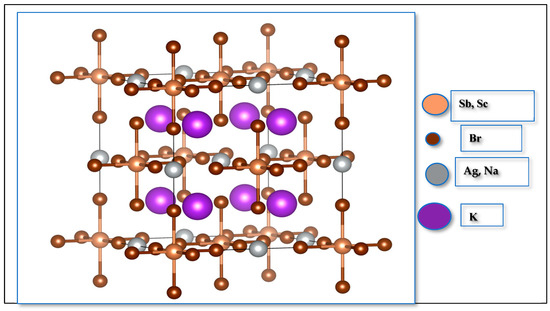
Figure 1.
Crystal structure of K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.
The bond lengths present in the octahedra are displayed in Table 1, and they mirror those of the bond lengths observed in the two compounds and may represent slight structural deviations in both compounds. Such differences underline the critical role of site substitution in shaping the material’s geometry and stability.
The lattice parameters and atomic positions of K2AgSbBr6 and K2NaScBr6, detailed in Table 2 and Table 3, further emphasize the influence of cationic substitution on the overall structural framework.

Table 2.
Selected geometrical (lattice, volumetric, density….) properties of K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.

Table 3.
Atom positions in the crystal structure of the K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.
The perovskite structure of compounds such as [25], , and [26] is often determined by evaluating the values of the octahedral factor () and the Goldschmidt tolerance factor (), as defined by Equation (1):
Here , , , and are the corresponding ionic radii of the ions of the K, B″ (Sb, Sc), B′ (Ag, Na), and Br atoms, respectively, and .
In perovskite structures (AMX3 and A2MM′X6), the octahedral factor () and the tolerance factor () play key roles in assessing structural stability. Stable perovskites typically fall within the ranges of and . Those with a value near are more likely to adopt a cubic phase. If these values deviate significantly from these ranges, it can suggest distortion or instability in the perovskite structure [26].
Following the calculation of the tolerance factor () and octahedral factor () for the two double perovskites, K2AgSbBr6 and K2NaScBr6, a comparative study of their structural, electronic, and optical properties reveals important insights. The values of and for the two compounds are presented in Table 4. Both materials exhibit structural stability, with tolerance factors of 0.86 for K2AgSbBr6 and 0.84 for K2NaScBr6, placing them within the stable range for perovskite structures. However, the slightly lower tolerance factor of K2NaScBr6 suggests a minor deviation from the ideal cubic phase, potentially leading to more subtle structural distortions.

Table 4.
of ions, , , and for K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.
The octahedral factors are 0.48 for K2AgSbBr6 and 0.45 for K2NaScBr6, showing that both materials have relatively stable octahedral geometries, but there may be slightly more structural distortion in K2NaScBr6 because of the smaller cations being used. Overall, both of these materials are still relatively stable and comparable to the typical ranges of stability for perovskite phases, particularly K2AgSbBr6, which is defined as being at least closer to an ideal cubic structure.
Looking at their electrical behavior, both materials seem promising, but existing differences in cationic sizes (cations exhibiting different structural influencing behaviors) may still affect respective electronic functionality and conductivity characteristics. For optical characteristics/filtering applications, both materials should perform actually somewhat similarly to one another based on our observations regarding their feasibility, but K2AgSbBr6 should produce more consistent optical behavior when compared to K2NaScBr6 due to its relatively more stable structure.
The geometric stability is verified through computed tolerance and octahedral factors; however we recognize that intrinsic point defects such as halide vacancies or B site antisites could have an effect on the synthesis and function of these materials. A defect formation energy analysis in detail would be more than the present study is able to conduct and is left for another study.
Thermodynamic stability was confirmed through the calculation of formation energy () [27], which was determined using the formula provided in [22,28]:
Here, represents the total energy of (B′B″ = AgSb, NaSc) compounds, while , , , and denote the individual energies of the K, Ag, B′ (Ag, Na), B″ (Sb, Sc), and Br atoms, respectively. The calculated formation energy () values are negative for all compounds, confirming their thermodynamic stability, as shown in Table 4.
While phonon dispersion or AIMD at finite temperatures could yield more information about dynamic stability, our discussion relies solely on structural criteria and formation energies, consistent with this comparative study performed at 0 K.
2.2. Mechanical Properties
The study of elastic behavior holds considerable relevance in both academic research and industrial applications due to its relevance in various applications [29,30]. The parameters of elastic behavior are essential for evaluating a material’s ductility, brittleness, atomic bonding, anisotropy, and stiffness. For cubic crystals, only three independent elastic constants (, , and ) are needed to define the stiffness matrix , which describes the material’s directional mechanical responses to different applied forces. The longitudinal distortion constant, C11, corresponds to hardness as it relates to longitudinal compression. The transverse constant, C12, is associated with transverse expansion and is tied to . Lastly, the constant, C44, reflects the crystal’s resistance to shearing and is tied to the shear modulus [31,32].
Our calculations confirm that all of the matrix are real and positive for both K2AgSbBr6 and K2NaScBr6 systems. Furthermore, the Born stability for cubic systems are satisfied: (i) ; (ii) ; and (iii) [31,33]. Together with the real and positive eigenvalues of the stiffness matrix, these results indicate that both K2AgSbBr6 and K2NaScBr6 meet the mechanical stability conditions.
According to the elastic parameters reported in Table 5, we observe notable differences between the mechanical properties of K2AgSbBr6 and K2NaScBr6. Both materials have similar values, with K2AgSbBr6 at 36.98 GPa and K2NaScBr6 slightly higher at 37.22 GPa, indicating comparable resistance to longitudinal compression.

Table 5.
Calculated mechanical parameters of K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.
The transverse modulus, C12, differs more significantly: 11.94 GPa for K2AgSbBr6 and 7.92 GPa for K2NaScBr6. This variation suggests that K2NaScBr6 is less resistant to transverse strain, potentially influencing its elastic behavior under applied stress.
For the shear modulus, C44, K2AgSbBr6 shows a higher value of 9.02 GPa compared to 5.65725 GPa for K2NaScBr6. This suggests that K2AgSbBr6 has more resistance to shear deformation which indicates greater mechanical preparedness when experiencing shear stress.
These differences in mechanical performance, shown in Table 5, demonstrate the differing mechanical properties of K2AgSbBr6 and K2NaScBr6, which in turn conveys valuable information about their structural stability and where they could work best in applications.
The mechanical properties of K2AgSbBr6 and K2NaScBr6 were analyzed to gain information about their structural stability, stiffness, and ductility. All mechanical properties were based on elastic constants , , and , which provide information on the mechanical behavior of cubic crystals (see Table 6). Young’s modulus () calculated using quantifies the stiffness of a material [23,34]. While K2AgSbBr6 (26.69 GPa) is stiffer than K2NaScBr6 (21.70 GPa), this is consistent with its greater resistance to tensile strain which can be attributed to the greater ionic size of and compared to and , which aids in the bond network stability.

Table 6.
Calculated values of , , , B, , , , , , λ, and .
The () indicates resistance to uniform compression [34,35], with K2AgSbBr6 (20.29 GPa) slightly outperforming K2NaScBr6 (17.68 GPa). This finding is consistent with K2AgSbBr6 due to its stronger bonding and tighter packing resisting volumetric deformation. The shear modulus () also confirms that K2AgSbBr6 has a greater resistance against shear deformation (10.42 GPa) than K2NaScBr6 (8.37 GPa) in accordance with its greater stiffness.
Poisson’s ratio () was found to be 0.28 for K2AgSbBr6 and 0.29 for K2NaScBr6, suggesting comparable ductility. With respect to Pugh’s ratio (), both materials fall into the ductile category (i.e., ) and have values of K2AgSbBr6 = 1.95 and K2NaScBr6 = 2.11, with K2NaScBr6 being more ductile than K2AgSbBr6. The positive Cauchy pressure () values in K2AgSbBr6 at 2.92 GPa and in K2NaScBr6 at 2.27 GPa also support ductility. The Cauchy pressure parameters suggest that metallic bonding contributions are important for both systems [23,34].
The anisotropy factor () reveals that K2AgSbBr6 (0.72) is more anisotropic than K2NaScBr6 (0.38). This indicates that K2NaScBr6 has relatively more isotropic mechanical behavior and may be useful for targeted applications that are insensitive to crystallographic directionality and require homogeneous mechanical performance [23,35].
