Carbonic Anhydrases in Photosynthesizing Cells of C3 Higher Plants
Abstract
1. Introduction
2. CA Gene Expression in Arabidopsis Leaves
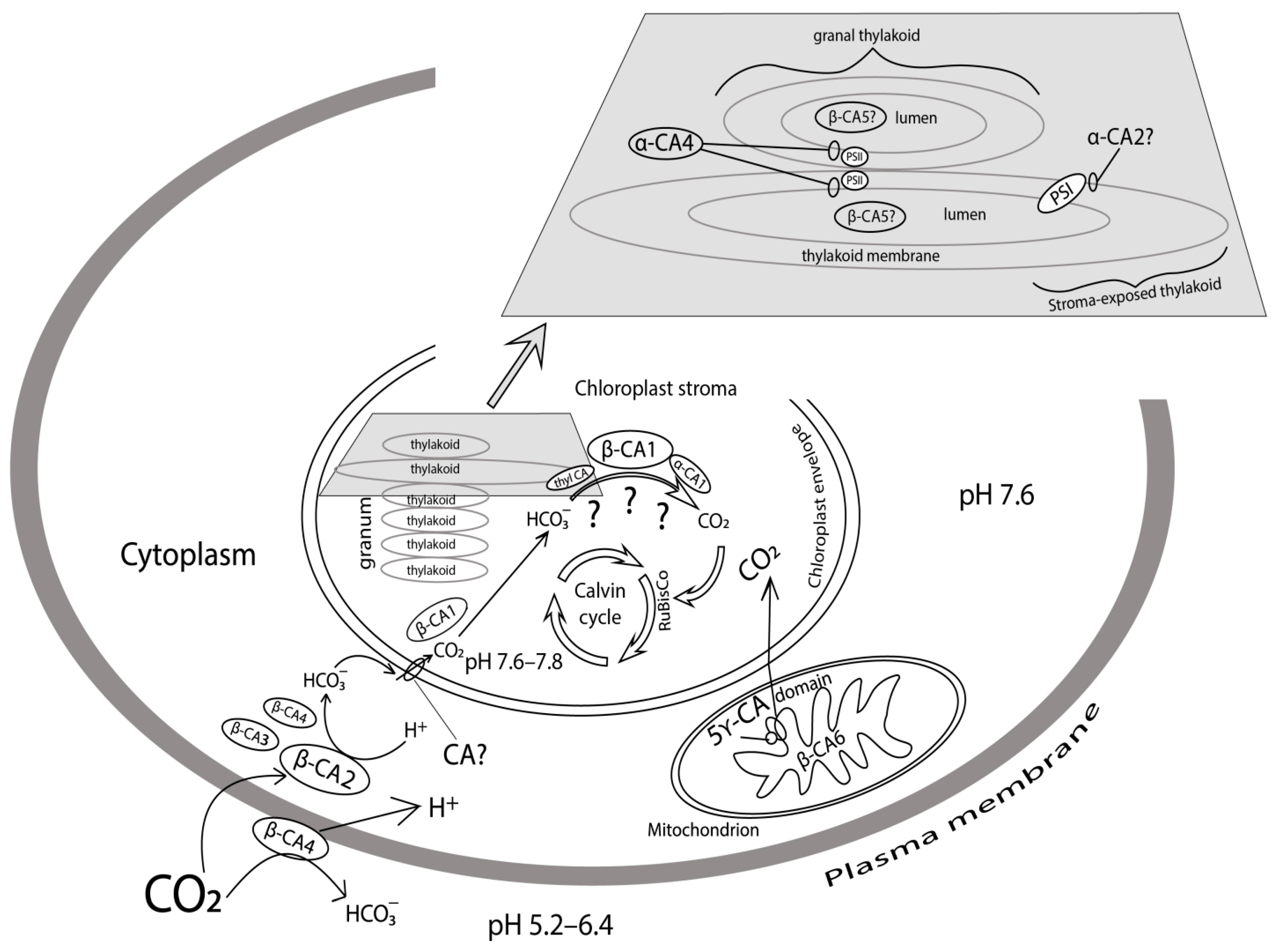
3. Carbonic Anhydrase in Plasma Membrane
4. Carbonic Anhydrases in Cytoplasm
5. Carbonic Anhydrases in Mitochondria
6. Carbonic Anhydrases in Chloroplasts
6.1. Carbonic Anhydrases in Chloroplasts Stroma
6.2. Carbonic Anhydrases in Thylakoids
6.2.1. Carbonic Anhydrases in Thylakoid Membranes
6.2.2. The Role of Carbonic Anhydrase in the Stimulation of Photophosphorylation by Bicarbonate
6.2.3. Carbonic Anhydrase in Thylakoid Lumen
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meldrum, N.U.; Roughton, F.J.W. Carbonic anhydrase. Its preparation and properties. J. Physiol. 1933, 80, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Keilin, D.; Mann, T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem. J. 1940, 34, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, D.; Pan, W.; Beggs, M.R.; Trepiccione, F.; Chambrey, R.; Eladari, D.; Cordat, E.; Dimke, H.; Alexander, R.T. Deficiency of carbonic anhydrase II results in a urinary concentrating defect. Front. Physiol. 2018, 8, 1108. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic Anhydrases and Metabolism. Metabolites 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, M.; Szahidewicz-Krupska, E.; Doroszko, A. The Human Carbonic Anhydrase II in Platelets: An Underestimated Field of Its Activity. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Sly, W.S.; Hu, P.Y. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu. Rev. Biochem. 1995, 64, 375–401. [Google Scholar] [CrossRef] [PubMed]
- Hewett-Emmett, D.; Tashian, R.E. Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol. Phylogenet Evol. 1996, 5, 50–77. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.C. Studies on chloroplasts: Their chemical composition and the distribution of certain metabolites between the chloroplasts and the remainder of the leaf. Biochem. J. 1939, 33, 300–308. [Google Scholar] [CrossRef]
- Moroney, J.V.; Bartlett, S.J.; Samuelson, G. Carbonic Anhydrases in Plants and Algae. Plant. Cell Environ. 2001, 24, 141–153. [Google Scholar] [CrossRef]
- Fabre, N.; Reiter, I.M.; Becuwe-Linka, N.; Genty, B.; Rumeau, D. Characterization and expression analysis of genes encoding α and β carbonic anhydrases in Arabidopsis. Plant. Cell Environ. 2007, 30, 617–629. [Google Scholar] [CrossRef]
- Missner, A.; Kügler, P.; Saparov, S.M.; Sommer, K.; Mathai, J.C.; Zeidel, M.L.; Pohl, P. Carbon dioxide transport through membranes. J. Biol. Chem. 2008, 283, 25340–25347. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Liu, F.C.; Xie, W.F.; Wang, G.D.; Wang, J.; Gao, Q.H.; Duan, K. Family-wide expression characterization of Arabidopsis beta-carbonic anhydrase genes using qRT-PCR and Promoter:: GUS fusions. Biochimie 2014, 97, 219–227. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Vetoshkina, D.V.; Fedorchuk, T.P.; Ivanov, B.N. Effect of light intensity under different photoperiods on expression level of carbonic anhydrase genes of the α-and β-families in Arabidopsis thaliana leaves. Biochem. Mosc. 2017, 82, 1025–1035. [Google Scholar] [CrossRef]