The Debye temperature calculated from elastic constants and sound velocity is an index of vibrational stability and thermal conduction [36,37]. K2AgSbBr6’s Debye temperature θ of 169.90 K is larger than K2NaScBr6’s Debye temperature of 165.40 K, which indicates that K2AgSbBr6 is capable of better phonon propagation and thermal transport. Accordingly, the average sound velocity () of K2AgSbBr6 (1877.65 ) is larger than that of K2NaScBr6 (1783.52 ), corroborating the increased stiffness and denser structure of K2AgSbBr6 compared to K2NaScBr6.
Lame’s parameter () further confirms the volumetric stability of K2AgSbBr6 (13.34 GPa) over K2NaScBr6 (12.10 GPa). On the other hand, compressibility () indicates that K2NaScBr6 (0.05653 1/GPa) is more compressible than K2AgSbBr6 (0.0493 1/GPa), which correlates with its lower bulk modulus and thus a softer bonding environment [38,39].
In conclusion, K2AgSbBr6 has better stiffness, resistance to shape change, and thermal and acoustic properties and is appropriate for applications that require mechanical stability and thermal transport. Conversely, K2NaScBr6 has better ductility, isotropy, compressibility, and robustness of applicable bending and deformity, while K2AgSbBr6 has stiffness and rigidity better suited to applications where pliancy is not a requirement for effectiveness. The noted differences are thus a consequence of substituting Ag+/Sb3+ with Na+/Sc3+ at the B′ and B″ sites, which relates directly to bond strength and lattice ties.
The relatively low bulk and shear moduli values determined from both K2AgSbBr6 and K2NaScBr6 are in agreement with previously reported DFT values for lead-free halide double perovskites featuring Ag+, Bi3+, Sb3+, or In3+ cations and showing weak inter-atomic bonding with open frameworks, i.e., softness. The Cs2AgSbCl6 and Cs2AgInCl6 compounds have also shown similar mechanical softness, which have been shown to have bulk moduli below 25 GPa [Ref]. In addition, the elastic constants predicted here satisfy the Born mechanical stability criteria for cubic systems of which both materials are confirmed stable. The stress convergence criterion used for the structural relaxations was 0.1 GPa, which should be sufficiently small to provide numerically stable and physically meaningful results for the size of systems considered. However, it is acknowledged that by further tightening the stress convergence, the elastic moduli values will be better estimates, and this will be addressed in future work [23,40,41,42].
2.3. Electronic Properties
A material’s performance in various applications is influenced by its electronic and optoelectronic properties. The electronic properties of K2AgSbBr6 and K2NaScBr6 were studied using their band structures [22,23], total density of states (DOS), and projected density of states (PDOS). These analyses provide important information about the electronic bandgap, electronic transition, and orbital participation of the contributing orbitals near the Fermi level, which is important to understand their functional properties [43,44].
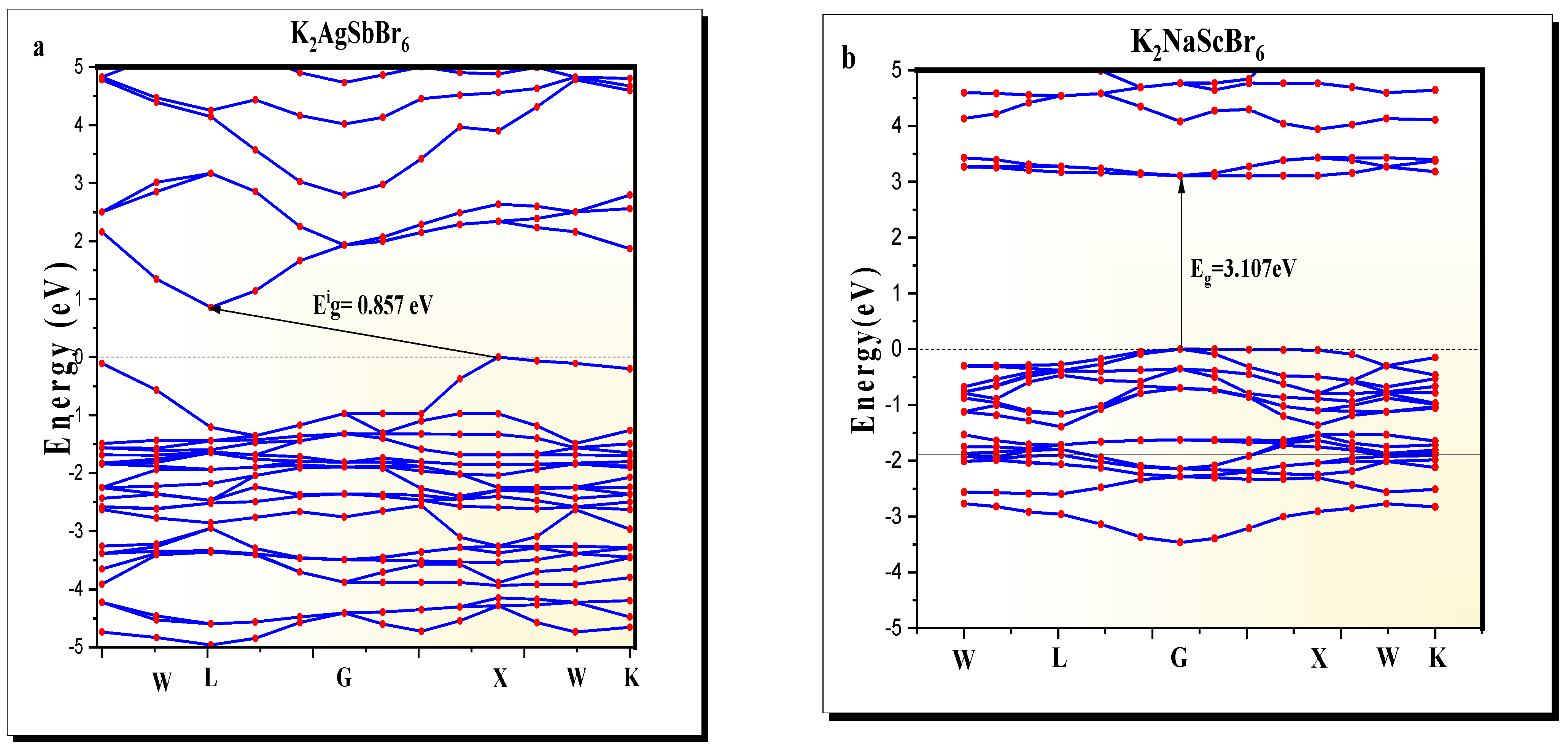
The band structure of K2AgSbBr6 (Figure 2a) exhibits an indirect bandgap of , a value representing semiconducting behavior and ideal for optoelectronic applications such as photovoltaics and photodetection. This indirect transition is due to the valence band maximum aligning with the conduction band minimum at different k-points in the zone. K2NaScBr6 (Figure 2b), on the other hand, exhibits a direct bandgap of at the point, which is indicative of a material favorable for wide-bandgap applications, such as photodetectors and insulating layers. Its direct bandgap suggests that electronic transitions are optically allowed for, implying suitable potential for light-based applications.
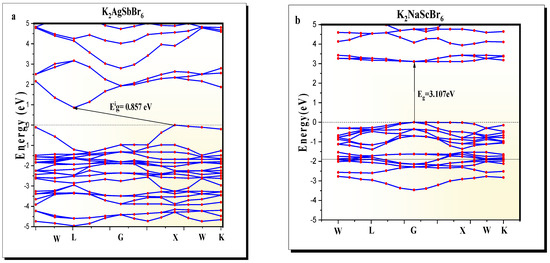
Figure 2.
Band structure of (a) K2AgSbBr6 and (b) K2NaScBr6 double perovskites.
It is well-known that the GGA-PBE approximation systematically underestimates bandgap values as it conducts an approximate treatment of the exchange–correlation effects when compared to other methods. Many other calculations use more robust levels of theory such as hybrid functionals (i.e., HSE06) or GW, and generally, the calculated values are closer to the experimental values. For example, in other similar compounds such as Cs2AgBiBr6 or Cs2AgInCl6, the bandgap typically increases from about 1.3 eV (PBE) to approximately 2.0 eV (HSE06) [3,19]. Therefore, while absolute values can be better corrected with higher levels of theory, the relative comparison of K2AgSbBr6 vs. K2NaScBr6 was valid because both materials were treated at the same theory level.
While spin–orbit coupling (SOC) was not considered in this study, it has been shown to have a non-negligible effect on halide perovskites that contain heavy atoms (such as Sb and Br). In general, SOC will reduce the bandgap through the splitting of the valence and conduction band edges and will also, in some instances, change the nature of the bandgap. For example, for Cs2AgBiBr6, the inclusion of SOC lowers the bandgap by approximately 0.4 eV and shifts the extrema of the bands slightly [19]. In this study, we excluded SOC to be consistent with our previous GGA-PBE study of K2AgSbBr6 [22]. However, SOC may be included in future studies to improve the quantitative accuracy of the band structure, especially for applications where precise knowledge of the electronic transitions is required.
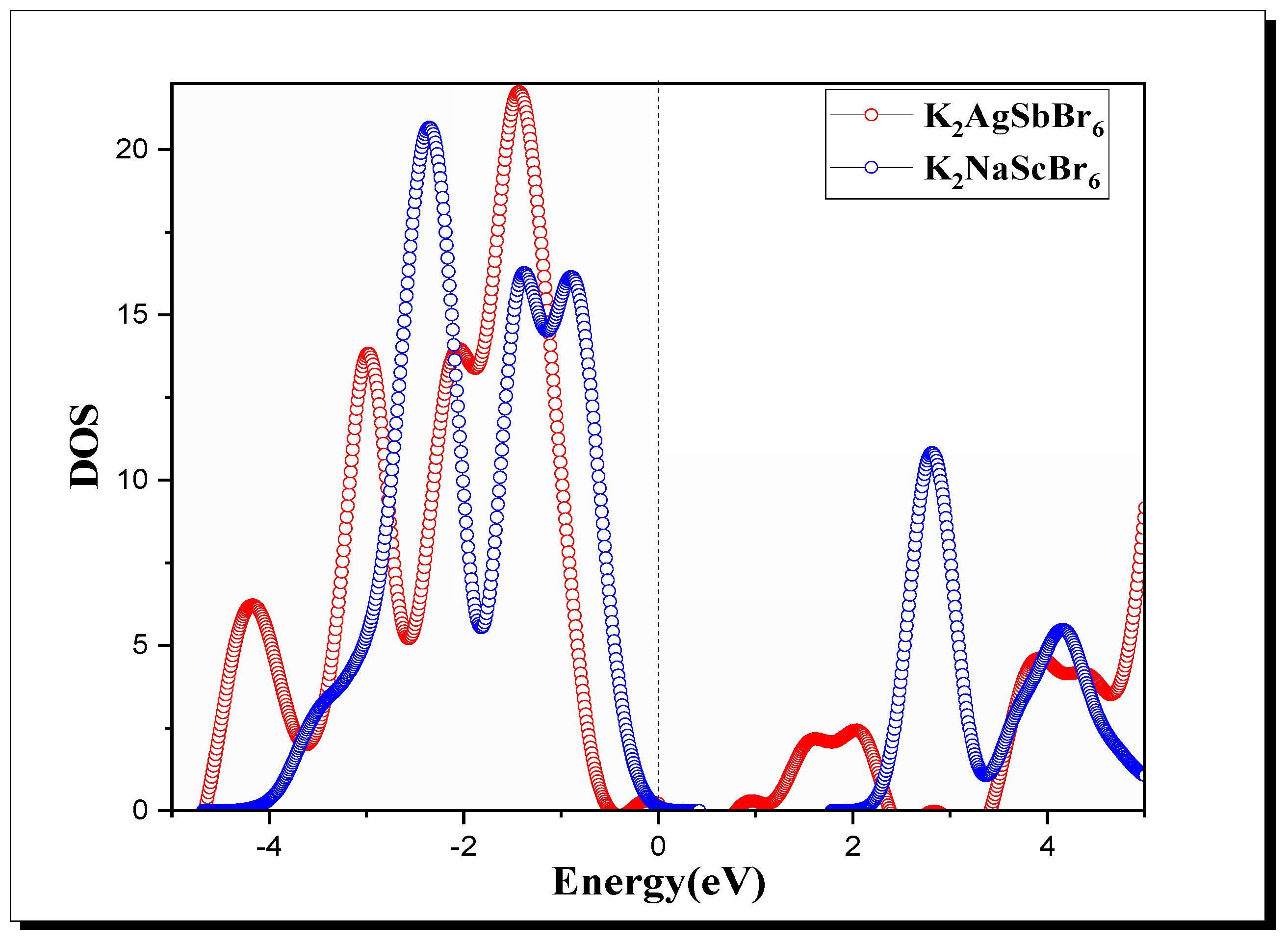
The (Figure 3) highlights the distribution of electronic states for both materials [45,46]. For K2AgSbBr6, the states near the VBM are primarily composed of contributions from the orbitals of and , with additional input from ’s orbitals. The is characterized by antibonding interactions involving the Sb and Br orbitals. In K2NaScBr6, the reveals that the is predominantly composed of states, while the is influenced by states, resulting in a less dense distribution of states near the level compared to K2AgSbBr6.
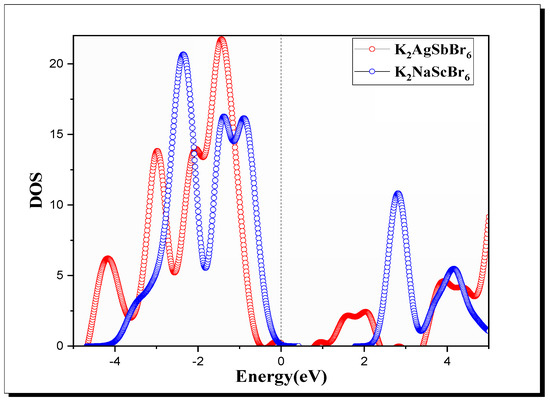
Figure 3.
DOS of K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.
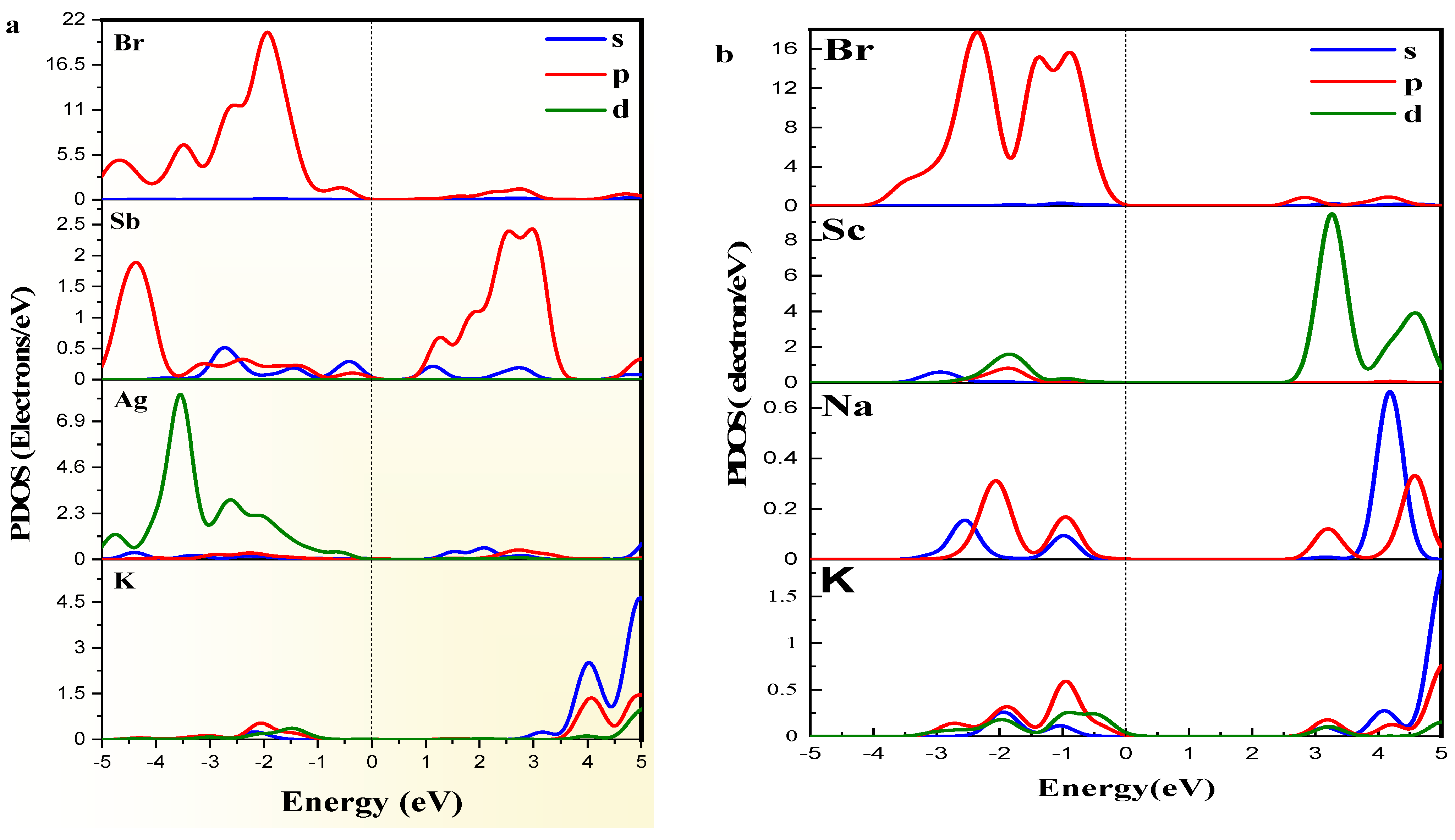
The (Figure 4a,b) provides a deeper understanding of the atomic contributions to the electronic structure [45]. For K2AgSbBr6, the shows strong contributions from the orbitals of and , alongside the orbitals of , resulting in a denser and more hybridized electronic structure. The is primarily dominated by Sb-p and Br-p states, reflecting strong interactions. Conversely, for K2NaScBr6, the is mainly driven by orbitals, while the features states with minimal hybridization. This reduced interaction leads to a noticeable increase in the bandgap and associated effects on electronic transitions in the material.