- Friso, G.; Giacomelli, L.; Ytterberg, A.J.; Peltier, J.B.; Rudella, A.; Sun, Q.; van Wijk, K.J. In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: New proteins, new functions, and a plastid proteome database. Plant. Cell 2004, 16, 478–499. [Google Scholar] [CrossRef]
- Makita, Y.; Shimada, S.; Kawashima, M.; Kondou-Kuriyama, T.; Toyoda, T.; Matsui, M. MOROKOSHI: Transcriptome database in Sorghum bicolor. Plant. Cell Physiol. 2014, 56, e6. [Google Scholar] [CrossRef]
- Pfanz, H. Apoplastic and symplastic proton concentrations and their significance for metabolism. In Ecophysiology of Photosynthesis, 2nd ed.; Schulze, E.-D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1994; pp. 103–122. ISBN 13: 978-3-540-58571-8. [Google Scholar]
- Faurholt, C. Etudes sur les solutions aqueuses d’anhydride carbonique et d’acide carbonique. J. Chim. Phys. 1923, 20, 400–455. [Google Scholar] [CrossRef]
- Mitz, M.A. CO2 biodynamics: A new concept of cellular control. J. Theor. Biol. 1979, 80, 537–551. [Google Scholar] [CrossRef]
- Ignatova, L.K.; Romanova, A.K. Carbonic anhydrase involvement in the pea protoplast photosynthesis inhibition by CO2 excess. Russ. J. Plant. Physiol. 1992, 39, 82–88. [Google Scholar]
- Enser, U.; Heber, U. Metabolic regulation by pH gradients. Inhibition of photosynthesis by indirect proton transfer across the chloroplast envelope. Biochim. Biophys. Acta 1980, 592, 577–591. [Google Scholar] [CrossRef]
- Utsunomia, E.; Muto, S. Carbonic anhydrase in the plasma membranes from leaves of C3 and C4 plants. Physiol. Plant. 1993, 88, 413–419. [Google Scholar] [CrossRef]
- Ignatova, L.K.; Moskvin, O.V.; Ivanov, B.N.; Romanova, A.K. The effect of CO2 uptake by pea protoplasts on the CO2 evolution rate and parameters of chlorophyll fluorescence quenching. Plant. Physiol. Biochem. 1993, 31, 295–301. [Google Scholar]
- Ignatova, L.K.; Moskvin, O.V.; Romanova, A.K.; Ivanov, B.N. Carbonic anhydrases in the C3-plant leaf cell. Funct. Plant. Biol. 1998, 25, 673–677. [Google Scholar] [CrossRef]
- Ignatova, L.K.; Moskvin, O.V.; Ivanov, B.N. Effects of carbonic anhydrase inhibitors on proton exchange and photosynthesis in pea protoplasts. Russ. J. Plant. Physiol. 2001, 48, 467–472. [Google Scholar] [CrossRef]
- DiMario, R.J.; Quebedeaux, J.C.; Longstreth, D.; Dassanayake, M.; Hartman, M.M.; Moroney, J.V. The cytoplasmic carbonic anhydrases βCA2 and βCA4 are required for optimal plant growth at low CO2. Plant. Physiol. 2016, 171, 280–293. [Google Scholar] [CrossRef]
- Kachru, R.; Anderson, L. Chloroplast and cytoplasmic enzymes. Pea-leaf carbonic anhydrase. Planta 1974, 118, 235–240. [Google Scholar] [CrossRef]
- Ku, S.B.; Edwards, G.E. Photosynthesis in mesophyll protoplasts and bundle sheath cells of various types of C4 plants IV. Enzymes of respiratory metabolism and energy utilizing enzymes of photosynthetic pathways. Zeitschrift für Pflanzenphysiologie 1975, 77, 16–32. [Google Scholar] [CrossRef]
- Fawcett, T.W.; Browse, J.A.; Volokita, M.; Bartlett, S.G. Spinach carbonic anhydrase primary structure deduced from the sequence of a cDNA clone. J. Biol. Chem. 1990, 265, 5414–5417. [Google Scholar]
- Von Caemmerer, S.; Quinn, V.; Hancock, N.C.; Price, G.D.; Furbank, R.T.; Ludwig, M. Carbonic anhydrase and C4 photosynthesis: A transgenic analysis. Plant. Cell Environ. 2004, 27, 697–703. [Google Scholar] [CrossRef]
- Burnell, J.N.; Hatch, M.D. Low bundle sheath carbonic anhydrase is apparently essential for effective C4 pathway operation. Plant. Physiol. 1988, 86, 1252–1256. [Google Scholar] [CrossRef]
- Rumeau, D.; Cuine, S.; Fina, L.; Gault, N.; Nicole, M.; Peltier, G. Subcellular distribution of carbonic anhydrase in Solanum tuberosum L. leaves: Characterization of two compartment—Specific isoforms. Planta 1996, 199, 79–88. [Google Scholar]
- Fett, J.P.; Coleman, J.R. Characterization and expression of two cDNAs encoding carbonic anhydrase in Arabidopsis thaliana. Plant. Physiol. 1994, 105, 707–713. [Google Scholar] [CrossRef]
- Moroney, J.V.; Ma, Y.; Frey, W.D.; Fusilier, K.A.; Pham, T.T.; Simms, T.A.; DiMario, R.J.; Yang, J.; Mukherjee, B. The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles. Photosynth. Res. 2011, 109, 133–149. [Google Scholar] [CrossRef]
- Sunderhaus, S.; Dudkina, N.V.; Jänsch, L.; Klodmann, J.; Heinemeyer, J.; Perales, M.; Zabaleta, E.; Boekema, E.J.; Braun, H.P. Carbonic anhydrase subunits form a matrix-exposed domain attached to the membrane arm of mitochondrial complex I in plants. J. Biol. Chem. 2006, 281, 6482–6488. [Google Scholar] [CrossRef]
- Riazunnisa, K.; Padmavathi, L.; Bauwe, H.; Raghavendra, A.S. Markedly low requirement of added CO2 for photosynthesis by mesophyll protoplasts of pea (Pisum sativum): Possible roles of photorespiratory CO2 and carbonic anhydrase. Physiol. Plant. 2006, 128, 763–772. [Google Scholar] [CrossRef]
- Zabaleta, E.; Martin, M.V.; Braun, H.-P. A basal carbon concentrating mechanism in plants? Plant. Sci. 2012, 187, 97–104. [Google Scholar] [CrossRef]
- Soto, D.; Córdoba, J.P.; Villarreal, F.; Bartoli, C.; Schmitz, J.; Maurino, V.G.; Braun, H.P.; Pagnussat, G.C.; Zabaleta, E. Functional characterization of mutants affected in the carbonic anhydrase domain of the respiratory complex I in Arabidopsis thaliana. Plant J. 2015, 83, 831–844. [Google Scholar] [CrossRef]
- Jiang, C.; Tholen, D.; Xu, J.M.; Xin, C.; Zhang, H.; Zhu, X.; Zhao, Y. Increased expression of mitochondria-localized carbonic anhydrase activity resulted in an increased biomass accumulation in Arabidopsis thaliana. J. Plant. Biol. 2014, 57, 366–374. [Google Scholar] [CrossRef]
- Giordano, M.; Norici, A.; Forssen, M.; Eriksson, M.; Raven, J.A. An anaplerotic role for mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant. Physiol. 2003, 132, 2126–2134. [Google Scholar] [CrossRef]