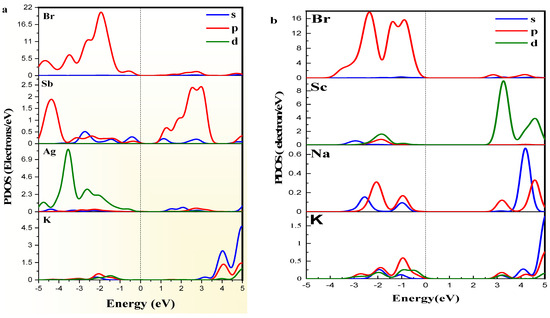
Figure 4.
PDOS of (a) K2AgSbBr6 and (b) K2NaScBr6 double perovskites.
The differences between K2AgSbBr6 and K2NaScBr6 arise because of the substitution of and at the and sites. K2NaScBr6 significantly reduced the overlapping orbitals between the metal and halide orbitals, which correlates to a larger direct bandgap and a different electronic structure. These observed differences demonstrate the extent to which cation substitution can change the electronic properties of double perovskites.
As a final point, K2AgSbBr6’s relatively smaller indirect bandgap and high density of electronic states near the Fermi level make it quite amenable to integration into optoelectronic systems, for example, as light absorbers in photovoltaic cells or photodetectors. On the other hand, K2NaScBr6, with its larger direct bandgap and weaker hybridization, will be useful in wide-bandgap applications including photodetectors or insulating layers in electronic devices. These results demonstrate the plethora of tunable electronic properties in halide double perovskites and how they can be used to solve complications in engineering for application-specific compositions.
2.4. Optical Properties
The optical properties of materials, particularly their response to electromagnetic radiation, are critical for determining their suitability in optoelectronic applications. These properties are commonly described by the dielectric function [47,48]:
where is the real part (representing dispersion), and is the imaginary part (representing absorption). The dielectric function provides insights into electronic transitions, energy loss mechanisms, and light–matter interactions.
The real part describes the ability of the material to store energy under an applied electric field from outside, while the imaginary part represents the use of electromagnetic radiation through absorption, which comes from interband electronic transitions. These quantities are generated from first-principles calculations based on the formalism, which connects to the electronic structure of the material [49].
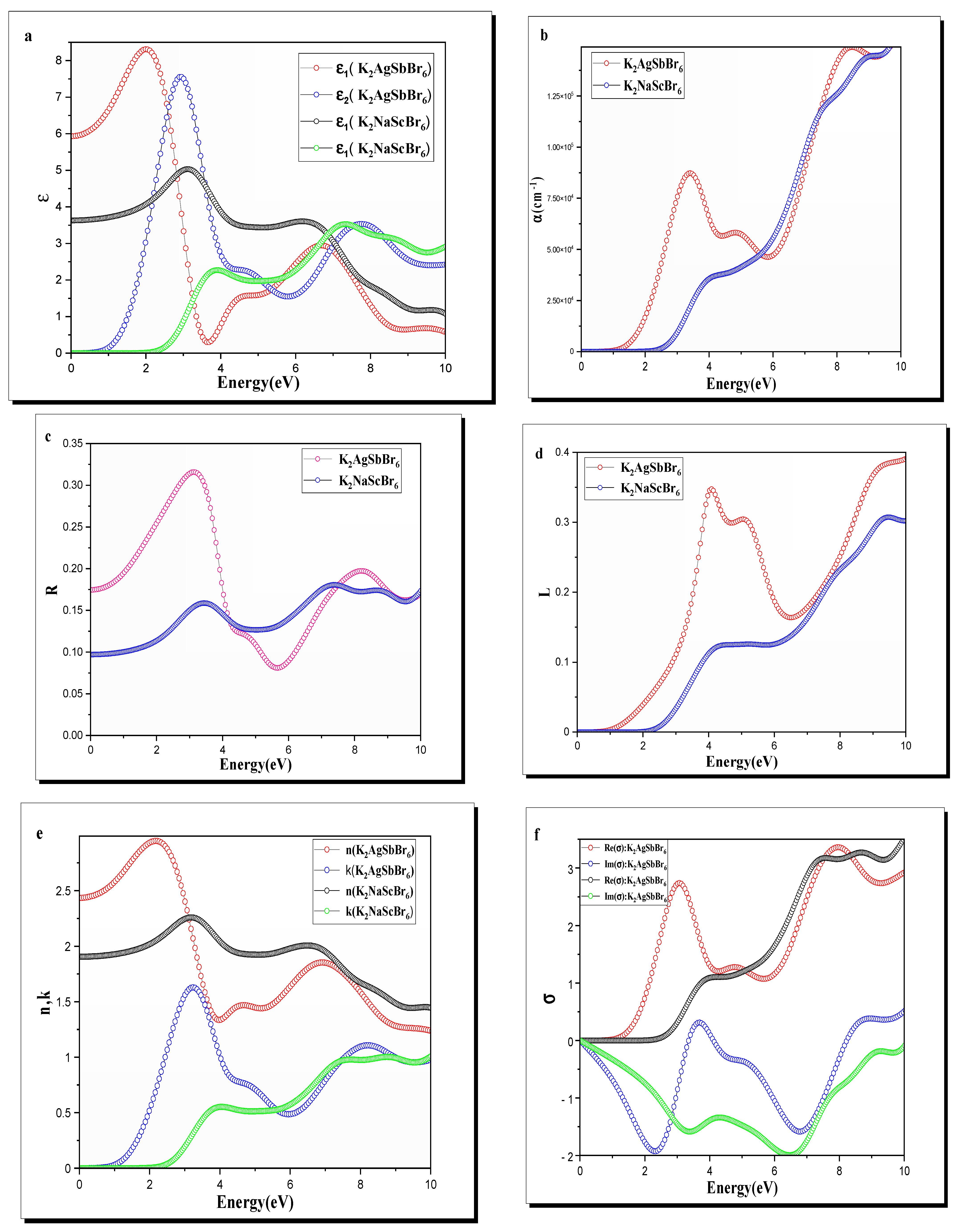
Figure 5a shows the calculated dielectric functions for K2AgSbBr6 and K2NaScBr6. The static dielectric constant , which is equal to at the zero-frequency limit, is greater for K2AgSbBr6 than for K2NaScBr6. This is due to the greater hybridization of the and orbitals in K2AgSbBr6 and simply a larger polarizability. K2NaScBr6 has lower values for the static dielectric constant because of a larger energy bandgap and less hybridization of the and orbitals.