- Jacobson, B.S.; Fong, F.; Heath, R.L. Carbonic anhydrase of spinach: Studies on its location, inhibition, and physiological function. Plant. Physiol. 1975, 55, 468–474. [Google Scholar] [CrossRef][Green Version]
- Price, G.D.; von Caemmerer, S.; Evans, J.R.; Yu, J.-W.; Lloyd, J.; Oja, V.; Harrison, K.; Gallagher, A.; Badger, M.R. Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation. Planta 1994, 193, 331–340. [Google Scholar] [CrossRef]
- Ferreira, F.J.; Guo, C.; Coleman, J.R. Reduction of plastid localized carbonic anhydrase activity results in reduced Arabidopsis seedling survivorship. Plant. Physiol. 2008, 147, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Werdan, K.; Heldt, H.W.; Geller, G. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim. Biophys. Acta 1972, 283, 430–441. [Google Scholar] [CrossRef]
- Anderson, L.E.; Carol, A.A. Enzyme co-localization with Rubisco in pea leaf chloroplasts. Photosyn. Res. 2004, 82, 49–58. [Google Scholar] [CrossRef]
- Lazova, G.N.; Stemler, A.J. A 160 kDa protein with carbonic anhydrase activity is complexed with rubisco on the outer surface of thylakoids. Cell Biol. Int. 2008, 32, 646–653. [Google Scholar] [CrossRef]
- Jebanathirajah, J.A.; Coleman, J.R. Association of carbonic anhydrase with a Calvin cycle enzyme complex in Nicotiana tabacum. Planta 1998, 204, 177–182. [Google Scholar] [CrossRef]
- Villarejo, A.; Buren, S.; Larsson, S.; Dejardin, A.; Monne, M.; Rudhe, C.; Karlsson, J.; Jansson, S.; Lerouge, P.; Rolland, N.; et al. Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat. Cell Biol. 2005, 7, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Buren, S. Targeting and Function of CAH1 Characterisation of a Novel Protein Pathway to the Plant Cell Chloroplast. Ph.D. Thesis, Umea University, Umea, Sweden, 29 January 2010. [Google Scholar]
- Restrepo, S.; Myers, K.L.; del Pozo, O.; Martin, G.B.; Hart, A.L.; Buell, C.R.; Fry, W.E.; Smart, C.D. Gene profiling of a compatible interaction between Phytophthora infestans and Solanum tuberosum suggests a role for carbonic anhydrase. Mol. Plant. Microbe Interact. 2005, 18, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Hoang, C.V.; Chapman, K.D. Biochemical and molecular inhibition of plastidial carbonic anhydrase reduces the incorporation of acetate into lipids in cotton embryos and tobacco cell suspensions and leaves. Plant. Physiol. 2002, 128, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Slaymaker, D.H.; Navarre, D.A.; Clark, D.; del Pozo, O.; Martin, G.B.; Klessig, D. The tobacco salicylic acid binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 11640–11645. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Boisson-Dernier, A.; Israelsson-Nordstrom, M.; Bohmer, M.; Xue, S.; Ries, A.; Godoski, J.; Kuhn, J.M.; Schroeder, J.I. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef]
- Komarova, Y.M.; Doman, N.G.; Shaposhnikov, G.L. Two forms of carbonic anhydrase from bean chloroplasts. Biochem. Mosc. 1982, 47, 856–862. [Google Scholar]
- Vaklinova, S.G.; Goushtina, L.M.; Lazova, G.N. Carboanhydrase activity in chloroplasts and chloroplast fragments. C R Acad. Bulg. Sci. 1982, 35, 1721–1724. [Google Scholar]
- Stemler, A. The case for chloroplast thylakoid carbonic anhydrase. Physiol. Plant. 1997, 99, 348–353. [Google Scholar] [CrossRef]
- Moubarak-Milad, M.; Stemler, A. Oxidation-reduction potential dependence of photosystem II carbonic anhydrase in maize thylakoids. Biochemistry 1994, 33, 4432–4438. [Google Scholar] [CrossRef]
- Moskvin, O.V.; Ignatova, L.K.; Ovchinnikova, V.I.; Ivanov, B.N. Membrane associated carbonic anhydrase of pea thylakoids. Biochem. Mosc. 1995, 60, 859–864. [Google Scholar]
- Moskvin, O.V.; Shutova, T.V.; Khristin, M.S.; Ignatova, L.K.; Villarejo, A.; Samuelsson, G.; Klimov, V.V.; Ivanov, B.N. Carbonic anhydrase activities in pea thylakoids. Photosynth Res. 2004, 79, 93–100. [Google Scholar] [CrossRef]
- Pronina, N.A.; Allakhverdiev, S.I.; Kupriyanova, E.V.; Klyachko-Gurvich, G.L.; Klimov, V.V. Carbonic anhydrase in subchloroplast particles of pea plants. Russ. J. Plant. Physiol. 2002, 49, 303–310. [Google Scholar] [CrossRef]
- Karlsson, J.; Hiltonen, T.; Husic, H.D.; Ramazanov, Z.; Samuelsson, G. Intracellular carbonic anhydrase of Chlamydomonas reinhardtii. Plant. Physiol. 1995, 109, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.N.; Ignatova, L.K.; Ivanov, B.N. Multiple sources of carbonic anhydrase activity in pea thylakoids: Soluble and membrane-bound forms. Photosynth. Res. 2007, 91, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, L.K.; Rudenko, N.N.; Mudrik, V.A.; Ivanov, B.N. Carbonic anhydrase activity in Arabidopsis thaliana thylakoid membrane and fragments enriched with PSI or PSII. Photosynth. Res. 2011, 110, 89–98. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Ignatova, L.K.; Kamornitskaya, V.B.; Ivanov, B.N. Pea Leaf Thylakoids Contain Several Carbonic Anhydrases. Dokl. Biochem. Biophys. 2006, 408, 155–157. [Google Scholar] [CrossRef]
- Ignatova, L.K.; Rudenko, N.N.; Khristin, M.S.; Ivanov, B.N. Heterogeneous origin of carbonic anhydrase activity of thylakoid membranes. Biochem. Mosc. 2006, 71, 525–532. [Google Scholar] [CrossRef]
- Shitov, A.V.; Terentyev, V.V.; Zharmukhamedov, S.K.; Rodionova, M.V.; Karacan, M.; Karacan, N.; Klimov, V.V.; Allakhverdiev, S.I. Is carbonic anhydrase activity of photosystem II required for its maximum electron transport rate? Biochim. Biophys. Acta Bioenergy 2018, 1859, 292–299. [Google Scholar] [CrossRef]
- Graham, D.; Perry, G.L.; Atkins, C.A. In search of a role for carbonic anhydrase in photosynthesis. In Mechanisms of Regulation of Plant Growth, 2nd ed.; Bieleski, R.L., Ferguson, A.R., Cresswell, M.M., Eds.; Royal Society of New Zealand: Wellington, New Zealand, 1974; Volume 12, pp. 251–258. [Google Scholar]
- Swader, J.A.; Jacobson, B.S. Acetazolamide inhibition of photosystem II in isolated spinach chloroplasts. Phytochemistry 1972, 11, 65–70. [Google Scholar] [CrossRef]