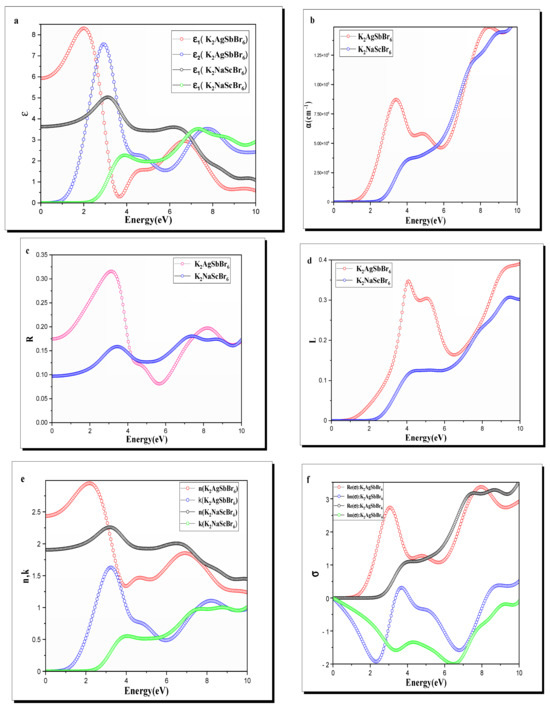
Figure 5.
(a) Dielectric function, (b) absorption coefficient, (c) reflectivity, (d) loss function, (e) complex refractive index, and (f) conductivity of K2B′B″Br6 (B′B″ = AgSb, NaSc) double perovskite.
The imaginary part reflects the range of energies where absorption occurs due to interband transitions. Primarily, for K2AgSbBr6, the largest absorption peak is found at close to , which 2.5*corresponds to electronic transitions from the maximum of the valence band (with states that are heavily and ) to the minimum of the conduction band (involving states). K2NaScBr6 on the other hand has its first absorption peak near 4 eV, consistent with its larger bandgap and electronic transitions involving mainly the and orbitals.
The larger value of for K2AgSbBr6 in the visible region indicates that it absorbs more light in the visible range than K2NaScBr6, making it a more favorable option for growing visible light-absorbing layers in photovoltaic devices. In contrast, the smaller amount of in the visible range for K2NaScBr6 suggests that the material is well-suited for devices that require transparency or an active material that interacts with ultraviolet light, like ultraviolet photodetectors or insulating layers.
The absorption coefficient conveys how well a material absorbs light as a function of its photon energy and is important in evaluating materials for optoelectronic applications. The absorption coefficient is derived from the dielectric function as follows [50,51]:
As shown in Figure 5b, the absorption spectra of K2AgSbBr6 and K2NaScBr6 are depicted. For the K2AgSbBr6 material, strong absorption is observed from energies of approximately , consistent with its narrow bandgap material and relevant transitions involving the hybridized and orbitals. The absorption intensity increases quickly through the near-visible region and peaks in the visible regime, around , which is what makes it great for applications that harvest light energy, such as photovoltaics and photodetectors.
K2NaScBr6, on the other hand, starts absorbing at a higher energy (), which is expected from the larger direct bandgap. The absorption steadily increases and peaks around , primarily due to the transitions involving and states. Given this behavior, the K2NaScBr6 material is better suited for applications that absorb high-energy UV light energy, such as UV photodetectors and UV transparent electronics.
The broader absorption range of K2AgSbBr6 in the visible spectrum confirms its potential for effective solar energy conversion applications, while the narrower range and higher energy onset of K2NaScBr6 reflect its potential to be a material for optical applications in which it could exhibit transparency or UV activity.
The optical spectra that are detailed in this paper were calculated in the independent-particle approximation, which does not include any excitonic effects or scissor correction. Hence, we undoubtedly biased some calculated absorption onset, which appears to be underestimated for the PBE functional, largely due to the intrinsic bandgap of the PBE functional approximated for this material class. This will somewhat limit the quantitative accuracy for optical constants, but the relative comparison of K2AgSbBr6 to K2NaScBr6 remains valid and helps to consolidate ideas. Note, previous work on Cs2AgBiBr6 and Cs2AgInCl6 has indicated that scissor shifts and hybrid functionals such as HSE06 can very much improve agreement with experiments [3,42]. Including many-body effects such as electron–hole interactions (using the Bethe–Salpeter equation) would provide an even more accurate representation of the predicted spectra and would be a suitable target for future work.
Reflectivity quantifies the amount of incident light rejected from a material at its surface and is important for applications like photovoltaics where the goal is to reduce reflection energy loss to improve efficiency. Reflectivity is determined from the real and imaginary parts of the dielectric function ( and ) using the following [22,47]:
The reflectivity spectra of K2AgSbBr6 and K2NaScBr6 are shown in Figure 5c. K2AgSbBr6 has a higher reflectivity in the visible region, with a peak near , which corresponds to the strong optical transitions after hybridizing the contribution of the , , and orbitals. This behavior is consistent with its high absorption in the visible range, meaning that only a very small amount of light is reflected, and the remaining light is absorbed, which makes it a good candidate for applications in light-harvesting devices.
K2NaScBr6, on the other hand, shows a relatively lower reflectivity across the visible spectrum, with peaks occurring at higher photon energies (~4 eV) due to transitions involving the Br-p and Sc-d orbitals. The reduced reflectivity at visible wavelengths highlights its potential for transparency-focused applications or UV-sensitive devices, where minimal reflection in the visible range is desirable.
At higher energies (>5 eV), the reflectivity of both materials converges, indicating similar optical behavior in this region, likely dominated by high-energy interband transitions. The distinct reflectivity profiles of the two materials further emphasize their diverse optical applications.
Following the discussion on reflectivity, the energy loss function offers additional insight into the electronic excitation behavior of K2AgSbBr6 and K2NaScBr6. The loss function is defined as follows [45,47]:
represents the energy dissipation of fast-moving electrons interacting with the material. Peaks in are directly linked to plasmon resonances, revealing energy ranges of significant collective electronic oscillations.
The loss spectra for both compounds, depicted in Figure 5d, exhibit distinct behaviors. For K2AgSbBr6, the primary loss peak occurs at approximately 6.2 eV, corresponding to the plasmon resonance frequency. This peak is associated with the collective oscillations of free electrons, strongly influenced by the hybridized Ag-d, Sb-p, and Br-p orbitals. The sharp and intense nature of the peak indicates lower dielectric screening and strong electronic excitation within this energy range.
Conversely, K2NaScBr6 shows a plasmon resonance peak at a higher energy of . The shift in to increases the bandgap and changes the electronic environment. The broader and less intense peak of K2NaScBr6 suggests reduced plasmonic activity and higher dielectric screening, consistent with its lower free carrier density.
The differences in the loss spectra align with the contrasting dielectric properties of the two materials. K2AgSbBr6, with its smaller bandgap and higher polarizability, exhibits a lower-energy, more pronounced plasmonic resonance. Meanwhile, the higher-energy resonance of K2NaScBr6 reflects its wider bandgap and reduced electronic excitations.
Building on the energy loss function , the refractive index and the extinction coefficient (complex refractive index), as optical parameters, depend on the dielectric function. These parameters describe how light propagates through a material, with representing the material’s ability to bend light and characterizing the material’s absorption of light. The refractive index and extinction coefficient are expressed as follows [52,53]:
Figure 5e illustrates the calculated and spectra for K2AgSbBr6 and K2NaScBr6. The static refractive index , corresponding to the zero-frequency limit, is higher for K2AgSbBr6 compared to K2NaScBr6, reflecting its enhanced polarizability due to the hybridized , , and orbitals. The peak value of for K2AgSbBr6 occurs at approximately 2.5 eV, indicating strong dispersion in the visible range, which is beneficial for light-harvesting applications.
The refractive index of K2NaScBr6 is lower than that of other compounds throughout the visible spectrum, with a maximum at roughly 4 eV. This trend in is due its larger energy bandgap and lower polarizability, as in K2AgSbBr6 is replaced by which induces less hybridization of the and orbitals.
The extinction coefficient , which characterizes the absorption of a material, displays the same trends as absorption coefficient measurements. for K2AgSbBr6 exhibits a considerable increase around 2.5 eV, which suggests electronic transitions from and orbital contributions to the conduction band. The calculated K2NaScBr6 shows a lower in the visible range and a noticeable increase around , confirming the limiting of transparent conductors for UV applications.
The complex optical conductivity reflects the response of the material to optical excitations and accounts for the contribution of the material to conduction under an applied electromagnetic field. It is expressed as follows [54,55]:
In optoelectronic studies, optical conductivity is consistently expressed with the real and imaginary parts of complex conductivity, defined by the following, where is the real part (dissipative (conductive), and is the imaginary part (energy storage). These components are directly derived from the dielectric function using the following [40,56]:
Figure 5f shows the complex optical conductivity spectra for K2AgSbBr6 and K2NaScBr6.