- Shitov, A.V.; Zharmukhamedov, S.K.; Shutova, T.V.; Allakhverdiev, S.I.; Samuelsson, G.; Klimov, V.V. A carbonic anhydrase inhibitor induces bicarbonate-reversible suppression of electron transfer in pea photosystem 2 membrane fragments. J. Photochem. Photobiol. Biol. 2011, 104, 366–371. [Google Scholar] [CrossRef]
- Fedorchuk, T.P.; Opanasenko, V.K.; Rudenko, N.N.; Ivanov, B.N. Bicarbonate-induced stimulation of photophosphorylation in isolated thylakoids: Effect of carbonic anhydrase inhibitors. Biol. Membr. 2018, 35, 34–41. [Google Scholar] [CrossRef]
- Lu, Y.K.; Stemler, A.J. Extrinsic photosystem II carbonic anhydrase in maize mesophyll chloroplasts. Plant. Physiol. 2002, 266, 16746–16754. [Google Scholar] [CrossRef]
- Khristin, M.S.; Ignatova, L.K.; Rudenko, N.N.; Ivanov, B.N.; Klimov, V.V. Photosystem II associated carbonic anhydrase activity in higher plants is situated in core complex. FEBS Lett. 2004, 577, 305–308. [Google Scholar] [CrossRef]
- Shitov, A.V.; Pobeguts, O.V.; Smolova, T.N.; Allakhverdiev, S.I.; Klimov, V.V. Manganese dependent carbonic anhydrase activity of photosystem II proteins. Biochem. Mosc. 2009, 74, 509–517. [Google Scholar] [CrossRef]
- Ignatova, L.K.; Ivanov, B.N. Carbonic anhydrases of higher plant thylakoids and their participation in photosynthesis. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 193–200. [Google Scholar]
- Van Rensen, J.J.; Tonk, W.J.M.; Bruijn, S.M. Involvement of bicarbonate in the protonation of the secondary quinone electron acceptor of photosystem II via the non-heme iron of the quinone-iron acceptor complex. FEBS Lett. 1988, 226, 347–351. [Google Scholar] [CrossRef]
- Lu, Y.K.; Stemler, A.J. Differing responses of the two forms of photosystem II carbonic anhydrase to chloride, cations, and pH. Biochim. Biophys. Acta 2007, 1767, 633–638. [Google Scholar] [CrossRef]
- Bricker, T.M.; Frankel, L.K. Auxiliary functions of the PsbO, PsbP and PsbQ proteins of higher plant photosystem II: A critical analysis. J. Photochem. Photobiol. B Biol. 2011, 104, 165–178. [Google Scholar] [CrossRef]
- Ignatova, L.; Zhurikova, E.; Ivanov, B. The presence of low molecular mass carbonic anhydrase in photosystem II of C3 higher plant. J. Plant. Physiol. 2019, 232, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhurikova, E.M.; Ignatova, L.K.; Semenova, G.; Rudenko, N.N.; Mudrik, V.A.; Ivanov, B.N. Effect of knockout of α-carbonic anhydrase 4 gene on photosynthetic characteristics and starch accumulation in leaves of Arabidopsis thaliana. Russ. J. Plant. Physiol. 2015, 62, 564–569. [Google Scholar] [CrossRef]
- Zhurikova, E.M.; Ignatova, L.K.; Rudenko, N.N.; Mudrik, V.A.; Vetoshkina, D.V.; Ivanov, B.N. The participation of two carbonic anhydrases of alpha family in photosynthetic reactions in Arabidopsis thaliana. Biochem. Mosc. 2016, 81, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Ilies, M.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase activators. In Carbonic Anhydrase: Its Inhibitors and Activators; Supuran, C.T., Scozzafava, A., Conway, G., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 317–352. ISBN 9780415306737. [Google Scholar]