For K2AgSbBr6, the real part shows a strong peak at about 2.5 eV that arises from very strong optical transitions involving the and orbitals at the valence band and the conduction band minimum which correspond to the high absorption and high extinction coefficient of visible light where this material is consistently capable of conducting excitation energy from light. The imaginary part reached its peak slightly below an energy of 2.5 eV, in part representing the energy stored by interband transitions.
The real part for K2NaScBr6 shows a much lower intensity peak at the expected higher energy (~4 eV) based on a larger bandgap and expected UV-active behavior. This results in a weaker ability to conduct excitation energy in the visible range for K2NaScBr6 based on the observed lower hybridization of with . The imaginary part also has a peak in UV with a trend similar to its real part, which corresponds to the electronic transitions corresponding to higher energy states.
With its higher conductivity and wider response range in the visible spectrum, K2AgSbBr6 is an attractive option for optoelectronic devices, especially those requiring the efficient conversion of incident light to electrons, such as photodetectors and solar cells. In contrast, K2NaScBr6 has a narrower response range spectrum, with its conductivity being active in the UV range, making it more applicable in devices that utilize higher-energy light, such as UV photodetectors and transparent layers for optoelectronic devices.
The optical spectra in this work were computed using the Kubo–Greenwood formalism, which does not include excitonic effects arising from electron–hole (e–h) interactions. In bulk 3D halide double perovskites such as K2AgSbBr6 and K2NaScBr6, the relatively large dielectric screening typically results in low exciton binding energies, suggesting that excitonic effects may only slightly shift the absorption edge without significantly altering the overall spectral profile. Previous studies on related materials, such as Cs2AgBiBr6 and Cs2AgInCl6, have shown exciton binding energies on the order of tens of meV, indicating that the independent-particle approximation remains reasonable for qualitative optical analysis in these systems. Nonetheless, future work involving many-body perturbation theory (e.g., BSE) would help clarify the precise excitonic influence [50].
3. Materials and Methods
The calculations employed the Projected Augmented Wave () pseudopotential method incorporated in the code (CAmbridge Serial Total Energy Package) [57,58,59]. The model configurations of and were systematically relaxed, with periodic boundary conditions applied to the system. For exchange and correlation (xc) functionals, we utilized the with –– () in our computational approach [60,61]. The interactions between the core ions and valence electrons were accurately represented using norm-conserved pseudopotentials. The pseudopotentials considered the valence states of (), (), (), (), (), and () for the materials under study. Geometry optimization was performed using the ––– minimization procedure [62,63]. The atomic configurations were relaxed using a force convergence criterion of , with the conjugate gradient minimization algorithm. The zone was sampled using a of 4 × 4 × 4 for the optimization of the structures [64]. Energy calculations were carried out with a total energy tolerance of per atom, stress tolerance of , ionic displacement of , and an accuracy of for ionic relaxation. A plane wave energy cut-off of was employed for the calculations.
4. Conclusions
This study outlines a thorough analysis of the structural, mechanical, electronic, and optical properties of K2AgSbBr6 and K2NaScBr6, with a focus on how cation substitution impacts the materials’ properties. The structural stability of both materials was verified through reasonable tolerance and octahedral factors, as well as negative formation energies, indicating good thermodynamic feasibility. The mechanical properties of each material reveal K2AgSbBr6 to have higher stiffness and volumetric compressibility, while K2NaScBr6 possesses better ductility and nearly isotropic mechanical properties. This finding indicates that by varying the cation, there is a trade-off between rigidity and flexibility, depending on the intended application. From an electronic perspective, K2AgSbBr6 has an indirect bandgap of 0.857 eV, which would be useful for visible light-harvesting technologies, while K2NaScBr6 has a direct bandgap of 3.107 eV, which would work well for ultraviolet applications. The density of states analysis and orbital hybridization data further highlighted the mere fact that the choice of cation changes the nature of the electronic transitions and influences the overall band structure.
With respect to optical performance, K2AgSbBr6 shows strong absorption in the visible range, and its reflectivity and plasmonic properties could allow for its use in photovoltaics and photodetectors. In contrast, K2NaScBr6 shows considerable UV absorption and conductivity, suggesting that it could have applications in UV photonic devices and transparent optoelectronic applications.
Ultimately, the tunable properties afforded by the cation engineering of K2AgSbBr6 and K2NaScBr6 indicate the potential for their use in many optoelectronic applications. The customizability of structural integrity, electronic properties, and optical properties positions K2AgSbBr6 and K2NaScBr6 as strong candidates to usher developments in the photonic and electronic space.
Author Contributions
Conceptualization, A.E. and O.B.; methodology, A.E. and M.K.; software, M.K., H.E. and A.E.; validation, O.B. and Y.L.; formal analysis, A.E. and H.E.; investigation, A.E.h. and A.L.; resources, H.E. and M.K.; data curation, O.B.; writing—original draft preparation, A.E.; writing—review and editing, A.E.; visualization, O.B.; supervision, O.B. and Y.L.; project administration, O.B. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, S.; Yin, H.; Liu, P.; Wang, Y.; Zhao, H. Stabilization and Performance Enhancement Strategies for Halide Perovskite Photocatalysts. Adv. Mater. 2023, 35, 2203836. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, J.; Yang, X.; Duan, Y.; Yang, P.; Tang, Q. Review on Recent Progress of Lead-Free Halide Perovskites in Optoelectronic Applications. Nano Energy 2021, 80, 105526. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Saravana Karthikeyan, S.K.S.; Kong, C.Y.; Zhang, F.; Guo, X.; Dang, N.N.; Ramaraj, S.G.; Liu, X. Structural, Electronic, Optical, Elastic, Thermodynamic and Thermal Transport Propertie of Cs2AgInCl6 and Cs2AgSbCl6 Double Perovskite Semiconductors Using a First-Principles Study. Phys. Chem. Chem. Phys. 2023, 25, 31848–31868. [Google Scholar] [CrossRef]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and Crystal Chemistry of the Hybrid Perovskite (CH3NH3)PbI3 for Solid-State Sensitised Solar Cell Applications. J. Mater. Chem. A Mater. 2013, 1, 5628. [Google Scholar] [CrossRef]
- Bakulin, A.A.; Selig, O.; Bakker, H.J.; Rezus, Y.L.A.; Müller, C.; Glaser, T.; Lovrincic, R.; Sun, Z.; Chen, Z.; Walsh, A.; et al. Real-Time Observation of Organic Cation Reorientation in Methylammonium Lead Iodide Perovskites. J. Phys. Chem. Lett. 2015, 6, 3663–3669. [Google Scholar] [CrossRef]
- deQuilettes, D.W.; Zhang, W.; Burlakov, V.M.; Graham, D.J.; Leijtens, T.; Osherov, A.; Bulović, V.; Snaith, H.J.; Ginger, D.S.; Stranks, S.D. Photo-Induced Halide Redistribution in Organic–Inorganic Perovskite Films. Nat. Commun. 2016, 7, 11683. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Zhou, W.-K.; Zhou, X.; Liu, K.-H.; Yu, D.-P.; Zhao, Q. Quantification of Light-Enhanced Ionic Transport in Lead Iodide Perovskite Thin Films and Its Solar Cell Applications. Light Sci. Appl. 2016, 6, e16243. [Google Scholar] [CrossRef]
- Igbari, F.; Xu, F.-F.; Shao, J.-Y.; Ud-Din, F.; Siffalovic, P.; Zhong, Y.-W. Stacking Interactions and Photovoltaic Performance of Cs2AgBiBr6 Perovskite. Sol. RRL 2023, 7, 2200932. [Google Scholar] [CrossRef]
- Mathew, N.P.; Rajeev Kumar, N.; Radhakrishnan, R. First Principle Study of the Structural and Optoelectronic Properties of Direct Bandgap Double Perovskite Cs2AgInCl6. Mater. Today Proc. 2020, 33, 1252–1256. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Q.; Shao, Y.; Lu, H.; Li, T.; Gruverman, A.; Huang, J. Electric-Field-Driven Reversible Conversion Between Methylammonium Lead Triiodide Perovskites and Lead Iodide at Elevated Temperatures. Adv. Energy Mater. 2016, 6, 4122–4128. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zeng, H. Lead-Free Halide Double Perovskites: Structure, Luminescence, and Applications. Small Struct. 2021, 2, 2000071. [Google Scholar] [CrossRef]
- Ji, F.; Boschloo, G.; Wang, F.; Gao, F. Challenges and Progress in Lead-Free Halide Double Perovskite Solar Cells. Sol. RRL 2023, 7, 2201112. [Google Scholar] [CrossRef]
- Sakhatskyi, K.; Turedi, B.; Matt, G.J.; Wu, E.; Sakhatska, A.; Bartosh, V.; Lintangpradipto, M.N.; Naphade, R.; Shorubalko, I.; Mohammed, O.F.; et al. Stable Perovskite Single-Crystal X-Ray Imaging Detectors with Single-Photon Sensitivity. Nat. Photonics 2023, 17, 510–517. [Google Scholar] [CrossRef]
- Bai, Y.; Hao, M.; Ding, S.; Chen, P.; Wang, L. Surface Chemistry Engineering of Perovskite Quantum Dots: Strategies, Applications, and Perspectives. Adv. Mater. 2022, 34, 2105958. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Y.; Zhao, J.; Liu, Q.; Xia, Z. Design Optimization of Lead-Free Perovskite Cs 2 AgInCl6:Bi Nanocrystals with 11.4% Photoluminescence Quantum Yield. Chem. Mater. 2019, 31, 3333–3339. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Sui, L.; Yan, J.; Wang, K.; Yuan, K.; Mao, W.L.; Zou, B. Tuning Emission and ElectronPhonon Coupling in Lead-Free Halide Double Perovskite Cs2AgBiCl6 under Pressure. ACS Energy Lett. 2019, 4, 2975–2982. [Google Scholar] [CrossRef]
- Dong, C.; Guan, X.; Wang, Z.; Zhao, H.; Kuai, Y.; Gao, S.; Chen, C.; Zou, W.; Lu, P. The Effects of Cation and Halide Anion on the Stability, Electronic and Optical Properties of Double Perovskite Cs2NaMX6 (M = In, Tl, Sb, Bi; X = Cl, Br, I). Comput. Mater. Sci. 2023, 220, 112058. [Google Scholar] [CrossRef]
- Shadabroo, M.S.; Abdizadeh, H.; Golobostanfard, M.R. Elpasolite Structures Based on A2AgBiX6 (A: MA, Cs, X: I, Br): Application in Double Perovskite Solar Cells. Mater. Sci. Semicond. Process. 2021, 125, 105639. [Google Scholar] [CrossRef]
- Tran, T.T.; Panella, J.R.; Chamorro, J.R.; Morey, J.R.; McQueen, T.M. Designing Indirect–Direct Bandgap Transitions in Double Perovskites. Mater. Horiz. 2017, 4, 688–693. [Google Scholar] [CrossRef]
- Munio, A.A.Z.; Liboon, A.Q., Jr.; Lagud, Y.J.; Patayon, U.B.; Pido, A.A.G.; Karouchi, M.; Ambolode II, L.C.C. A Density Functional Theory Study on the Interaction of Cellulose Biopolymer and Atomic Arsenic. Solid State Phenom. 2023, 352, 39–46. [Google Scholar] [CrossRef]
- Iqbal, J.; Ullah, H.; Archi, M.; Ullah, N.; Ullah, S.; Ullah, R.; Iqbal, Z. Enchanting Optical, Electronic, and Mechanical Properties of the Sodium Based Na2MgSiZ6 (Z = I, Br, Cl) Halides Have Been Explored through DFT. Phys. Scr. 2024, 99, 115949. [Google Scholar] [CrossRef]
- Ejjabli, A.; Karouchi, M.; Al-Hattab, M.; Bajjou, O.; Rahmani, K.; Lachtioui, Y. Investigation of Lead-Free Halide K2AgSbBr6 Double Perovskite’s Structural, Electronic, and Optical Properties Using DFT Functionals. Chem. Phys. Impact 2024, 9, 100656. [Google Scholar] [CrossRef]
- Jamshaid, S.; Nasarullah; Aldaghfag, S.A.; K Butt, M.; Yaseen, M.; Murtaza, A.; Ishfaq, M.; Hegazy, H.H. Investigation of Cubic K2NaXBr6(X=Sc, Y) Double Perovskites for Optical and Thermoelectric Devices. J. Phys. Chem. Solids 2023, 178, 111341. [Google Scholar] [CrossRef]
- Wallace, D.C.; Patrick, J.L. Stability of Crystal Lattices. Phys. Rev. 1965, 137, A152–A160. [Google Scholar] [CrossRef]
- Feng, L.M.; Jiang, L.Q.; Zhu, M.; Liu, H.B.; Zhou, X.; Li, C.H. Formability of ABO3 Cubic Perovskites. J. Phys. Chem. Solids 2008, 69, 967–974. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef]
- Emery, A.A.; Wolverton, C. High-Throughput DFT Calculations of Formation Energy, Stability and Oxygen Vacancy Formation Energy of ABO3 Perovskites. Sci. Data 2017, 4, 170153. [Google Scholar] [CrossRef] [PubMed]
- Laassouli, A.; Karouchi, M.; Ejjabli, A.; Lachtioui, Y.; Bajjou, O. DFT Study on K2AgSbI6: Exploring the Electronic, Optical, and Elastic Properties of a Double Perovskite. Solid State Commun. 2025, 402, 115947. [Google Scholar] [CrossRef]
- Arikan, N.; DikiCi Yildiz, G.; Yildiz, Y.G.; İyigör, A. Electronic, Elastic, Vibrational and Thermodynamic Properties of HfIrX (X = As, Sb and Bi) Compounds: Insights from DFT-Based Computer Simulation. J. Electron. Mater. 2020, 49, 3052–3062. [Google Scholar] [CrossRef]
- Laassouli, A.; Moulaoui, L.; Najim, A.; Archi, M.; Karouchi, M.; Rahmani, K.; Lachtioui, Y.; Bajjou, O. Electronic and Optical Properties of Cl-Doped CH3NH3SnI3 Perovskite: A DFT Study. E3S Web Conf. 2025, 601, 00013. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.-X. Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Archi, M.; Moulaoui, L.; Karouchi, M.; Darkaoui, E.; Laassouli, A.; Bajjou, O.; Rahmani, K.; Manaut, B.; Elhadadi, B. First-Principles Study of Co-Doped Wurtzite ZnO: Insights into Carrier Dynamics, Visible Light Absorption, and Structural Properties for Solar Cell Applications. Opt. Quantum Electron. 2025, 57, 269. [Google Scholar] [CrossRef]
- Archi, M.; Bajjou, O.; Rahmani, K.; Elhadadi, B. Investigation of Structural, Phonon, Thermodynamic, Electronic, and Mechanical Properties of Non-Toxic XZnH3 (X = Li, Na, K) Perovskites for Solid-State Hydrogen Storage: A DFT and AIMD Approach. J. Energy Storage 2025, 112, 115492. [Google Scholar] [CrossRef]
- Archi, M.; Bajjou, O.; Rahmani, k.; Elhadadi, B. A Comparative Ab Initio Analysis of the Stability, Electronic, Thermodynamic, Mechanical, and Hydrogen Storage Properties of SrZnH3 and SrLiH3 Perovskite Hydrides through DFT and AIMD Approaches. Int. J. Hydrogen Energy 2025, 105, 759–770. [Google Scholar] [CrossRef]
- Razzaq, M.A. Mechanical, Electronic, Optical, and Thermoelectric Properties of Rb 2 TlGaX 6 (X = F, I) Lead-Free Double Perovskites: A First-Principles Study. Phys. Scr. 2025, 100, 015906. [Google Scholar] [CrossRef]