- Rudenko, N.N.; Fedorchuk, T.P.; Vetoshkina, D.V.; Zhurikova, E.M.; Ignatova, L.K.; Ivanov, B.N. Influence of knockout of At4g20990 gene encoding α-CA4 on photosystem II light harvesting antenna in plants grown under different light intensities and day lengths. Protoplasma 2018, 255, 69–78. [Google Scholar] [CrossRef]
- Zhurikova, E.M. Study of Participation of Alpha-Carbonic Anhydrase 2 and Alpha-Carbonic Anhydrase 4 in Photosynthetic Metabolism Arabidopsis thaliana. Ph.D. Thesis, IBBP RAS, Pushchino, Russia, 2016. [Google Scholar]
- Punnett, T.; Iyer, R.V. The enhancement of photophosphorylation and the Hill reaction by carbon dioxide. J. Biol. Chem. 1964, 239, 2335–2339. [Google Scholar]
- Cohen, W.S.; Jagendorf, A.T. Inhibition of energy-linked reactions in chloroplasts by polygalacturonate. Arch. Biochem. Biophys. 1972, 150, 235–243. [Google Scholar] [CrossRef]
- Cohen, W.S.; MacPeek, W.A. A proposed mechanism for the stimulatory effect of bicarbonate ions on ATP synthesis in isolated chloroplasts. Plant. Physiol. 1980, 66, 242–245. [Google Scholar] [CrossRef]
- Podorvanov, V.V.; Zolotareva, E.K.; Chernoshtan, A.A. The role of bicarbonate in light-dependent proton absorption by isolated chloroplasts. Fiziol. Biochem. Cult. Plants 2005, 37, 326–332. [Google Scholar]
- Onoiko, E.B.; Polishchuck, A.V.; Zolotareva, E.K. The stimulation of photophosphorylation in isolated spinach chloroplasts by exogenous bicarbonate: The role of carbonic anhydrase. Rep. Nat. Acad. Sci. Ukr. 2010, 10, 161–165. [Google Scholar]
- Fedorchuk, T.; Rudenko, N.; Ignatova, L.; Ivanov, B. The presence of soluble carbonic anhydrase in the thylakoid lumen of chloroplasts from Arabidopsis leaves. J. Plant. Physiol. 2014, 171, 903–906. [Google Scholar] [CrossRef]
- Moroney, J.V.; (Louisiana State University, Baton Rouge, LA, USA). Personal communication, 2010.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatova, L.; Rudenko, N.; Zhurikova, E.; Borisova-Mubarakshina, M.; Ivanov, B. Carbonic Anhydrases in Photosynthesizing Cells of C3 Higher Plants. Metabolites 2019, 9, 73. https://doi.org/10.3390/metabo9040073
Ignatova L, Rudenko N, Zhurikova E, Borisova-Mubarakshina M, Ivanov B. Carbonic Anhydrases in Photosynthesizing Cells of C3 Higher Plants. Metabolites. 2019; 9(4):73. https://doi.org/10.3390/metabo9040073
Chicago/Turabian StyleIgnatova, Lyudmila, Natalia Rudenko, Elena Zhurikova, Maria Borisova-Mubarakshina, and Boris Ivanov. 2019. "Carbonic Anhydrases in Photosynthesizing Cells of C3 Higher Plants" Metabolites 9, no. 4: 73. https://doi.org/10.3390/metabo9040073
APA StyleIgnatova, L., Rudenko, N., Zhurikova, E., Borisova-Mubarakshina, M., & Ivanov, B. (2019). Carbonic Anhydrases in Photosynthesizing Cells of C3 Higher Plants. Metabolites, 9(4), 73. https://doi.org/10.3390/metabo9040073