- Sajid, L.; Saeed, M.U.; Mashadi, S.H.; Abid, S.S.; Pervaiz, S.; Ali, Z.; Alanazi, Y.M.; Bacha, A.U.R.; Saeed, Y. Ab Initio Study of Electronic, Elastic, Thermodynamic, Photocatalytic Properties of Double Antiperovskite, Cs6AgBiX2 (X = Cl, Br, I). RSC Adv. 2024, 14, 35348–35359. [Google Scholar] [CrossRef]
- Ullah, H.M.N.; Rizwan, M.; Ali, S.S.; Usman, Z.; Cao, C. A DFT Study of Optical, Elastic, Mechanical, and Overall Water-Splitting Photocatalytic Properties of Pristine and Cd Substituted BaZrO3: A Lead Free Environment Friendly Material. Mater. Sci. Eng. B 2022, 286, 116041. [Google Scholar] [CrossRef]
- Karwasara, H.; Bhamu, K.C.; Kang, S.G.; Kushwaha, A.K.; Rai, D.P.; Sappati, S.; Sahariya, J.; Soni, A. Ab-Initio Investigations for Structural, Mechanical, Optoelectronic, and Thermoelectric Properties of Ba2SbXO6 (X[Dbnd]Nb, Ta) Compounds. J. Alloys Compd. 2022, 893, 162332. [Google Scholar] [CrossRef]
- Uddin, S.; Das, A.; Rayhan, M.A.; Ahmad, S.; Khokan, R.M.; Rasheduzzaman, M.; Das, R.; Ullah, A.; Arafat, Y.; Hasan, M.Z. Theoretical Prediction of the Mechanical, Electronic, Optical and Thermodynamic Properties of Antiperovskites A3BO (A = K, Rb and B = Au, Br) Using DFT Scheme: New Candidate for Optoelectronic Devices Application. J. Comput. Electron. 2024, 23, 1217–1237. [Google Scholar] [CrossRef]
- Alotaibi, N.H. Frist Principle Study of Double Perovskites Cs2AgSbX6 (X = Cl, Br, I) for Solar Cell and Renewable Energy Applications. J. Phys. Chem. Solids 2022, 171, 110984. [Google Scholar] [CrossRef]
- Saeed, M.; Haq, I.U.; Saleemi, A.S.; Rehman, S.U.; Haq, B.U.; Chaudhry, A.R.; Khan, I. First-Principles Prediction of the Ground-State Crystal Structure of Double-Perovskite Halides Cs2AgCrX6 (X = Cl, Br, and I). J. Phys. Chem. Solids 2022, 160, 110302. [Google Scholar] [CrossRef]
- Alotaibi, N.H.; Mustafa, G.M.; Kattan, N.A.; Mahmood, Q.; Albalawi, H.; Morsi, M.; Somaily, H.H.; Hafez, M.A.; Mahmoud, H.I.; Amin, M.A. DFT Study of Double Perovskites Cs2AgBiX6 (X = Cl, Br): An Alternative of Hybrid Perovskites. J. Solid State Chem. 2022, 313, 123353. [Google Scholar] [CrossRef]
- Karouchi, M.; Bajjou, O.; Jabraoui, H.; Ejjabli, A.; Archi, M.; Moulaoui, L.; Rahmani, K.; Lachtioui, Y. Increasing Electro-Optical Properties of Perovskite FAPbI3 Under the Effect of Doping by Sn. In Proceedings of the 2023 3rd International Conference on Innovative Research in Applied Science, Engineering and Technology (IRASET), Mohammedia, Morocco, 18–19 May 2023; pp. 1–7. [Google Scholar]
- Archi, M.; Bajjou, O.; Moulaoui, L.; Najim, A.; Karouchi, M.; Rahmani, K.; El haddadi, B. Electronic and Optical Properties of Different Concentrations of Ga Doped ZnO: CASTEP Study. In Proceedings of the 2023 3rd International Conference on Innovative Research in Applied Science, Engineering and Technology (IRASET), Mohammedia, Morocco, 18–19 May 2023; pp. 1–7. [Google Scholar]
- Ejjabli, A.; Karouchi, M.; Errahoui, H.; Bajjou, O.; Guerroum, J.; Elhajji, A.; Rahmani, K.; Lachtioui, Y. Electronic and Optical Properties of Double Perovskite Oxide LaFeWO6: A Theoretical Understanding from DFT Calculations. E3S Web Conf. 2024, 582, 02001. [Google Scholar] [CrossRef]
- Laassouli, A.; Moulaoui, L.; Najim, A.; Errahoui, H.; Rahmani, K.; Lachtioui, Y.; Omar Bajjou, O.B. Phosphorus Doping Effects on the Optoelectronic Properties of K2AgAsBr6 Double Perovskites for Photovoltaic Applications. Sol. Energy Sustain. Dev. J. 2024, 14, 1–11. [Google Scholar] [CrossRef]
- Errahoui, H.; Karouchi, M.; Ejjabli, A.; El haji, A.; Laassouli, A.; Ait El Alia, O.; Chaji, S.; Lachtioui, Y.; Bajjou, O. Impact of Calcium Doping on the Electronic and Optical Characteristics of Strontium Hydride (SrH2): A DFT Study. Atoms 2024, 12, 55. [Google Scholar] [CrossRef]
- Karouchi, M.; Ejjabli, A.; Bajjou, O.; Guerroum, J.; Al-Hattab, M.; Basyooni-M. Kabatas, M.A.; Rahmani, K.; Lachtioui, Y. Investigating the Structural, Electronic, and Optical Properties of the Novel Double Perovskite K2AgBiI6 Using DFT. Front. Mater. 2024, 11, 1448400. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Marques, H.M. The Cs2AgRhCl6 Halide Double Perovskite: A Dynamically Stable Lead-Free Transition-Metal Driven Semiconducting Material for Optoelectronics. Front. Chem. 2020, 8, 796. [Google Scholar] [CrossRef]
- Green, M.A.; Jiang, Y.; Soufiani, A.M.; Ho-Baillie, A. Optical Properties of Photovoltaic Organic–Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 4774–4785. [Google Scholar] [CrossRef]
- Karouchi, M.; Ejjabli, A.; Samine, S.; Bajjou, O.; Lachtioui, Y. Enhancing the Optoelectronic Properties of TiPbO3 Perovskite through Lanthanum Doping. Sol. Energy Sustain. Dev. J. 2024, 14, 142–155. [Google Scholar] [CrossRef]
- Ambrosch-Draxl, C.; Sofo, J.O. Linear Optical Properties of Solids within the Full-Potential Linearized Augmented Planewave Method. Comput. Phys. Commun. 2006, 175, 1–14. [Google Scholar] [CrossRef]
- Pingak, R.K.; Harbi, A.; Bouhmaidi, S.; Johannes, A.Z.; Hauwali, N.U.J.; Khan, W.; Nitti, F.; Tambaru, D.; Moutaabbid, M.; Setti, L. Novel Tl2GeX6 (X = Cl,Br) Double Perovskites for Solar Cell, Optoelectronic, and Thermoelectric Applications: A DFT Investigation. Chem. Phys. Impact 2024, 9, 100749. [Google Scholar] [CrossRef]
- Ayyaz, A.; Murtaza, G.; Umer, M.; Usman, A.; Raza, H.H. Structural, Elastic, Optoelectronic, and Transport Properties of Na-Based Halide Double Perovskites Na2CuMX6 (M = Sb, Bi, and X = Cl, Br) as Renewable Energy Materials: A DFT Insight. J. Mater. Res. 2023, 38, 4609–4624. [Google Scholar] [CrossRef]
- Al-Daraghmeh, T.M.; Nazir, G.; Zayed, O.; Kattan, N.A.; Rouf, S.A.; Albalawi, H.; Aljameel, A.I.; Boukhris, I. Tuning of Optical, Thermodynamic, and Thermoelectric Properties of Cs2CuBiX6 (X = Cl, Br, I) Halide Perovskites for Solar Cells and Energy Harvesting Applications. Phys. Scr. 2024, 99, 105969. [Google Scholar] [CrossRef]
- Hedhili, F.; Khan, H.; Sohail, M.; Rahman, N.; Khan, R.; Alahmad, W.; Albaqawi, H.S.; Al-Shomar, S.M.; Alsalmi, O. Structural, Electronic, Elastic, and Optical Characteristics of AgZF3 (Z = Sb and Bi) Fluoro-Perovskites: Using a Computational Approach for Energy Generation. Molecules 2023, 28, 4418. [Google Scholar] [CrossRef] [PubMed]
- Hasnip, P.J.; Refson, K.; Probert, M.I.J.; Yates, J.R.; Clark, S.J.; Pickard, C.J. Density Functional Theory in the Solid State. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130270. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-Principles Simulation: Ideas, Illustrations and the CASTEP Code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.M.; Gázquez, J.L.; Trickey, S.B.; Vela, A. Non-Empirical Improvement of PBE and Its Hybrid PBE0 for General Description of Molecular Properties. J. Chem. Phys. 2012, 136, 104108. [Google Scholar] [CrossRef]
- Liu, D.C.; Nocedal, J. On the Limited Memory BFGS Method for Large Scale Optimization. Math Program 1989, 45, 503–528. [Google Scholar] [CrossRef]
- Bhattarai, S.; Hossain, I.; Maiti, M.; Pandey, R.; Madan, J. Performance Analysis and Optimization of All-Inorganic CsPbI3-Based Perovskite Solar Cell. Indian J. Phys. 2023, 97, 2629–2637. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).